

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 by Lineweaver-Burk plot | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50243745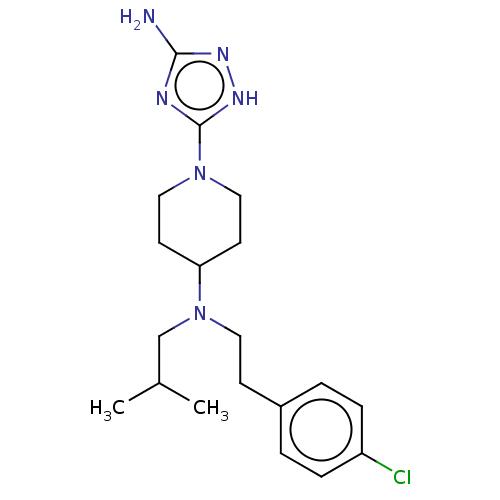 (CHEMBL4076989) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 14 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on rice alpha Glucosidase at pH 8.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243745 (CHEMBL4076989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280031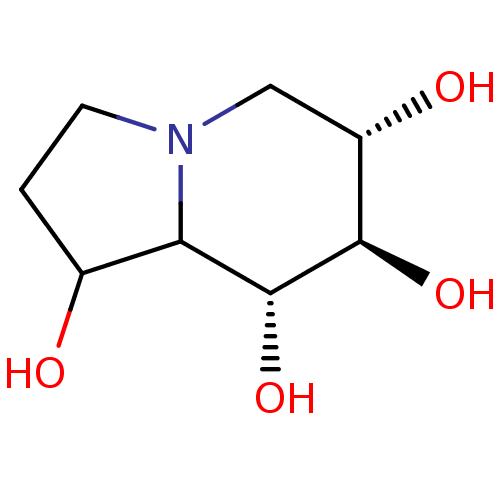 ((6S,7R,8R)-Octahydro-indolizine-1,6,7,8-tetraol | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 15 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 7.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50255702 (CHEMBL520612 | N-(4'-Methyl phenyl)-6,8-dimethoxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 by Lineweaver-Burk plot | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50255812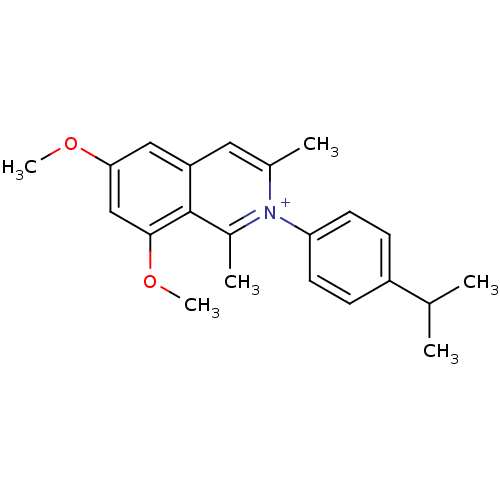 (CHEMBL481022 | N-(4'-i-Propylphenyl)-6,8-dimethoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 by Lineweaver-Burk plot | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 160 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50243745 (CHEMBL4076989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged chitotriosidase expressed in CHO-K1 cells using 4-methylumbelliferyl-beta-D-N,N',N\... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50198075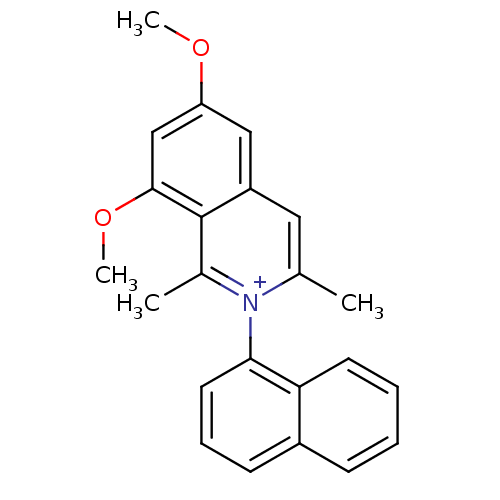 (6,8-dimethoxy-1,3-dimethyl-2-(naphthalen-1-yl)isoq...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 by Lineweaver-Burk plot | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50280031 ((6S,7R,8R)-Octahydro-indolizine-1,6,7,8-tetraol | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 900 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on Asp. Wentii beta Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50280031 ((6S,7R,8R)-Octahydro-indolizine-1,6,7,8-tetraol | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on almonds beta Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280029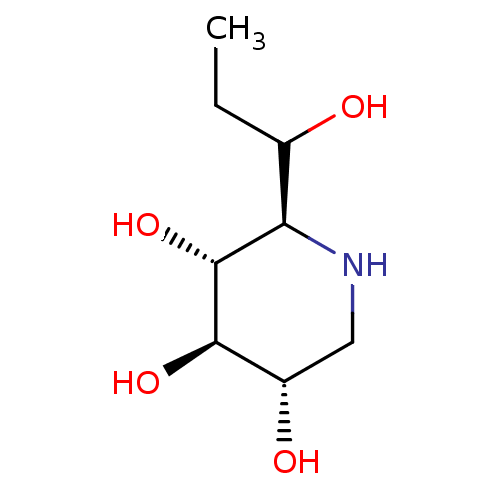 ((2R,3R,4R,5S)-2-(1-Hydroxy-propyl)-piperidine-3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chitotriosidase-1 (Mus musculus) | BDBM50243745 (CHEMBL4076989) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged chitotriosidase expressed in CHO-K1 cells using 4-methylumbelliferyl-beta-D-N,N',N\... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280030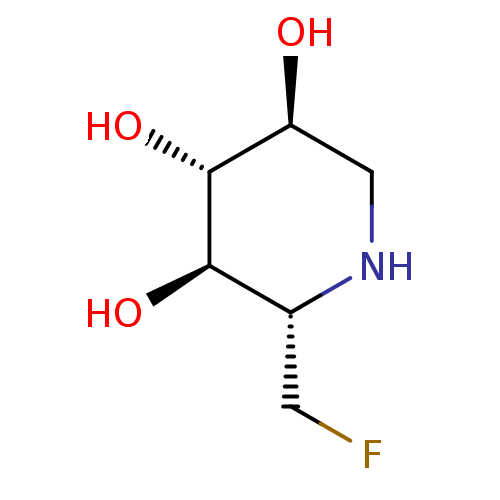 ((2S,3R,4R,5S)-2-Fluoromethyl-piperidine-3,4,5-trio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 7.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280030 ((2S,3R,4R,5S)-2-Fluoromethyl-piperidine-3,4,5-trio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 7.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 6.