

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM338467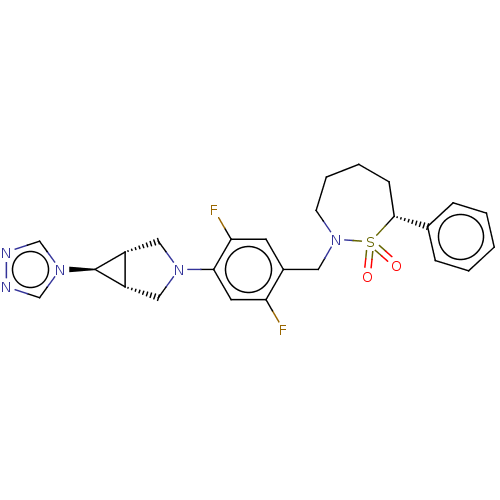 ((7R)-2-[[2,5-difluoro-4-[(1R,5S)- 6-(1,2,4-triazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM338460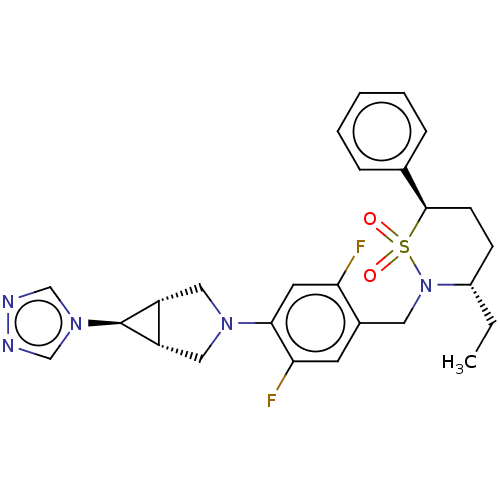 ((3S,6R)-2-[[2,5-difluoro-4-[(1R,5S)- 6-(1,2,4-tria...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM338462 ((3S,6R)-3-ethyl-2-[[2-fluoro-4- [(1R,5S)-6-(1,2,4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM338469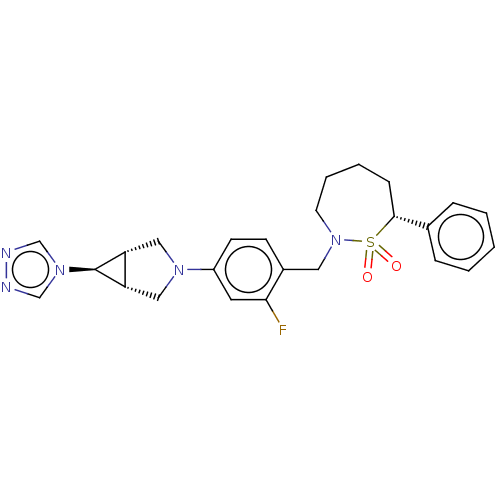 ((7R)-2-[[2-fluoro-4-[(1R,5S)-6- (1,2,4-triazol-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29782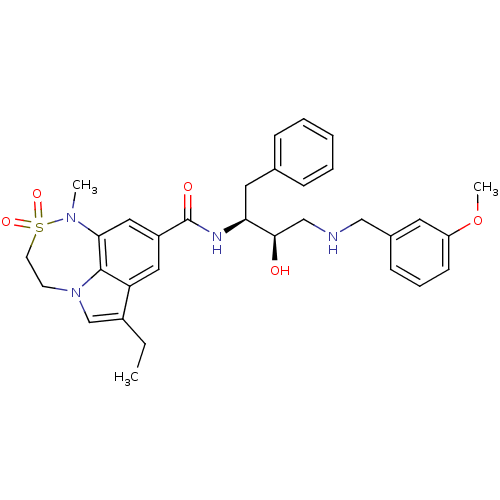 (7,6,5 tricyclic sulfonamide, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26503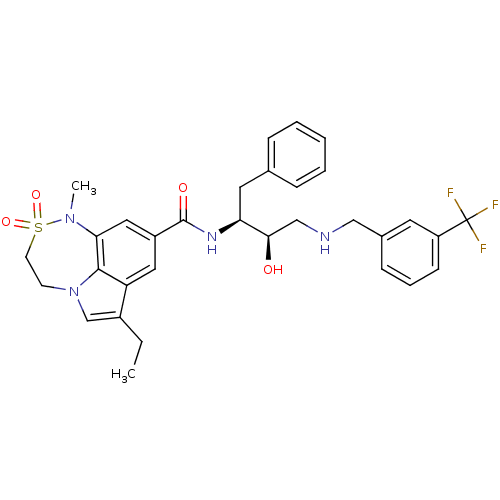 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26503 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26503 (3-ethyl-N-[(2S,3R)-3-hydroxy-1-phenyl-4-({[3-(trif...