

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242333 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260295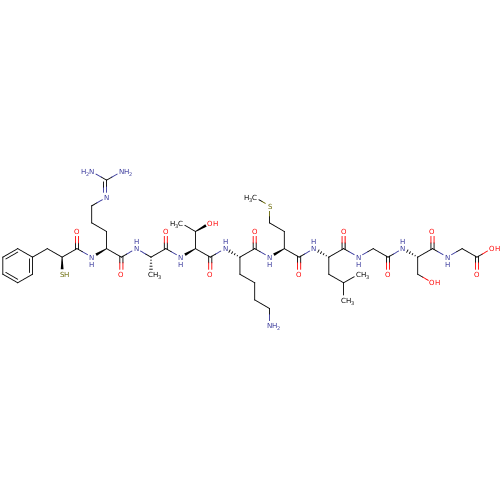 (2-((S)-2-(2-((S)-2-((S)-2-((S)-6-amino-2-((2S,3R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260294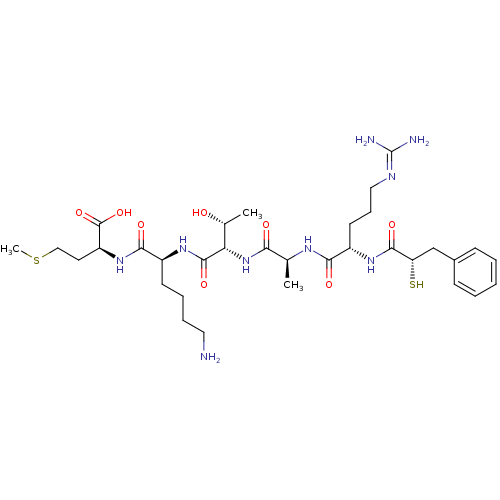 ((S)-2-((S)-6-amino-2-((2S,3R)-2-((S)-2-((S)-5-guan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242337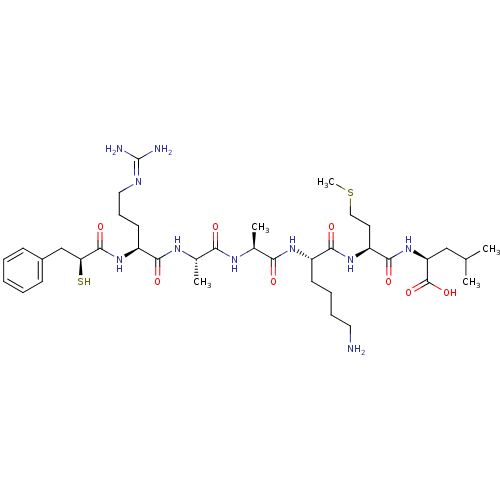 ((S)-2-{(S)-2-[(S)-6-Amino-2-((S)-2-{(S)-2-[(S)-5-g...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242339 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260296 (CHEMBL501525 | CRATKML) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242336 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50240902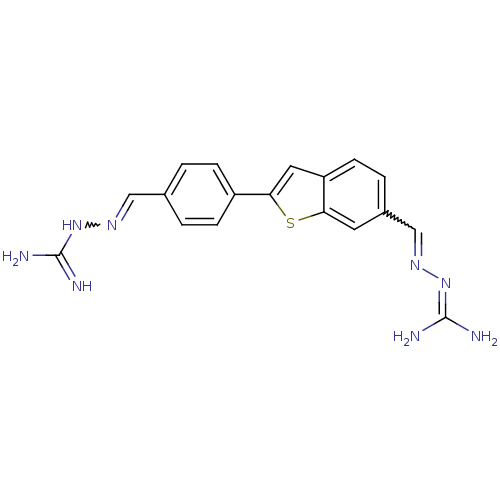 ((2E)-2-{4-[6-((E)-{[(E)-amino(imino)methyl]hydrazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242338 ((S)-2-{(S)-2-[(S)-2-((2S,3R)-2-{(S)-2-[(S)-5-Guani...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260293 ((S)-6-amino-2-((2S,3R)-2-((S)-2-((S)-5-guanidino-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50240901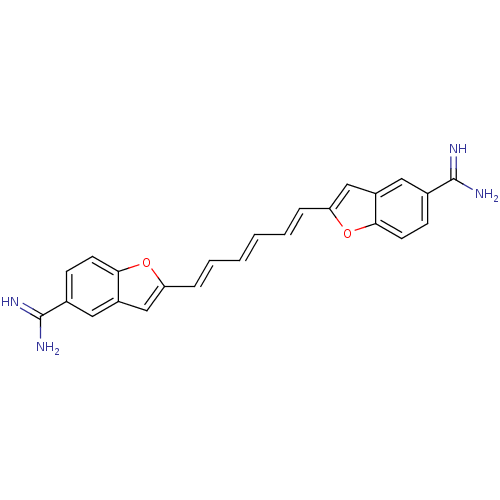 (2-((1E,3E,5E)-6-{5-[(E)-amino(imino)methyl]-1-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242334 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260300 (6-(4,5-dihydro-1H-imidazol-2-yl)-2-(2-(2-(4,5-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260292 ((2S,3R)-2-{(S)-2-[(S)-5-Guanidino-2-((S)-2-mercapt...