 Found 568 hits with Last Name = 'sugimoto' and Initial = 'y'
Found 568 hits with Last Name = 'sugimoto' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244370
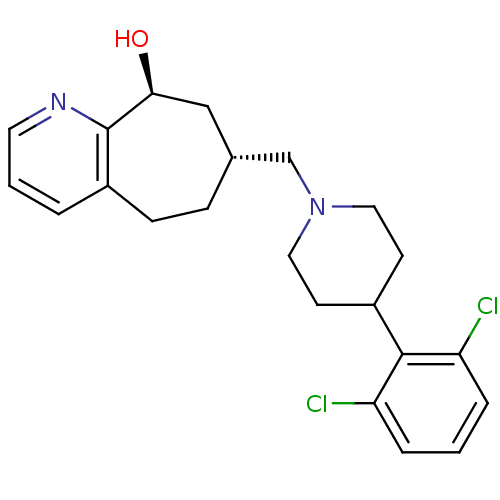
((7R,9S)-7-((4-(2,6-dichlorophenyl)piperidin-1-yl)m...)Show SMILES O[C@H]1C[C@H](CN2CCC(CC2)c2c(Cl)cccc2Cl)CCc2cccnc12 |r| Show InChI InChI=1S/C22H26Cl2N2O/c23-18-4-1-5-19(24)21(18)16-8-11-26(12-9-16)14-15-6-7-17-3-2-10-25-22(17)20(27)13-15/h1-5,10,15-16,20,27H,6-9,11-14H2/t15-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(MOUSE) | BDBM50008780
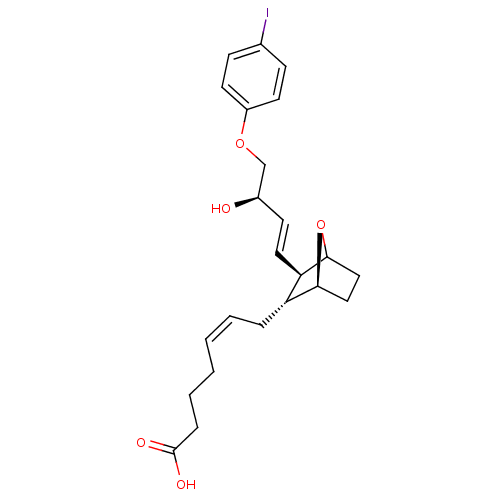
(7-{3-[3-Hydroxy-4-(4-iodo-phenoxy)-but-1-enyl]-7-o...)Show SMILES O[C@@H](COc1ccc(I)cc1)\C=C\[C@H]1C2CC[C@H](O2)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C23H29IO5/c24-16-7-10-18(11-8-16)28-15-17(25)9-12-20-19(21-13-14-22(20)29-21)5-3-1-2-4-6-23(26)27/h1,3,7-12,17,19-22,25H,2,4-6,13-15H2,(H,26,27)/b3-1-,12-9+/t17-,19-,20-,21+,22?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM85183
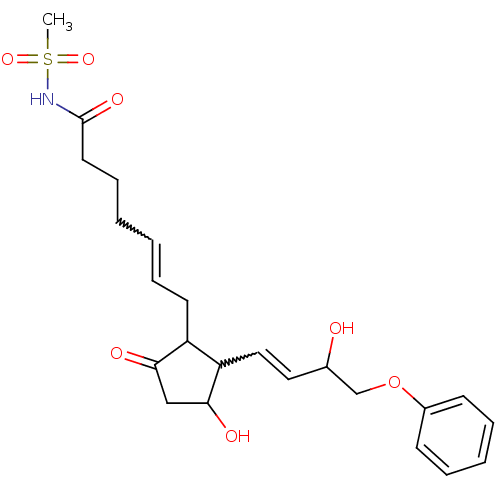
(CAS_60325-46-4 | NSC_43251 | SULPROSTONE)Show SMILES CS(=O)(=O)NC(=O)CCCC=CCC1C(C=CC(O)COc2ccccc2)C(O)CC1=O |w:10.9,15.14| Show InChI InChI=1S/C23H31NO7S/c1-32(29,30)24-23(28)12-8-3-2-7-11-19-20(22(27)15-21(19)26)14-13-17(25)16-31-18-9-5-4-6-10-18/h2,4-7,9-10,13-14,17,19-20,22,25,27H,3,8,11-12,15-16H2,1H3,(H,24,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(MOUSE) | BDBM50008805
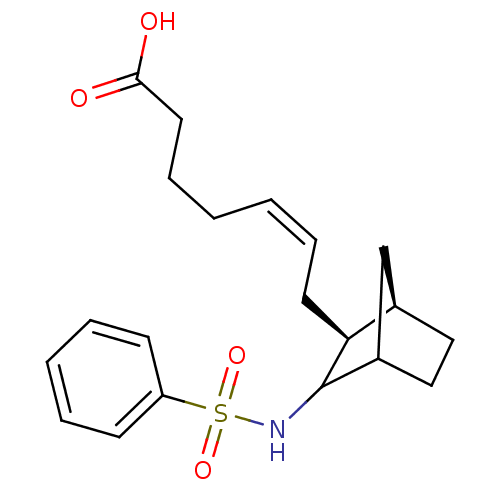
(7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...)Show SMILES OC(=O)CCC\C=C/C[C@H]1[C@@H]2CCC(C2)C1NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C20H27NO4S/c22-19(23)11-7-2-1-6-10-18-15-12-13-16(14-15)20(18)21-26(24,25)17-8-4-3-5-9-17/h1,3-6,8-9,15-16,18,20-21H,2,7,10-14H2,(H,22,23)/b6-1-/t15-,16?,18+,20?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM85177
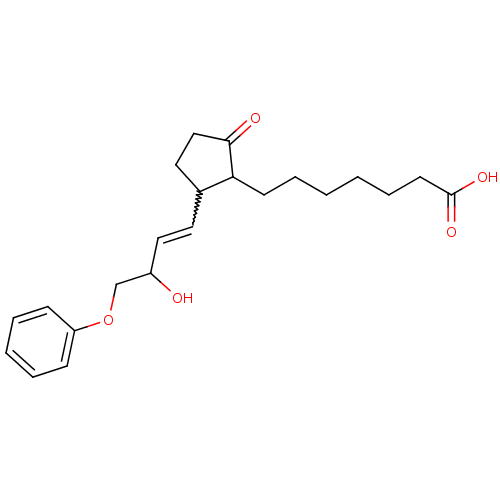
(CAS_80558-61-8 | M&B-28767 | NSC_119139)Show SMILES OC(COc1ccccc1)C=CC1CCC(=O)C1CCCCCCC(O)=O |w:11.12| Show InChI InChI=1S/C22H30O5/c23-18(16-27-19-8-4-3-5-9-19)14-12-17-13-15-21(24)20(17)10-6-1-2-7-11-22(25)26/h3-5,8-9,12,14,17-18,20,23H,1-2,6-7,10-11,13,15-16H2,(H,25,26) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244371