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26788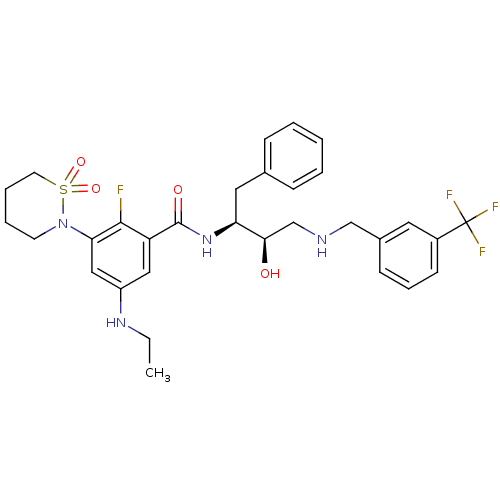 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Rattus norvegicus (Rat)) | BDBM50282632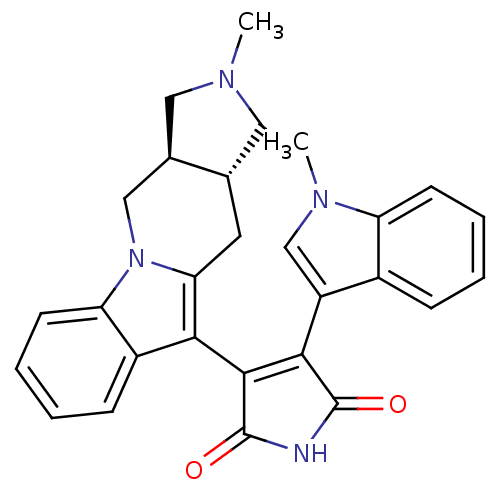 (3-((3aR,10aR)-2-Methyl-2,3,3a,4,10,10a-hexahydro-1...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.85 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Protein kinase C alpha | Bioorg Med Chem Lett 4: 1303-1308 (1994) Article DOI: 10.1016/S0960-894X(01)80349-8 BindingDB Entry DOI: 10.7270/Q27H1JJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM338448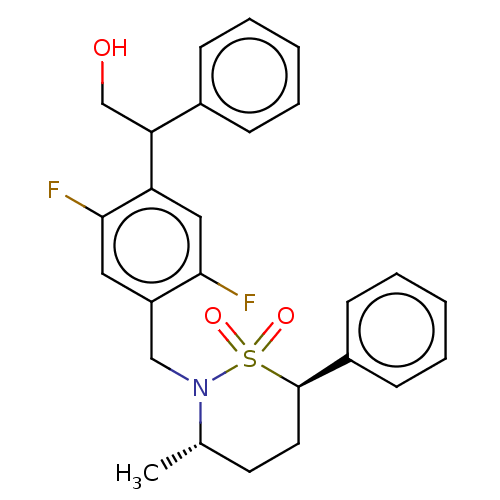 (2-[2,5-difluoro-4-[[(3S,6R)-3- methyl-1,1-dioxo-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM338458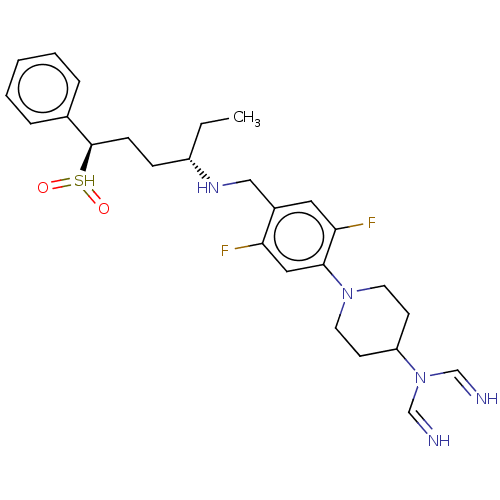 ((3S,6R)-2-[[2,5-difluoro-4-[4- (1,2,4-triazol-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50322883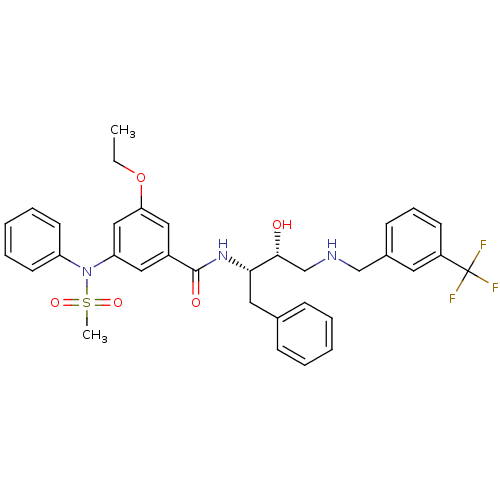 (3-ethoxy-N-((2S,3R)-3-hydroxy-1-phenyl-4-(3-(trifl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 4639-44 (2010) Article DOI: 10.1016/j.bmcl.2010.05.111 BindingDB Entry DOI: 10.7270/Q2RB75K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50322882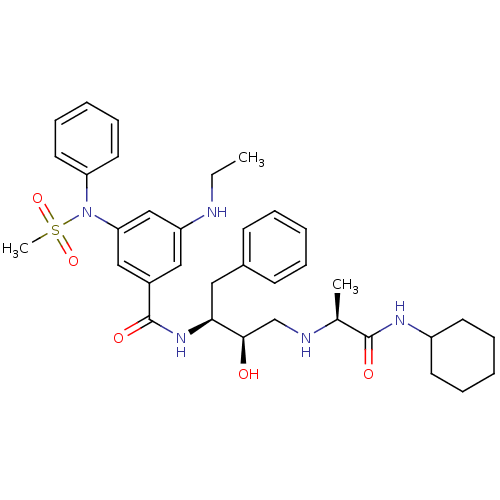 (CHEMBL1210359 | N-((1S,2R)-3-(((1S)-2-(CYCLOHEXYLA...