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280030 ((2S,3R,4R,5S)-2-Fluoromethyl-piperidine-3,4,5-trio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on rice alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50280029 ((2R,3R,4R,5S)-2-(1-Hydroxy-propyl)-piperidine-3,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on Asp. Wentii beta Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280030 ((2S,3R,4R,5S)-2-Fluoromethyl-piperidine-3,4,5-trio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on Asp. Wentii beta Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280030 ((2S,3R,4R,5S)-2-Fluoromethyl-piperidine-3,4,5-trio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 7.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280029 ((2R,3R,4R,5S)-2-(1-Hydroxy-propyl)-piperidine-3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on almonds beta Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on almonds beta Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 7.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50280030 ((2S,3R,4R,5S)-2-Fluoromethyl-piperidine-3,4,5-trio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on Asp. Wentii beta Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM18351 ((2R,3R,4R,5S)-2-(Hydroxymethyl)piperidine-3,4,5-tr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article | 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on rice alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50280030 ((2S,3R,4R,5S)-2-Fluoromethyl-piperidine-3,4,5-trio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50280029 ((2R,3R,4R,5S)-2-(1-Hydroxy-propyl)-piperidine-3,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on almonds beta Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50280032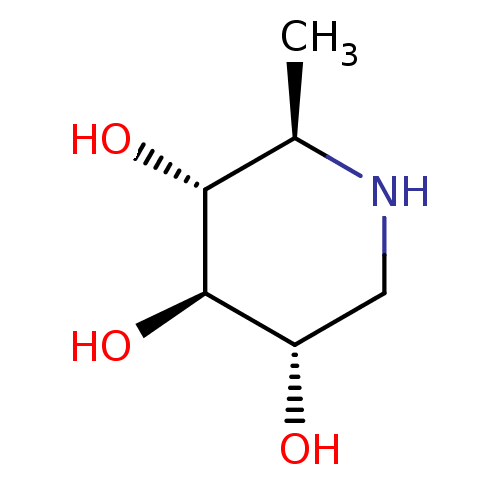 ((2R,3R,4R,5S)-2-Methyl-piperidine-3,4,5-triol | 1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 7.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on almonds beta Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280031 ((6S,7R,8R)-Octahydro-indolizine-1,6,7,8-tetraol | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | >1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on rice alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280032 ((2R,3R,4R,5S)-2-Methyl-piperidine-3,4,5-triol | 1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1.56E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral alpha-glucosidase AB (Homo sapiens (Human)) | BDBM50280029 ((2R,3R,4R,5S)-2-(1-Hydroxy-propyl)-piperidine-3,4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
TBA Curated by ChEMBL | Assay Description Competitive Inhibition constant of the compound was determined on yeast alpha Glucosidase at pH 5.0 | Bioorg Med Chem Lett 2: 27-32 (1992) Article DOI: 10.1016/S0960-894X(00)80648-4 BindingDB Entry DOI: 10.7270/Q2TH8MKB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243685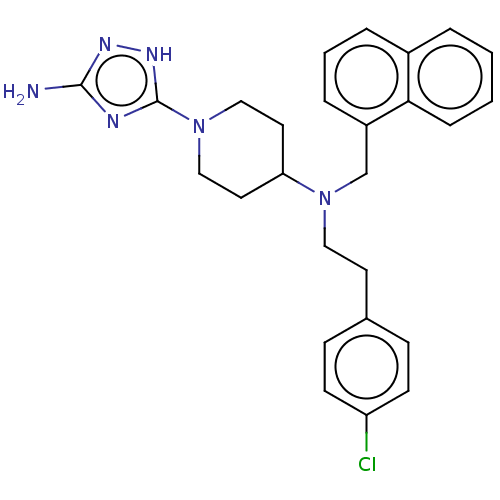 (CHEMBL4084573) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243795 (CHEMBL4077644) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243799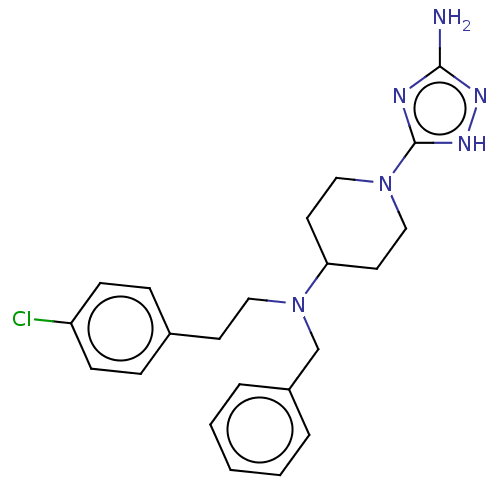 (CHEMBL4077482) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50243685 (CHEMBL4084573) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50243760 (CHEMBL4082060) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50243746 (CHEMBL4068944) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243746 (CHEMBL4068944) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243760 (CHEMBL4082060) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50243795 (CHEMBL4077644) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of [3H]WIN-35428 binding to the dopamine transporter | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50243799 (CHEMBL4077482) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of [3H]GBR-12935 binding to the dopamine transporter. | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Homo sapiens (Human)) | BDBM50243745 (CHEMBL4076989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50243745 (CHEMBL4076989) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50121975 ((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in baculovirus-infected insect cell system | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Chitotriosidase-1 (Homo sapiens (Human)) | BDBM50243685 (CHEMBL4084573) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of human recombinant full length C-terminal His-tagged chitotriosidase expressed in CHO-K1 cells using 4-methylumbelliferyl-beta-D-N,N',N\... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acidic mammalian chitinase (Mus musculus) | BDBM50243744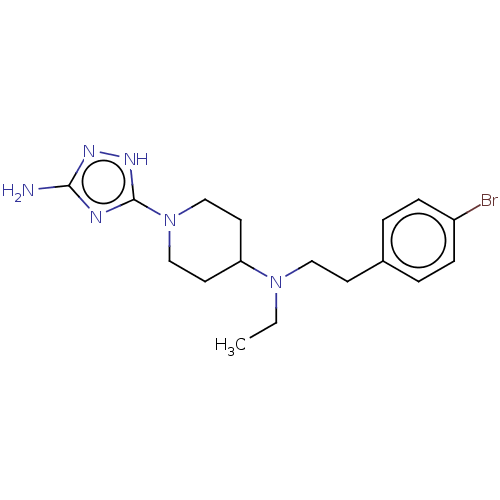 (CHEMBL4098997) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA Curated by ChEMBL | Assay Description Inhibition of mouse recombinant full length C-terminal His-tagged acidic mammalian chitinase expressed in CHO-K1 cells using 4-methylumbelliferyl-bet... | J Med Chem 61: 695-710 (2018) Article DOI: 10.1021/acs.jmedchem.7b01051 BindingDB Entry DOI: 10.7270/Q2J105J5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit epsilon (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of acetylcholinesterase | J Med Chem 48: 7496-9 (2005) Article DOI: 10.1021/jm058041z BindingDB Entry DOI: 10.7270/Q20864WZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50255702 (CHEMBL520612 | N-(4'-Methyl phenyl)-6,8-dimethoxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56.1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 expressed in baculovirus-infected insect cell system | J Med Chem 52: 626-36 (2009) Article DOI: 10.1021/jm801084u BindingDB Entry DOI: 10.7270/Q29C6X9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 150 total ) | Next | Last >> |