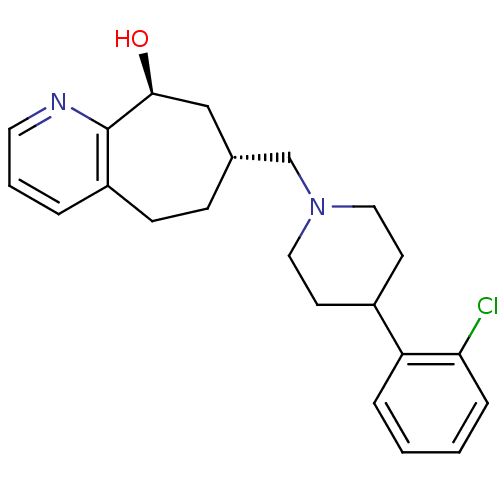
((7R,9S)-7-((4-(2-chlorophenyl)piperidin-1-yl)methy...)Show SMILES O[C@H]1C[C@H](CN2CCC(CC2)c2ccccc2Cl)CCc2cccnc12 |r| Show InChI InChI=1S/C22H27ClN2O/c23-20-6-2-1-5-19(20)17-9-12-25(13-10-17)15-16-7-8-18-4-3-11-24-22(18)21(26)14-16/h1-6,11,16-17,21,26H,7-10,12-15H2/t16-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50101828
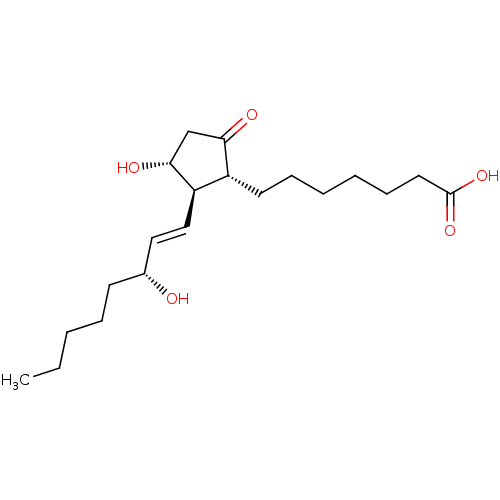
(7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-oct-1...)Show SMILES CCCCC[C@@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16-,17-,19-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM85189
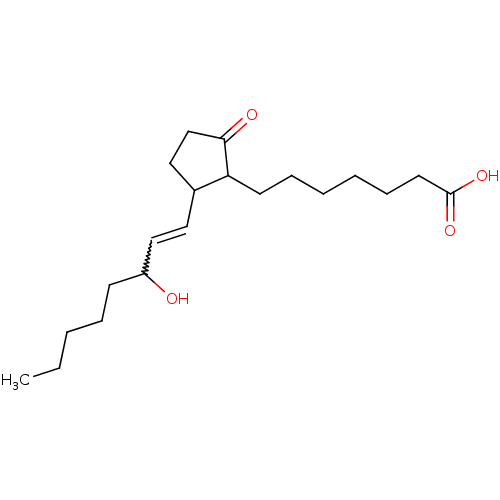
(PGE1,11-DEOXY)Show SMILES CCCCCC(O)C=CC1CCC(=O)C1CCCCCCC(O)=O |w:7.6| Show InChI InChI=1S/C20H34O4/c1-2-3-6-9-17(21)14-12-16-13-15-19(22)18(16)10-7-4-5-8-11-20(23)24/h12,14,16-18,21H,2-11,13,15H2,1H3,(H,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM85185
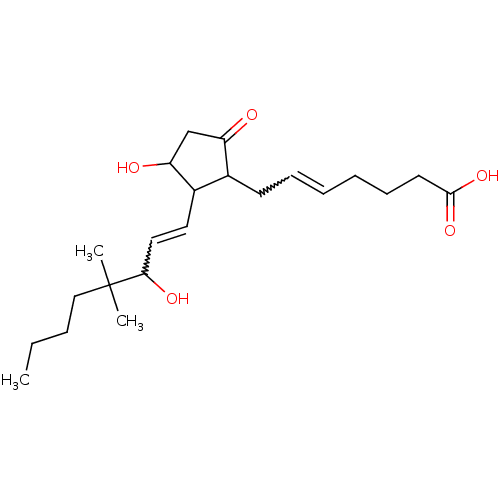
(PGE2,16,16-DIMETHYL)Show SMILES CCCCC(C)(C)C(O)C=CC1C(O)CC(=O)C1CC=CCCCC(O)=O |w:9.8,19.19| Show InChI InChI=1S/C22H36O5/c1-4-5-14-22(2,3)20(25)13-12-17-16(18(23)15-19(17)24)10-8-6-7-9-11-21(26)27/h6,8,12-13,16-17,19-20,24-25H,4-5,7,9-11,14-15H2,1-3H3,(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM85174
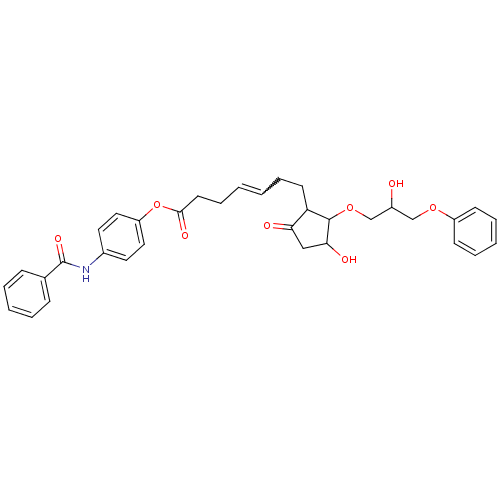
(CAS_5311224 | GR 63799X | NSC_5311224)Show SMILES OC(COC1C(O)CC(=O)C1CCC=CCCC(=O)Oc1ccc(NC(=O)c2ccccc2)cc1)COc1ccccc1 |w:13.13| Show InChI InChI=1S/C34H37NO8/c36-26(22-41-27-13-7-4-8-14-27)23-42-33-29(30(37)21-31(33)38)15-9-1-2-10-16-32(39)43-28-19-17-25(18-20-28)35-34(40)24-11-5-3-6-12-24/h1-8,11-14,17-20,26,29,31,33,36,38H,9-10,15-16,21-23H2,(H,35,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM50101828

(7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-oct-1...)Show SMILES CCCCC[C@@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16-,17-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(MOUSE) | BDBM50020300