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260291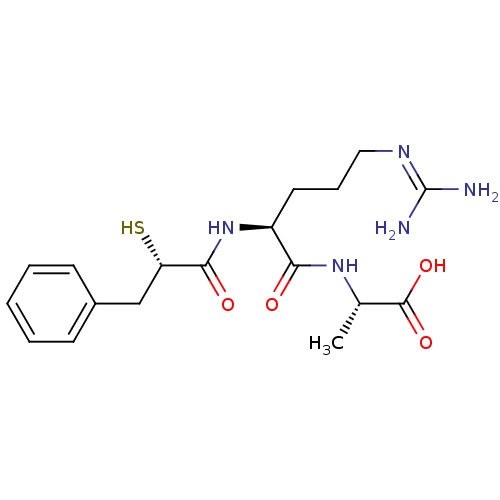 ((S)-2-[(S)-5-Guanidino-2-((S)-2-mercapto-3-phenyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242311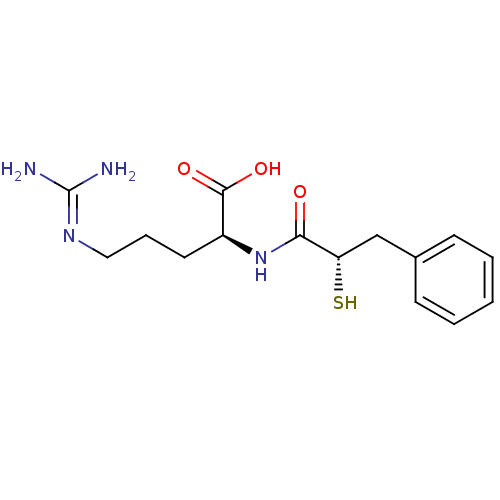 ((S)-5-Guanidino-2-((S)-2-mercapto-3-phenyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242335 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50158375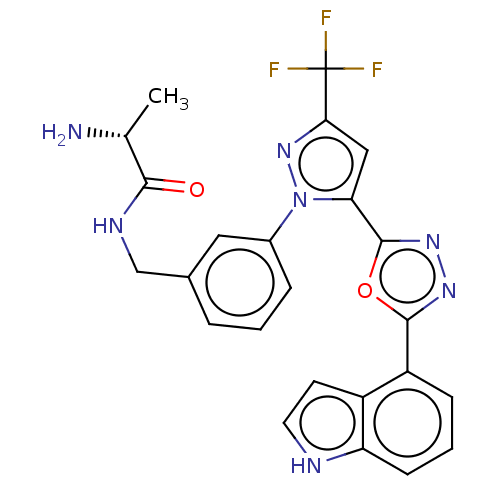 (CHEMBL3781284) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of GST-tagged CARM1 (unknown origin) using tritiated S-adenosylmethionine as substrate at 6 nM by methylation- based filter assay | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50158100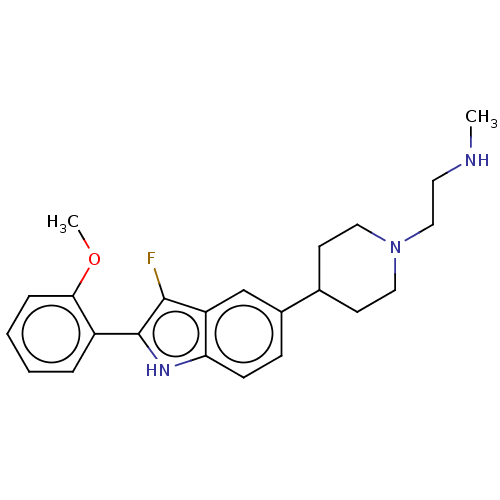 (CHEMBL1614812) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human PRMT4 using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation proximity as... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50158097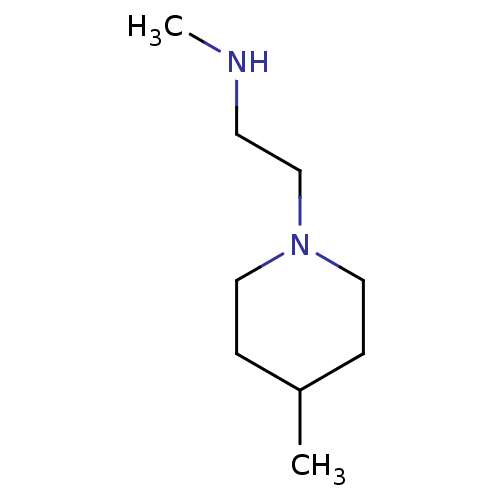 (CHEMBL3780701) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of CARM1 (unknown origin) using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation p... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50158097 (CHEMBL3780701) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human PRMT4 using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation proximity as... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50158094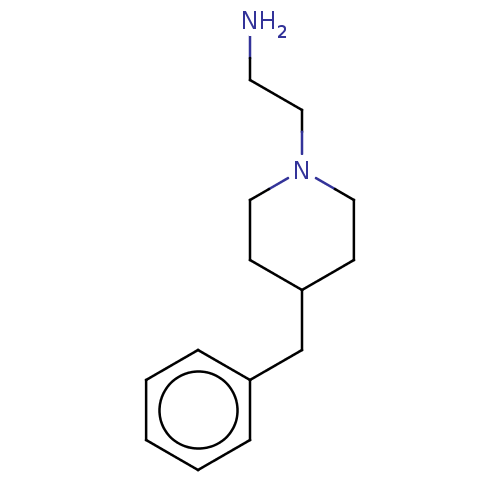 (CHEMBL3780926) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50158094 (CHEMBL3780926) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human PRMT4 using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation proximity as... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50158094 (CHEMBL3780926) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of CARM1 (unknown origin) using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation p... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50158097 (CHEMBL3780701) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM50158094 (CHEMBL3780926) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human PRMT8 using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation proximity as... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50158095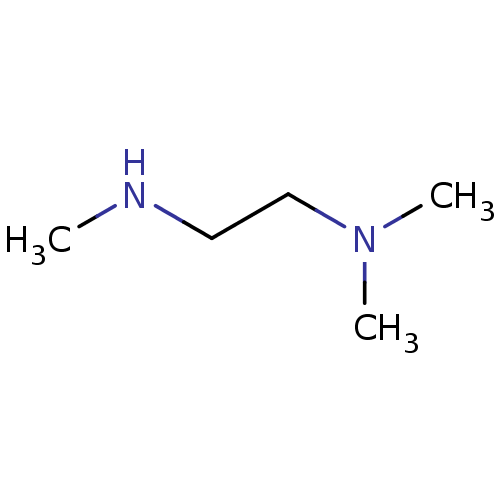 (CHEMBL3780444) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of CARM1 (unknown origin) using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation p... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50158095 (CHEMBL3780444) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human PRMT4 using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation proximity as... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50158095 (CHEMBL3780444) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM50158095 (CHEMBL3780444) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human PRMT8 using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation proximity as... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM50158094 (CHEMBL3780926) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human PRMT1 using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation proximity as... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 8 (Homo sapiens (Human)) | BDBM50158097 (CHEMBL3780701) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human PRMT8 using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation proximity as... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM50158094 (CHEMBL3780926) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human PRMT3 using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation proximity as... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50158094 (CHEMBL3780926) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of FLAG-tagged human PRMT6 expressed in HEK293 cells assessed as reduction in H3R2 global methylation incubated for 20 hrs by Western blot... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50145918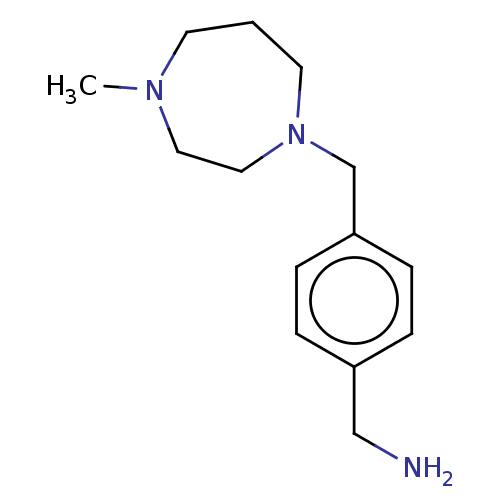 (CHEMBL3763993) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 3 [N508S] (Homo sapiens (Human)) | BDBM50158095 (CHEMBL3780444) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human PRMT3 using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation proximity as... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM50158095 (CHEMBL3780444) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human PRMT1 using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation proximity as... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50158369 (CHEMBL3781561) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 1 [11-371] (Homo sapiens (Human)) | BDBM50158097 (CHEMBL3780701) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human PRMT1 using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation proximity as... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50158371 (CHEMBL3780738) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50158372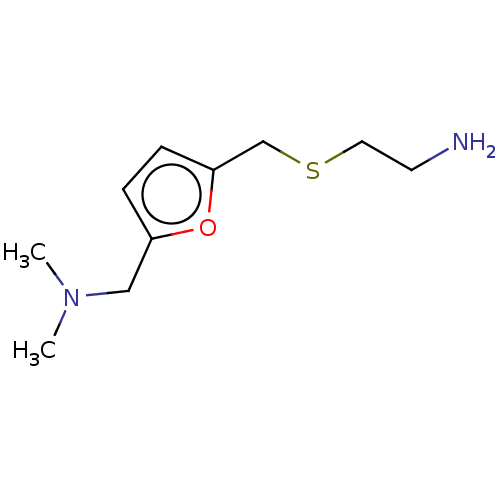 (CHEMBL3779956) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50158085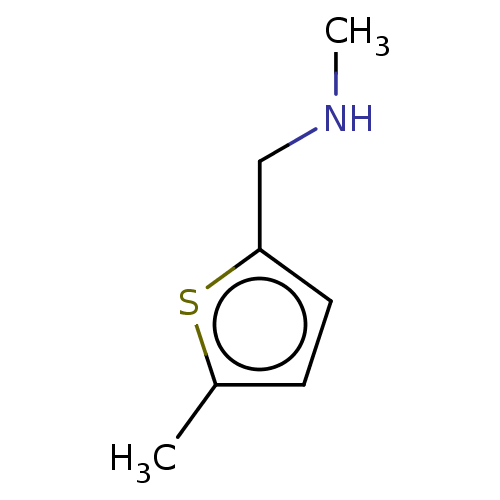 (CHEMBL3781201) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50158084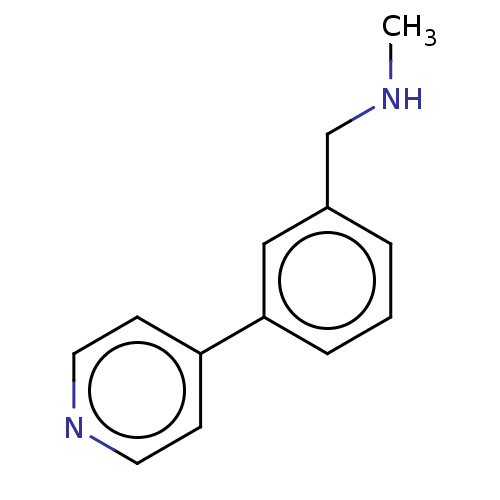 (CHEMBL3780979) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50068109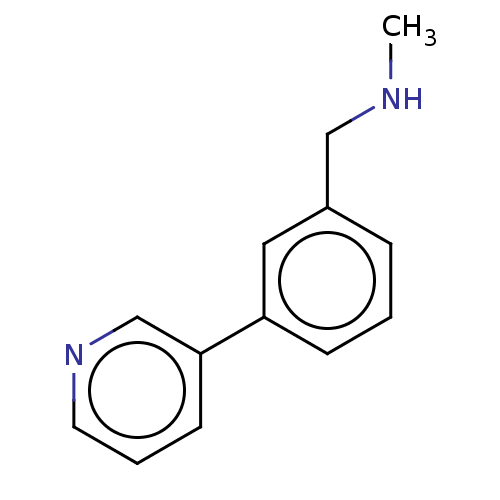 (CHEMBL3402235) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50158087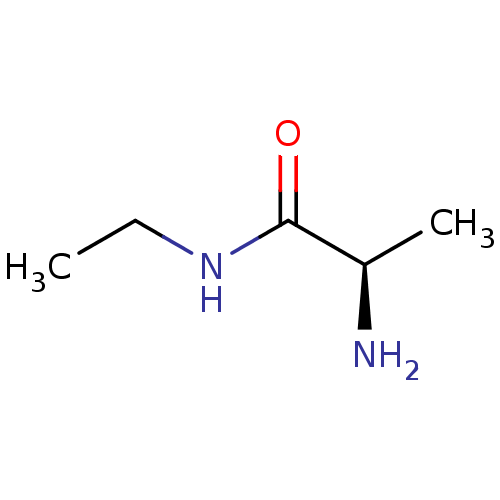 (CHEMBL3781841) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of CARM1 (unknown origin) using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation p... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM50158087 (CHEMBL3781841) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human PRMT4 using 24 residues of biotin labelled histone4 substrate and tritiated 3H-S-adenosylmethionine by scintillation proximity as... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50158370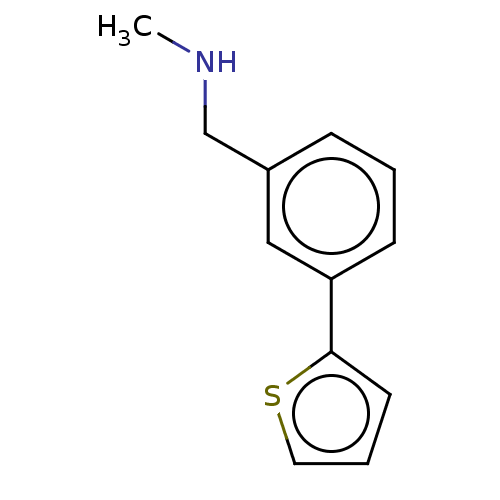 (CHEMBL3780615) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50158391 (CHEMBL3781682) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.36E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50158389 (CHEMBL3781603) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein arginine N-methyltransferase 6 (Homo sapiens (Human)) | BDBM50158377 (CHEMBL3781043) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of N-terminal hexa-His tagged human PRMT6 expressed in Sf9 cells using 24 residues of biotin labelled histone4 substrate and tritiated 3H-... | J Med Chem 59: 1176-83 (2016) Article DOI: 10.1021/acs.jmedchem.5b01772 BindingDB Entry DOI: 10.7270/Q2SF2Z11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |