

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM85093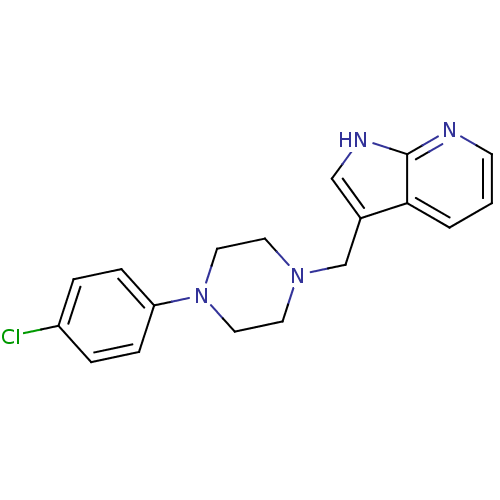 (CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D4 by [3H]-spiperone displacement. | J Med Chem 47: 2348-55 (2004) Article DOI: 10.1021/jm0305669 BindingDB Entry DOI: 10.7270/Q2M61M03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139374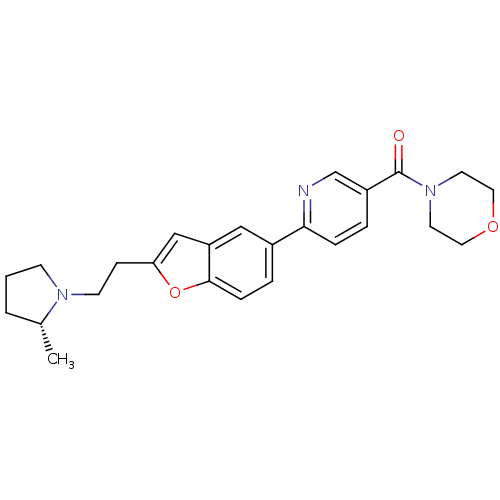 ((6-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139374 ((6-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139403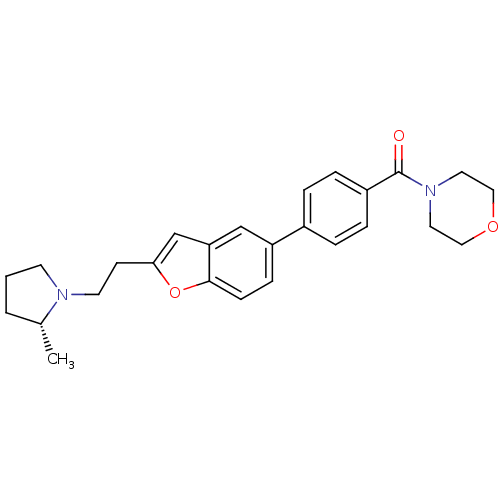 ((4-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50153255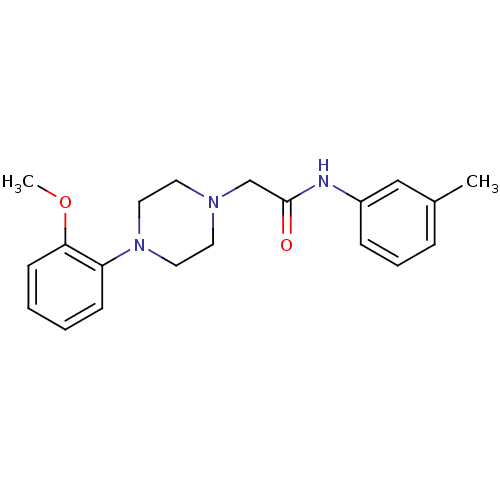 (2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-N-m-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-spiperone binding to human Dopamine receptor D4.4 allele | Bioorg Med Chem Lett 14: 5095-8 (2004) Article DOI: 10.1016/j.bmcl.2004.07.068 BindingDB Entry DOI: 10.7270/Q23X863Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139403 ((4-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139389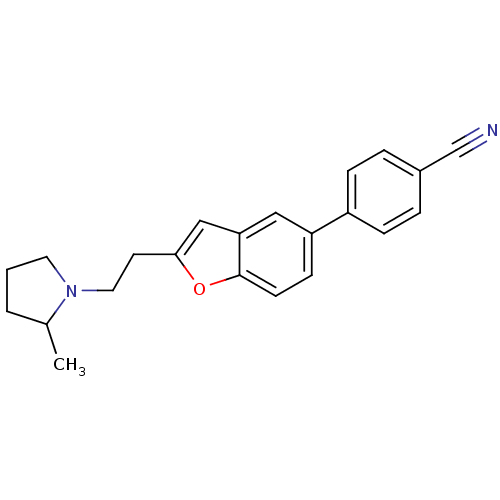 (4-{2-[2-(2-Methyl-pyrrolidin-1-yl)-ethyl]-benzofur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139389 (4-{2-[2-(2-Methyl-pyrrolidin-1-yl)-ethyl]-benzofur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.955 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50177063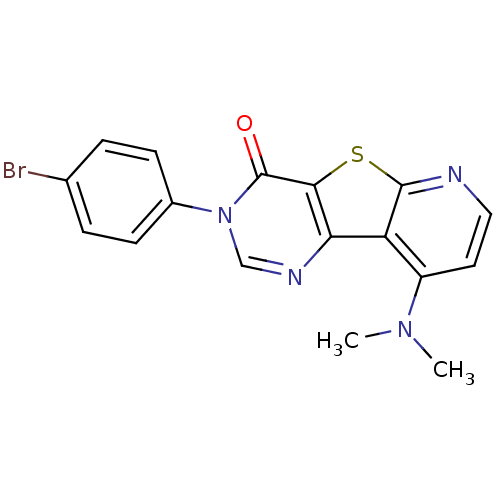 (3-(4-Bromo-phenyl)-9-dimethylamino-3H-pyrido[3',2'...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes | J Med Chem 48: 7374-88 (2005) Article DOI: 10.1021/jm0504407 BindingDB Entry DOI: 10.7270/Q27P8XX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139382 ((4-{2-[2-((2R,6S)-2,6-Dimethyl-piperidin-1-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139382 ((4-{2-[2-((2R,6S)-2,6-Dimethyl-piperidin-1-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50200028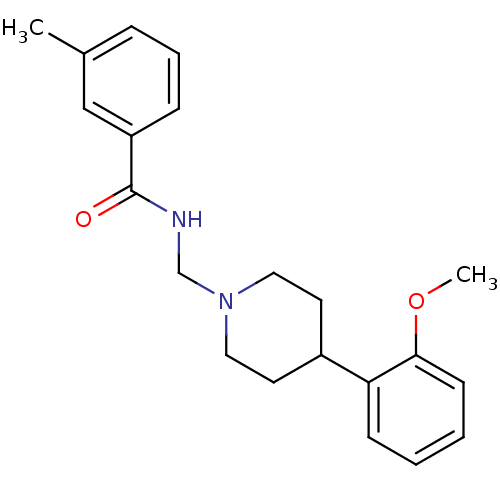 (2-methoxy-N-(3',4',5',6'-tetrahydro-2'H-[2,4'-bipy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]A369508 from human D4 receptor expressed in HEK293 cell membrane | J Med Chem 49: 7450-65 (2006) Article DOI: 10.1021/jm060662k BindingDB Entry DOI: 10.7270/Q25X28MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-spiperone binding to human Dopamine receptor D4.4 | Bioorg Med Chem Lett 14: 5095-8 (2004) Article DOI: 10.1016/j.bmcl.2004.07.068 BindingDB Entry DOI: 10.7270/Q23X863Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50150141 (2-[4-(3,4-Dimethyl-phenyl)-piperazin-1-ylmethyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity tested on HEK293 cells co-transfected with human D4.4 receptor using [3H]spiperone as a radioligand in FLIPR assay | J Med Chem 47: 3853-64 (2004) Article DOI: 10.1021/jm030505a BindingDB Entry DOI: 10.7270/Q24B3225 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139374 ((6-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139374 ((6-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50153255 (2-[4-(2-Methoxy-phenyl)-piperazin-1-yl]-N-m-tolyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-6b binding to human Dopamine receptor D4.4 | Bioorg Med Chem Lett 14: 5095-8 (2004) Article DOI: 10.1016/j.bmcl.2004.07.068 BindingDB Entry DOI: 10.7270/Q23X863Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139403 ((4-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139408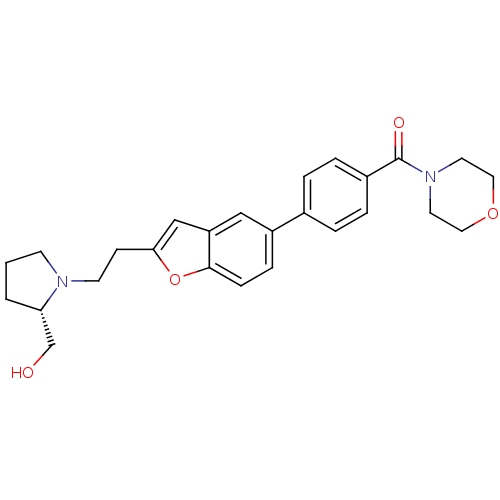 ((4-{2-[2-((S)-2-Hydroxymethyl-pyrrolidin-1-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-6b binding to human Dopamine receptor D4.4 | Bioorg Med Chem Lett 14: 5095-8 (2004) Article DOI: 10.1016/j.bmcl.2004.07.068 BindingDB Entry DOI: 10.7270/Q23X863Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139408 ((4-{2-[2-((S)-2-Hydroxymethyl-pyrrolidin-1-yl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139403 ((4-{2-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-ben...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description pBinding potency of the compound was determined by displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50177097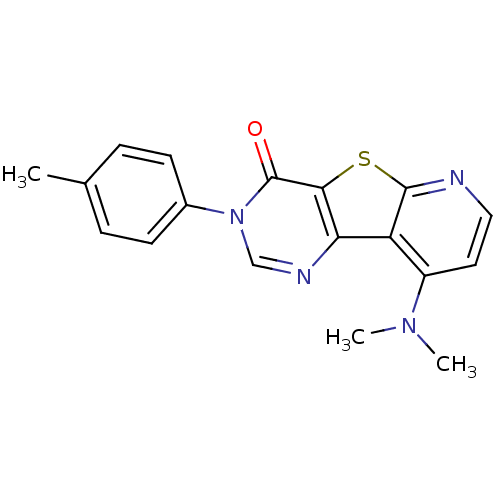 (9-Dimethylamino-3-p-tolyl-3H-pyrido[3',2':4,5]thie...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes | J Med Chem 48: 7374-88 (2005) Article DOI: 10.1021/jm0504407 BindingDB Entry DOI: 10.7270/Q27P8XX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139380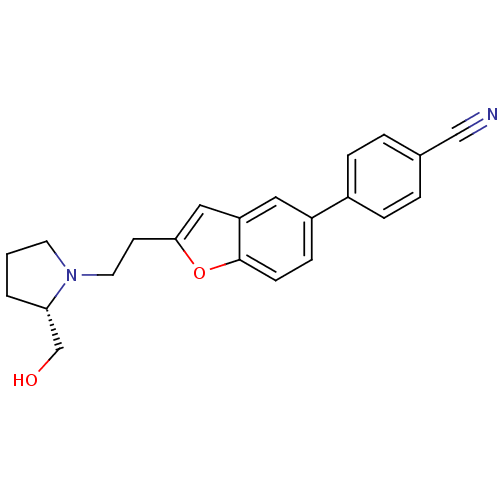 (4-{2-[2-((S)-2-Hydroxymethyl-pyrrolidin-1-yl)-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139380 (4-{2-[2-((S)-2-Hydroxymethyl-pyrrolidin-1-yl)-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139383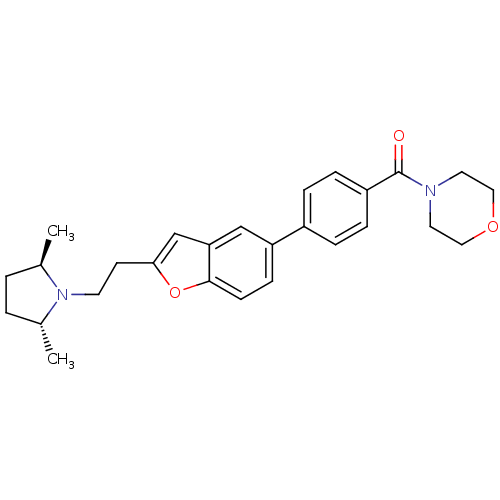 ((4-{2-[2-((2R,5R)-2,5-Dimethyl-pyrrolidin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139383 ((4-{2-[2-((2R,5R)-2,5-Dimethyl-pyrrolidin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139388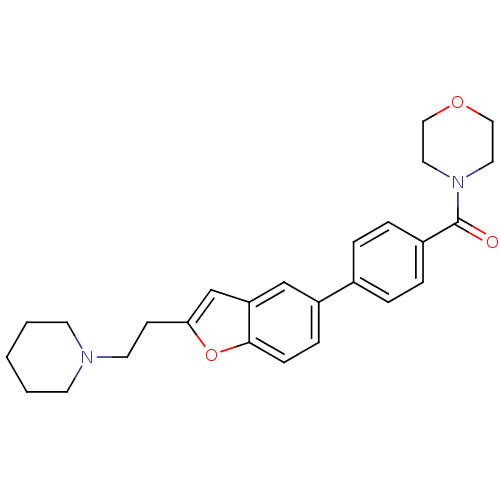 (CHEMBL159565 | Morpholin-4-yl-{4-[2-(2-piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139388 (CHEMBL159565 | Morpholin-4-yl-{4-[2-(2-piperidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139407 (4-{2-[2-((2R,6S)-2,6-Dimethyl-piperidin-1-yl)-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139407 (4-{2-[2-((2R,6S)-2,6-Dimethyl-piperidin-1-yl)-ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50153259 (2-[4-(4-[3H]-2-cyanophenyl)piperazinyl]-N-(2,4,6-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-spiperone binding to human Dopamine receptor D4.4 | Bioorg Med Chem Lett 14: 5095-8 (2004) Article DOI: 10.1016/j.bmcl.2004.07.068 BindingDB Entry DOI: 10.7270/Q23X863Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50102712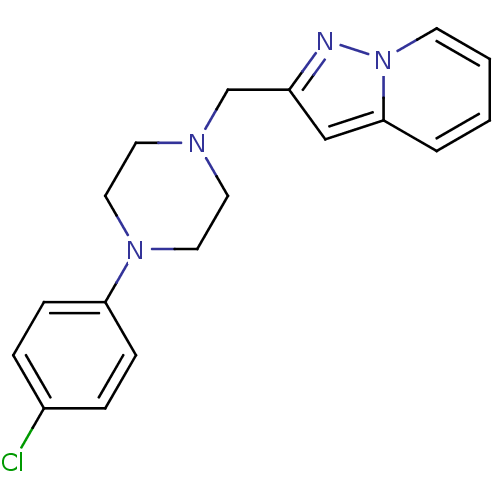 (2-((4-(4-chlorophenyl)piperazin-1-yl)methyl)-pyraz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D4 by [3H]-spiperone displacement. | J Med Chem 47: 2348-55 (2004) Article DOI: 10.1021/jm0305669 BindingDB Entry DOI: 10.7270/Q2M61M03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139402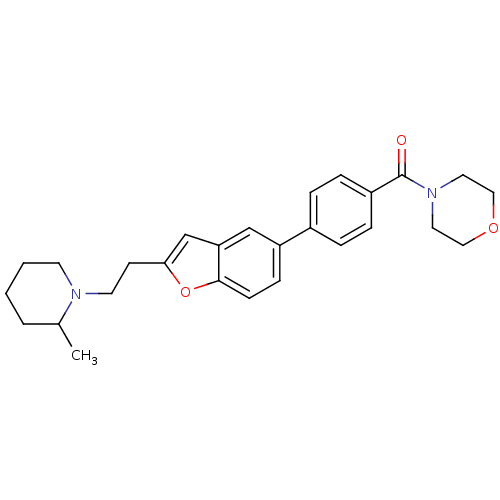 ((4-{2-[2-(2-Methyl-piperidin-1-yl)-ethyl]-benzofur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139402 ((4-{2-[2-(2-Methyl-piperidin-1-yl)-ethyl]-benzofur...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from histamine H3 receptor of rat cortical membranes | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139385 (CHEMBL346562 | {4-[2-(2-Azepan-1-yl-ethyl)-benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139385 (CHEMBL346562 | {4-[2-(2-Azepan-1-yl-ethyl)-benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50145073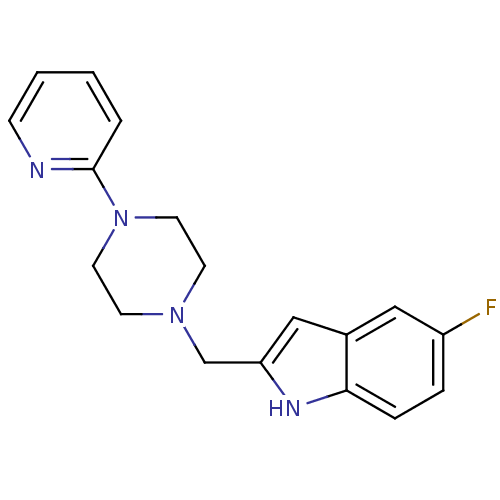 (5-Fluoro-2-(4-pyridin-2-yl-piperazin-1-ylmethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity towards human D4.4 receptor | J Med Chem 47: 3853-64 (2004) Article DOI: 10.1021/jm030505a BindingDB Entry DOI: 10.7270/Q24B3225 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]7-OH-PIPAT from human D2L receptor expressed in HEK293 cell membrane | J Med Chem 49: 7450-65 (2006) Article DOI: 10.1021/jm060662k BindingDB Entry DOI: 10.7270/Q25X28MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50150143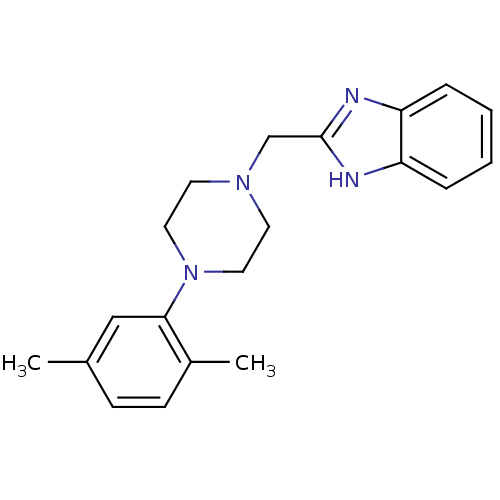 (2-[4-(2,5-Dimethyl-phenyl)-piperazin-1-ylmethyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity tested on HEK293 cells co-transfected with human D4.4 receptor using [3H]spiperone as a radioligand in FLIPR assay | J Med Chem 47: 3853-64 (2004) Article DOI: 10.1021/jm030505a BindingDB Entry DOI: 10.7270/Q24B3225 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139392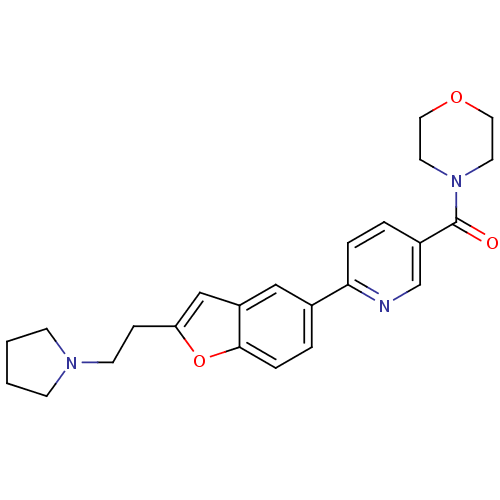 (CHEMBL422360 | Morpholin-4-yl-{6-[2-(2-pyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]-N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139392 (CHEMBL422360 | Morpholin-4-yl-{6-[2-(2-pyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50177076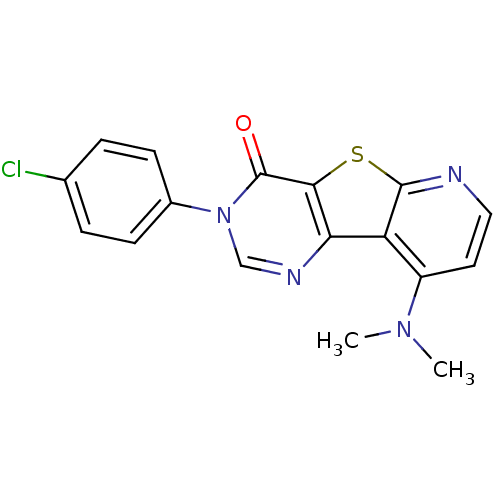 (3-(4-Chloro-phenyl)-9-dimethylamino-3H-pyrido[3',2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes | J Med Chem 48: 7374-88 (2005) Article DOI: 10.1021/jm0504407 BindingDB Entry DOI: 10.7270/Q27P8XX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (RAT) | BDBM50177065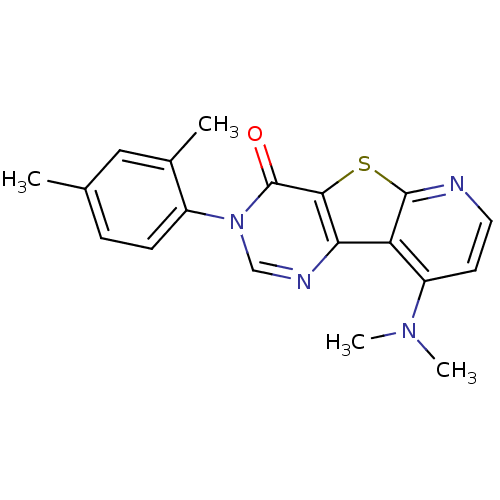 (9-Dimethylamino-3-(2,4-dimethyl-phenyl)-3H-pyrido[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]R214127 from mGluR1 in rat cerebellum membranes | J Med Chem 48: 7374-88 (2005) Article DOI: 10.1021/jm0504407 BindingDB Entry DOI: 10.7270/Q27P8XX0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139377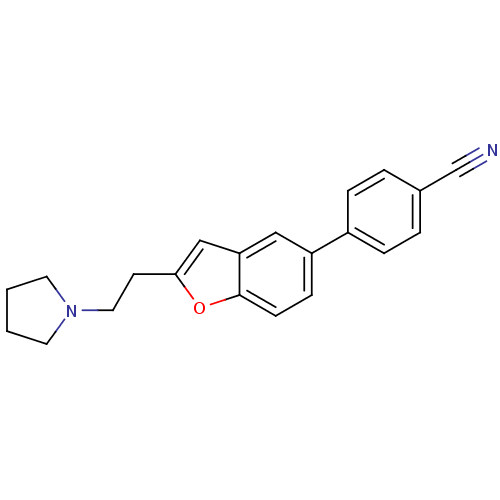 (4-[2-(2-Pyrrolidin-1-yl-ethyl)-benzofuran-5-yl]-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding potency was determined by displacement of [3H]N-alpha-methyl histamine from cloned human histamine H3 receptor expressed in C6 cells | Bioorg Med Chem Lett 14: 689-93 (2004) BindingDB Entry DOI: 10.7270/Q20C4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50145078 (2-[4-(2-Methoxy-phenyl)-piperazin-1-ylmethyl]-1H-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity against human Dopamine receptor D4 by [3H]-spiperone displacement. | J Med Chem 47: 2348-55 (2004) Article DOI: 10.1021/jm0305669 BindingDB Entry DOI: 10.7270/Q2M61M03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2297 total ) | Next | Last >> |