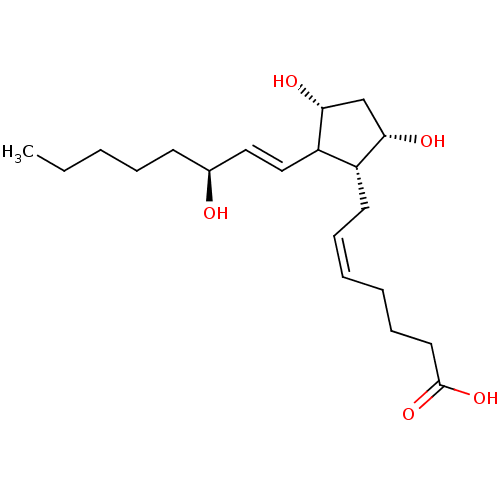
((S-isomer)7-[3,5-Dihydroxy-2-(3-hydroxy-oct-1-enyl...)Show SMILES CCCCC[C@H](O)\C=C\C1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-19,21-23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17?,18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243726
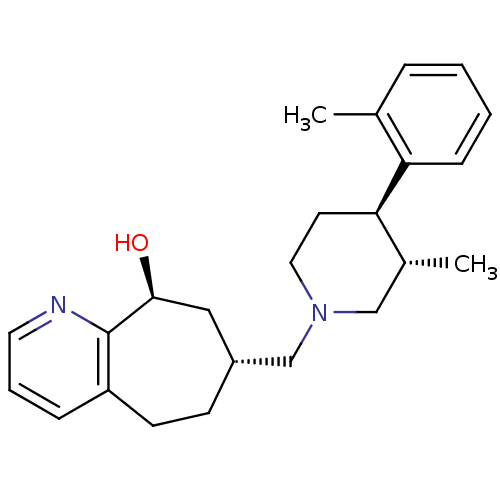
((7R,9S)-7-(((3S,4R)-3-methyl-4-o-tolylpiperidin-1-...)Show SMILES C[C@@H]1CN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)CC[C@H]1c1ccccc1C |r| Show InChI InChI=1S/C24H32N2O/c1-17-6-3-4-8-21(17)22-11-13-26(15-18(22)2)16-19-9-10-20-7-5-12-25-24(20)23(27)14-19/h3-8,12,18-19,22-23,27H,9-11,13-16H2,1-2H3/t18-,19-,22-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM82094
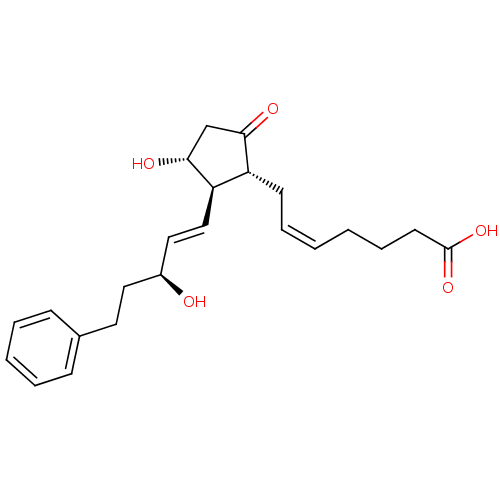
(17-PHENYL TRINOR PROSTAGLANDIN E2 | CAS_38315-43-4...)Show SMILES O[C@@H](CCc1ccccc1)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O |r| Show InChI InChI=1S/C23H30O5/c24-18(13-12-17-8-4-3-5-9-17)14-15-20-19(21(25)16-22(20)26)10-6-1-2-7-11-23(27)28/h1,3-6,8-9,14-15,18-20,22,24,26H,2,7,10-13,16H2,(H,27,28)/b6-1-,15-14+/t18-,19+,20+,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243729
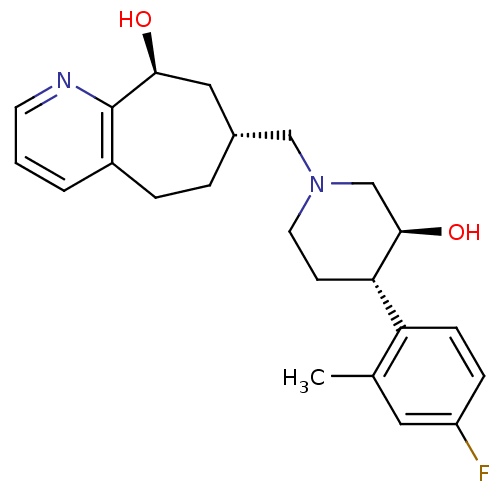
((7R,9S)-7-(((3S,4S)-4-(4-fluoro-2-methylphenyl)-3-...)Show SMILES Cc1cc(F)ccc1[C@@H]1CCN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)C[C@H]1O |r| Show InChI InChI=1S/C23H29FN2O2/c1-15-11-18(24)6-7-19(15)20-8-10-26(14-22(20)28)13-16-4-5-17-3-2-9-25-23(17)21(27)12-16/h2-3,6-7,9,11,16,20-22,27-28H,4-5,8,10,12-14H2,1H3/t16-,20+,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(MOUSE) | BDBM85173
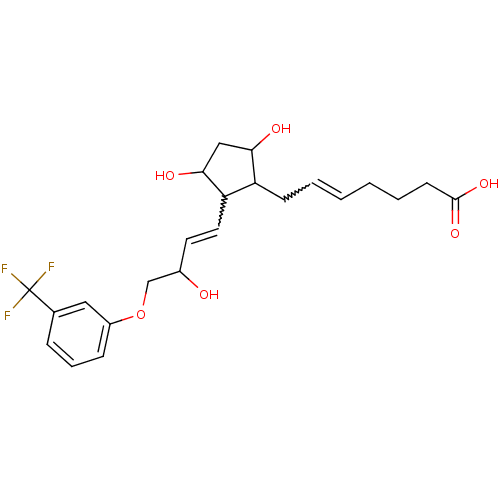
(CAS_40666-16-8 | FLUPROSTENOL | NSC_5311100)Show SMILES OC(COc1cccc(c1)C(F)(F)F)C=CC1C(O)CC(O)C1CC=CCCCC(O)=O |w:15.16,24.25| Show InChI InChI=1S/C23H29F3O6/c24-23(25,26)15-6-5-7-17(12-15)32-14-16(27)10-11-19-18(20(28)13-21(19)29)8-3-1-2-4-9-22(30)31/h1,3,5-7,10-12,16,18-21,27-29H,2,4,8-9,13-14H2,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243727
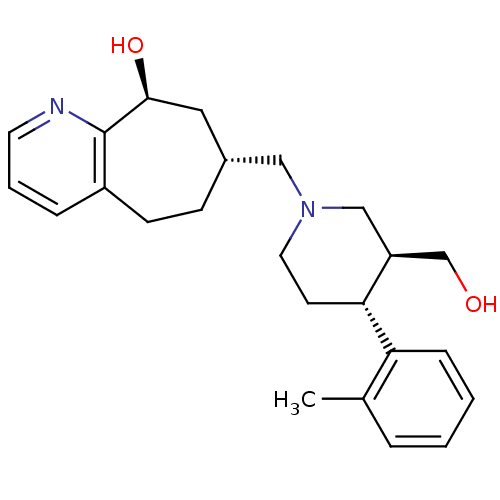
((7R,9S)-7-(((3S,4R)-3-(hydroxymethyl)-4-o-tolylpip...)Show SMILES Cc1ccccc1[C@@H]1CCN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)C[C@H]1CO |r| Show InChI InChI=1S/C24H32N2O2/c1-17-5-2-3-7-21(17)22-10-12-26(15-20(22)16-27)14-18-8-9-19-6-4-11-25-24(19)23(28)13-18/h2-7,11,18,20,22-23,27-28H,8-10,12-16H2,1H3/t18-,20+,22-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243730
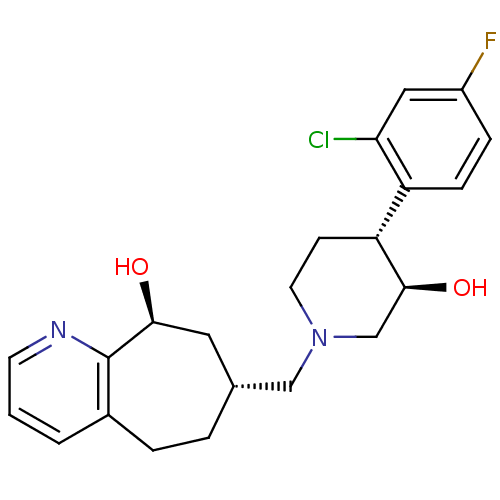
((7R,9S)-7-(((3R,4R)-4-(2-chloro-4-fluorophenyl)-3-...)Show SMILES O[C@H]1CN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)CC[C@@H]1c1ccc(F)cc1Cl |r| Show InChI InChI=1S/C22H26ClFN2O2/c23-19-11-16(24)5-6-17(19)18-7-9-26(13-21(18)28)12-14-3-4-15-2-1-8-25-22(15)20(27)10-14/h1-2,5-6,8,11,14,18,20-21,27-28H,3-4,7,9-10,12-13H2/t14-,18-,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244297
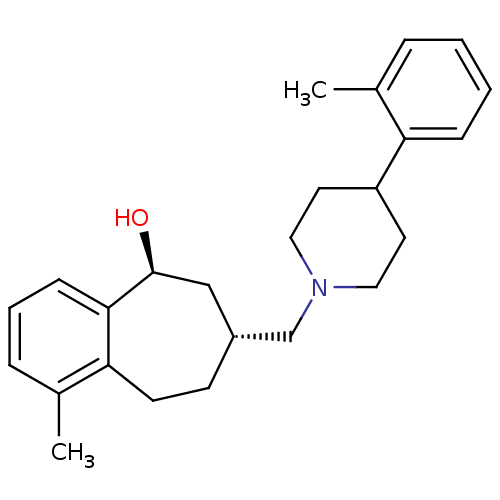
(CHEMBL513585 | cis-1-methyl-7-((4-o-tolylpiperidin...)Show SMILES Cc1ccccc1C1CCN(C[C@@H]2CCc3c(C)cccc3[C@@H](O)C2)CC1 |r| Show InChI InChI=1S/C25H33NO/c1-18-6-3-4-8-22(18)21-12-14-26(15-13-21)17-20-10-11-23-19(2)7-5-9-24(23)25(27)16-20/h3-9,20-21,25,27H,10-17H2,1-2H3/t20-,25+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243728
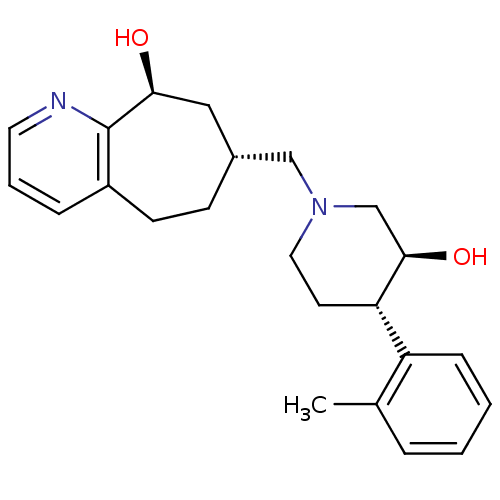
((7R,9S)-7-(((3S,4S)-3-hydroxy-4-o-tolylpiperidin-1...)Show SMILES Cc1ccccc1[C@@H]1CCN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)C[C@H]1O |r| Show InChI InChI=1S/C23H30N2O2/c1-16-5-2-3-7-19(16)20-10-12-25(15-22(20)27)14-17-8-9-18-6-4-11-24-23(18)21(26)13-17/h2-7,11,17,20-22,26-27H,8-10,12-15H2,1H3/t17-,20+,21+,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(MOUSE) | BDBM85179
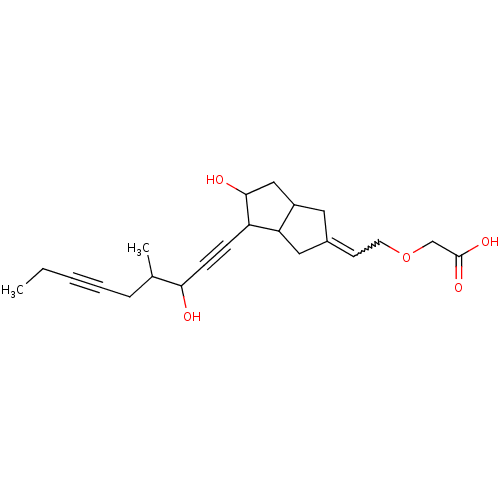
(CAS_94079-80-8 | CICAPROST | NSC_72023)Show SMILES CCC#CCC(C)C(O)C#CC1C(O)CC2CC(CC12)=CCOCC(O)=O |w:20.22| Show InChI InChI=1S/C22H30O5/c1-3-4-5-6-15(2)20(23)8-7-18-19-12-16(9-10-27-14-22(25)26)11-17(19)13-21(18)24/h9,15,17-21,23-24H,3,6,10-14H2,1-2H3,(H,25,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Mus musculus (Mouse)) | BDBM50101828

(7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-oct-1...)Show SMILES CCCCC[C@@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16-,17-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243887
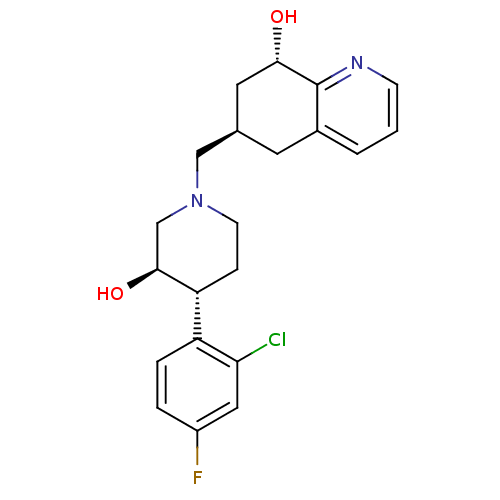
((-)-(3R,4R)-4-(2-Chloro-4-fluorophenyl)-3-hydroxy-...)Show SMILES O[C@H]1CN(C[C@H]2C[C@H](O)c3ncccc3C2)CC[C@@H]1c1ccc(F)cc1Cl |r| Show InChI InChI=1S/C21H24ClFN2O2/c22-18-10-15(23)3-4-16(18)17-5-7-25(12-20(17)27)11-13-8-14-2-1-6-24-21(14)19(26)9-13/h1-4,6,10,13,17,19-20,26-27H,5,7-9,11-12H2/t13-,17-,19+,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(MOUSE) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244336
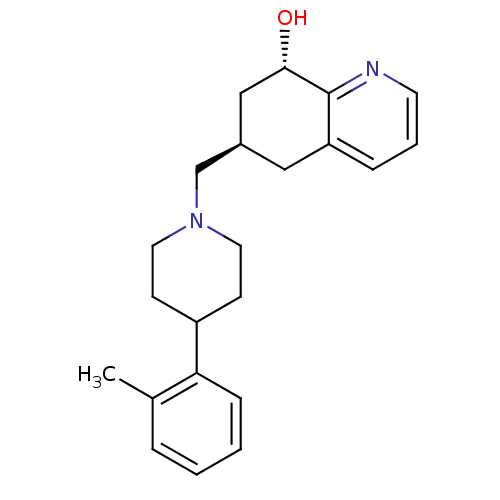
((6R,8S)-6-((4-o-tolylpiperidin-1-yl)methyl)-5,6,7,...)Show SMILES Cc1ccccc1C1CCN(C[C@H]2C[C@H](O)c3ncccc3C2)CC1 |r| Show InChI InChI=1S/C22H28N2O/c1-16-5-2-3-7-20(16)18-8-11-24(12-9-18)15-17-13-19-6-4-10-23-22(19)21(25)14-17/h2-7,10,17-18,21,25H,8-9,11-15H2,1H3/t17-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
(MOUSE) | BDBM50283049
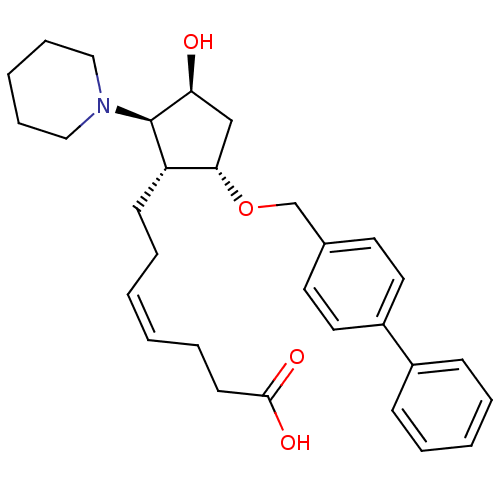
((Z)-7-[(1R,2R,3S,5S)-5-(Biphenyl-4-ylmethoxy)-3-hy...)Show SMILES O[C@H]1C[C@H](OCc2ccc(cc2)-c2ccccc2)[C@H](CC\C=C/CCC(O)=O)[C@H]1N1CCCCC1 Show InChI InChI=1S/C30H39NO4/c32-27-21-28(35-22-23-15-17-25(18-16-23)24-11-5-3-6-12-24)26(13-7-1-2-8-14-29(33)34)30(27)31-19-9-4-10-20-31/h1-3,5-6,11-12,15-18,26-28,30,32H,4,7-10,13-14,19-22H2,(H,33,34)/b2-1-/t26-,27-,28-,30+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Mus musculus (Mouse)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thromboxane-A synthase
(MOUSE) | BDBM50008781
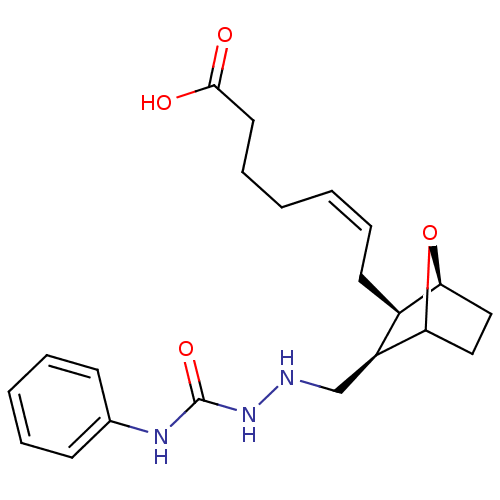
(7-(3-(2-ethyl-N-phenylhydrazinecarboxamide)-7-oxa-...)Show SMILES OC(=O)CCC\C=C/C[C@H]1[C@@H]2CCC(O2)[C@H]1CNNC(=O)Nc1ccccc1 Show InChI InChI=1S/C21H29N3O4/c25-20(26)11-7-2-1-6-10-16-17(19-13-12-18(16)28-19)14-22-24-21(27)23-15-8-4-3-5-9-15/h1,3-6,8-9,16-19,22H,2,7,10-14H2,(H,25,26)(H2,23,24,27)/b6-1-/t16-,17+,18+,19?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Thromboxane-A synthase
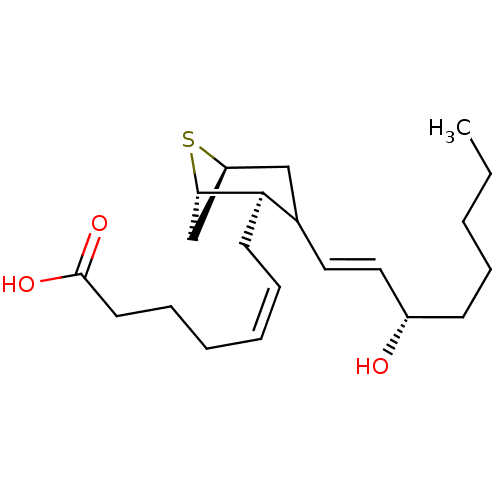
(MOUSE) | BDBM81948
(CAS_89617-02-7 | ONO 11,113 | ONO-11113 | STA2)Show SMILES CCCCC[C@H](O)\C=C\C1C[C@H]2C[C@H](S2)[C@@H]1C\C=C/CCCC(O)=O |r| Show InChI InChI=1S/C21H34O3S/c1-2-3-6-9-17(22)13-12-16-14-18-15-20(25-18)19(16)10-7-4-5-8-11-21(23)24/h4,7,12-13,16-20,22H,2-3,5-6,8-11,14-15H2,1H3,(H,23,24)/b7-4-,13-12+/t16?,17-,18-,19+,20-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM82094

(17-PHENYL TRINOR PROSTAGLANDIN E2 | CAS_38315-43-4...)Show SMILES O[C@@H](CCc1ccccc1)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O |r| Show InChI InChI=1S/C23H30O5/c24-18(13-12-17-8-4-3-5-9-17)14-15-20-19(21(25)16-22(20)26)10-6-1-2-7-11-23(27)28/h1,3-6,8-9,14-15,18-20,22,24,26H,2,7,10-13,16H2,(H,27,28)/b6-1-,15-14+/t18-,19+,20+,22+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(MOUSE) | BDBM85186
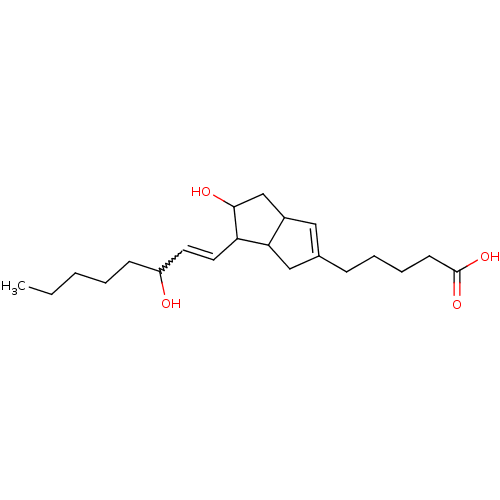
(CAS_5311244 | ISOCARBACYCLIN | NSC_5311244)Show SMILES CCCCCC(O)C=CC1C(O)CC2C=C(CCCCC(O)=O)CC12 |w:7.6,t:14| Show InChI InChI=1S/C21H34O4/c1-2-3-4-8-17(22)10-11-18-19-13-15(7-5-6-9-21(24)25)12-16(19)14-20(18)23/h10-12,16-20,22-23H,2-9,13-14H2,1H3,(H,24,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostacyclin receptor
(MOUSE) | BDBM85181
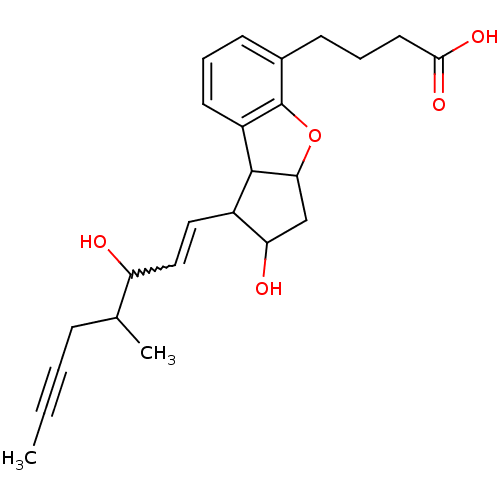
(BERAPROST | CAS_2352 | NSC_2352)Show SMILES CC#CCC(C)C(O)C=CC1C(O)CC2Oc3c(cccc3CCCC(O)=O)C12 |w:8.7| Show InChI InChI=1S/C24H30O5/c1-3-4-7-15(2)19(25)13-12-17-20(26)14-21-23(17)18-10-5-8-16(24(18)29-21)9-6-11-22(27)28/h5,8,10,12-13,15,17,19-21,23,25-26H,6-7,9,11,14H2,1-2H3,(H,27,28) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244334
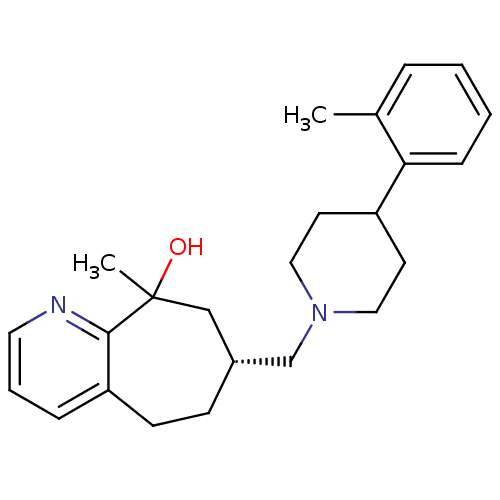
((7R)-9-methyl-7-((4-o-tolylpiperidin-1-yl)methyl)-...)Show SMILES Cc1ccccc1C1CCN(C[C@@H]2CCc3cccnc3C(C)(O)C2)CC1 |r| Show InChI InChI=1S/C24H32N2O/c1-18-6-3-4-8-22(18)20-11-14-26(15-12-20)17-19-9-10-21-7-5-13-25-23(21)24(2,27)16-19/h3-8,13,19-20,27H,9-12,14-17H2,1-2H3/t19-,24?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244334

((7R)-9-methyl-7-((4-o-tolylpiperidin-1-yl)methyl)-...)Show SMILES Cc1ccccc1C1CCN(C[C@@H]2CCc3cccnc3C(C)(O)C2)CC1 |r| Show InChI InChI=1S/C24H32N2O/c1-18-6-3-4-8-22(18)20-11-14-26(15-12-20)17-19-9-10-21-7-5-13-25-23(21)24(2,27)16-19/h3-8,13,19-20,27H,9-12,14-17H2,1-2H3/t19-,24?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Mus musculus (Mouse)) | BDBM85185

(PGE2,16,16-DIMETHYL)Show SMILES CCCCC(C)(C)C(O)C=CC1C(O)CC(=O)C1CC=CCCCC(O)=O |w:9.8,19.19| Show InChI InChI=1S/C22H36O5/c1-4-5-14-22(2,3)20(25)13-12-17-16(18(23)15-19(17)24)10-8-6-7-9-11-21(26)27/h6,8,12-13,16-17,19-20,24-25H,4-5,7,9-11,14-15H2,1-3H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244295
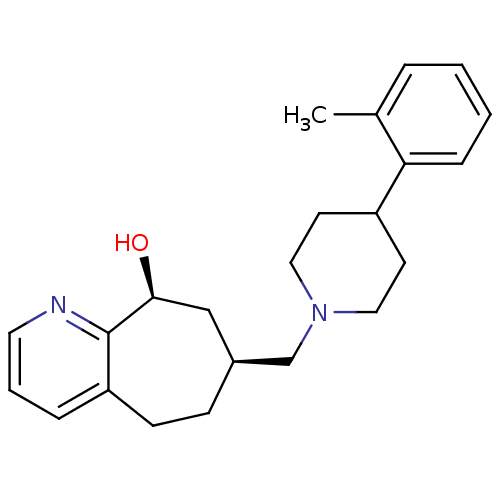
((-)-7-((4-o-tolylpiperidin-1-yl)methyl)-6,7,8,9-te...)Show SMILES Cc1ccccc1C1CCN(C[C@H]2CCc3cccnc3[C@@H](O)C2)CC1 |r| Show InChI InChI=1S/C23H30N2O/c1-17-5-2-3-7-21(17)19-10-13-25(14-11-19)16-18-8-9-20-6-4-12-24-23(20)22(26)15-18/h2-7,12,18-19,22,26H,8-11,13-16H2,1H3/t18-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244295

((-)-7-((4-o-tolylpiperidin-1-yl)methyl)-6,7,8,9-te...)Show SMILES Cc1ccccc1C1CCN(C[C@H]2CCc3cccnc3[C@@H](O)C2)CC1 |r| Show InChI InChI=1S/C23H30N2O/c1-17-5-2-3-7-21(17)19-10-13-25(14-11-19)16-18-8-9-20-6-4-12-24-23(20)22(26)15-18/h2-7,12,18-19,22,26H,8-11,13-16H2,1H3/t18-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244372
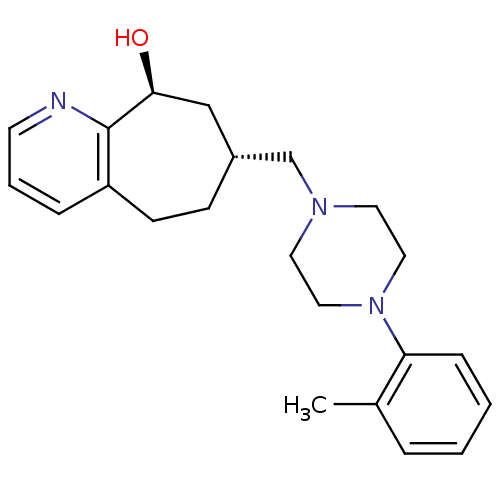
((7R,9S)-7-((4-o-tolylpiperazin-1-yl)methyl)-6,7,8,...)Show SMILES Cc1ccccc1N1CCN(C[C@@H]2CCc3cccnc3[C@@H](O)C2)CC1 |r| Show InChI InChI=1S/C22H29N3O/c1-17-5-2-3-7-20(17)25-13-11-24(12-14-25)16-18-8-9-19-6-4-10-23-22(19)21(26)15-18/h2-7,10,18,21,26H,8-9,11-16H2,1H3/t18-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM85183

(CAS_60325-46-4 | NSC_43251 | SULPROSTONE)Show SMILES CS(=O)(=O)NC(=O)CCCC=CCC1C(C=CC(O)COc2ccccc2)C(O)CC1=O |w:10.9,15.14| Show InChI InChI=1S/C23H31NO7S/c1-32(29,30)24-23(28)12-8-3-2-7-11-19-20(22(27)15-21(19)26)14-13-17(25)16-31-18-9-5-4-6-10-18/h2,4-7,9-10,13-14,17,19-20,22,25,27H,3,8,11-12,15-16H2,1H3,(H,24,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50243886
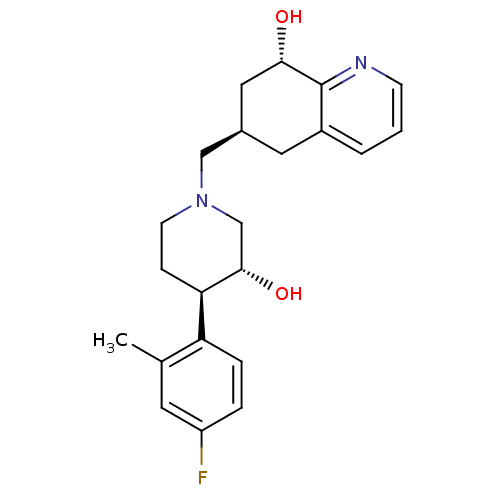
((6R,8S)-6-(((3R,4R)-4-(4-fluoro-2-methylphenyl)-3-...)Show SMILES Cc1cc(F)ccc1[C@H]1CCN(C[C@H]2C[C@H](O)c3ncccc3C2)C[C@@H]1O |r| Show InChI InChI=1S/C22H27FN2O2/c1-14-9-17(23)4-5-18(14)19-6-8-25(13-21(19)27)12-15-10-16-3-2-7-24-22(16)20(26)11-15/h2-5,7,9,15,19-21,26-27H,6,8,10-13H2,1H3/t15-,19-,20+,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(MOUSE) | BDBM82213
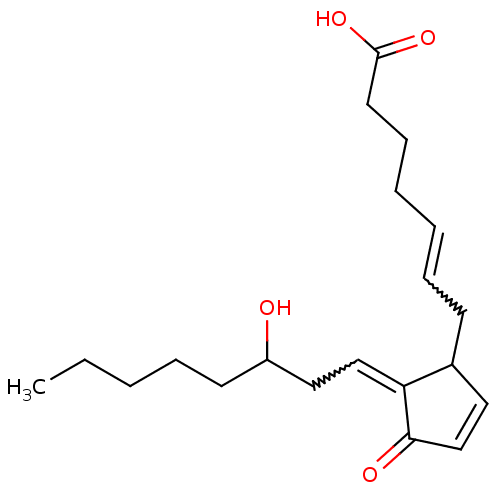
(CAS_41598-07-6 | NSC_114678 | PGD2)Show SMILES CCCCCC(O)CC=C1C(CC=CCCCC(O)=O)C=CC1=O |w:8.7,12.11,c:20| Show InChI InChI=1S/C20H30O4/c1-2-3-6-10-17(21)13-14-18-16(12-15-19(18)22)9-7-4-5-8-11-20(23)24/h4,7,12,14-17,21H,2-3,5-6,8-11,13H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM81948

(CAS_89617-02-7 | ONO 11,113 | ONO-11113 | STA2)Show SMILES CCCCC[C@H](O)\C=C\C1C[C@H]2C[C@H](S2)[C@@H]1C\C=C/CCCC(O)=O |r| Show InChI InChI=1S/C21H34O3S/c1-2-3-6-9-17(22)13-12-16-14-18-15-20(25-18)19(16)10-7-4-5-8-11-21(23)24/h4,7,12-13,16-20,22H,2-3,5-6,8-11,14-15H2,1H3,(H,23,24)/b7-4-,13-12+/t16?,17-,18-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Mus musculus (Mouse)) | BDBM85189

(PGE1,11-DEOXY)Show SMILES CCCCCC(O)C=CC1CCC(=O)C1CCCCCCC(O)=O |w:7.6| Show InChI InChI=1S/C20H34O4/c1-2-3-6-9-17(21)14-12-16-13-15-19(22)18(16)10-7-4-5-8-11-20(23)24/h12,14,16-18,21H,2-11,13,15H2,1H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244335
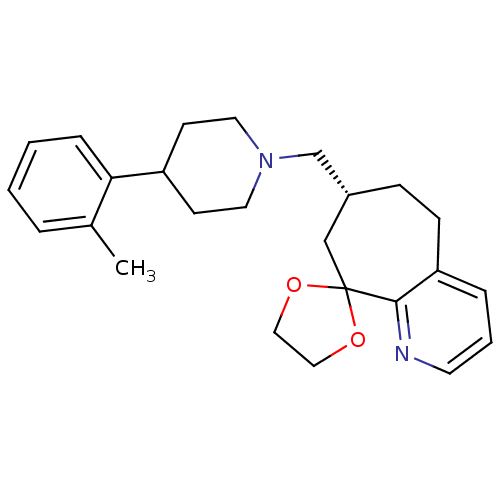
((3R)-3-{[4-(2-methylphenyl)piperidin-1-yl]methyl}-...)Show SMILES Cc1ccccc1C1CCN(C[C@@H]2CCc3cccnc3C3(C2)OCCO3)CC1 |r| Show InChI InChI=1S/C25H32N2O2/c1-19-5-2-3-7-23(19)21-10-13-27(14-11-21)18-20-8-9-22-6-4-12-26-24(22)25(17-20)28-15-16-29-25/h2-7,12,20-21H,8-11,13-18H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50244373
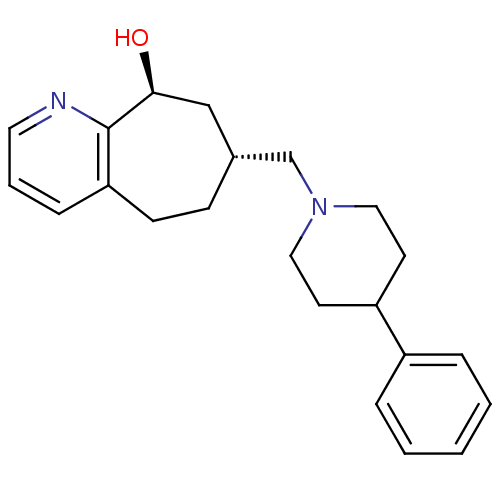
((7R,9S)-7-((4-phenylpiperidin-1-yl)methyl)-6,7,8,9...)Show SMILES O[C@H]1C[C@H](CN2CCC(CC2)c2ccccc2)CCc2cccnc12 |r| Show InChI InChI=1S/C22H28N2O/c25-21-15-17(8-9-20-7-4-12-23-22(20)21)16-24-13-10-19(11-14-24)18-5-2-1-3-6-18/h1-7,12,17,19,21,25H,8-11,13-16H2/t17-,21+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I][Tyr14]nociceptin from human ORL1 expressed in CHO cells |
J Med Chem 51: 4021-9 (2008)
Article DOI: 10.1021/jm701590h
BindingDB Entry DOI: 10.7270/Q2KW5FTW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data