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 4639-44 (2010) Article DOI: 10.1016/j.bmcl.2010.05.111 BindingDB Entry DOI: 10.7270/Q2RB75K7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50132477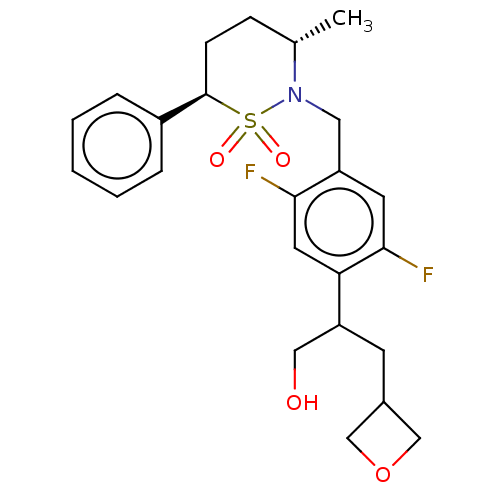 (CHEMBL3632716 | US9751873, Example 127) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM338466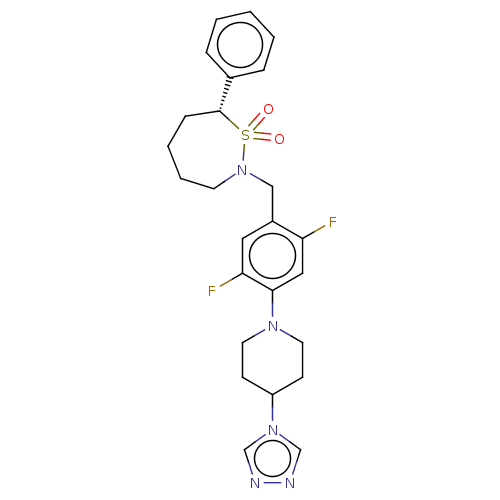 ((7R)-2-[[2,5-difluoro-4-[4-(1,2,4- triazol-4-yl)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26788 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29796 (sulfonamide tricyclic analogue, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26786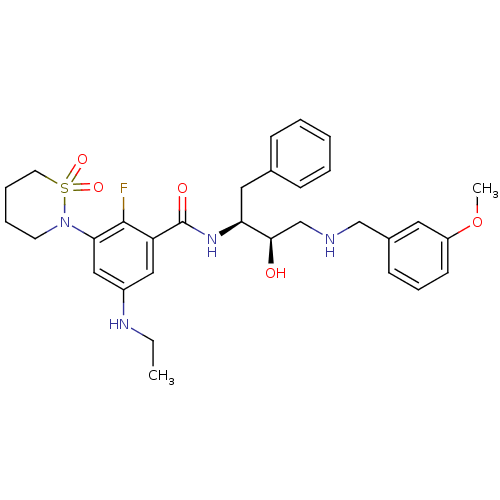 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50322916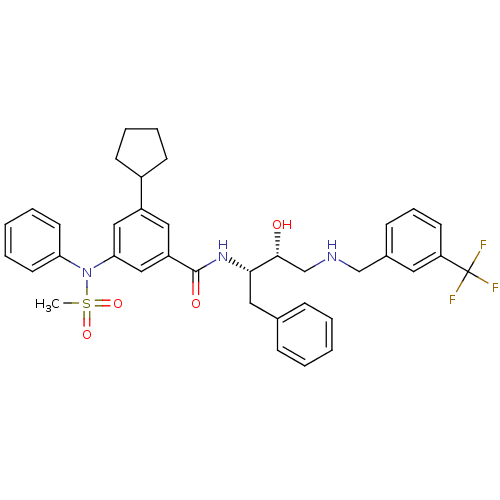 (3-cyclopentyl-N-((2S,3R)-3-hydroxy-1-phenyl-4-(3-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 4639-44 (2010) Article DOI: 10.1016/j.bmcl.2010.05.111 BindingDB Entry DOI: 10.7270/Q2RB75K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50132478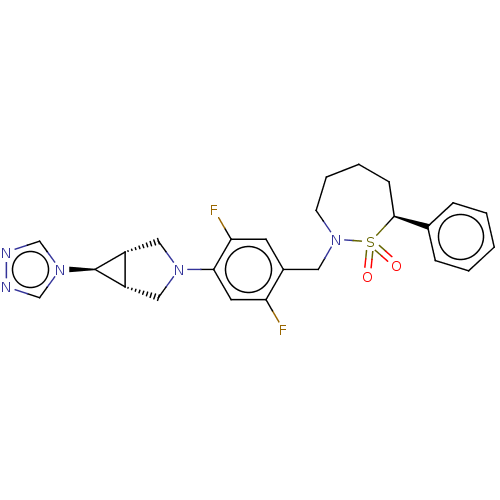 (CHEMBL3634609 | US9751873, Example 157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50322884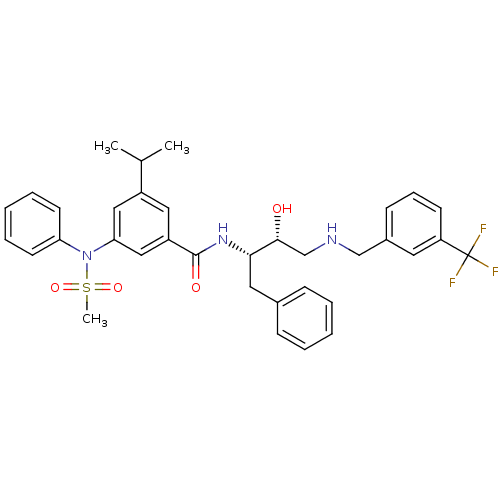 (CHEMBL1210361 | N-((2S,3R)-3-hydroxy-1-phenyl-4-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 4639-44 (2010) Article DOI: 10.1016/j.bmcl.2010.05.111 BindingDB Entry DOI: 10.7270/Q2RB75K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50132478 (CHEMBL3634609 | US9751873, Example 157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29789 (7,6,5 tricyclic sulfonamide, 35) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29794 (sulfonamide tricyclic analogue, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29815 (sulfonamide tricyclic analogue, 28) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3674-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.149 BindingDB Entry DOI: 10.7270/Q25H7DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM338335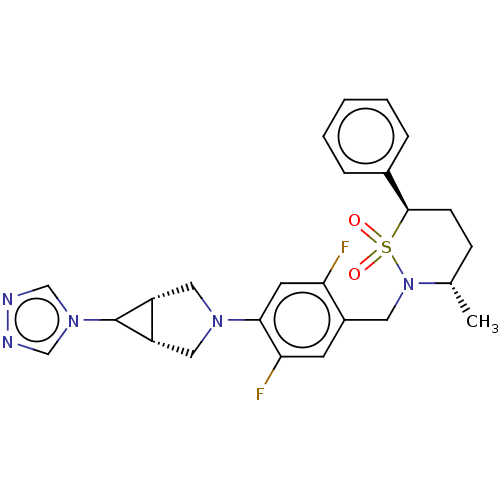 ((1R,5S,6S)-3-[2,5-Difluoro-4- ((3S,6R)-3-methyl-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26506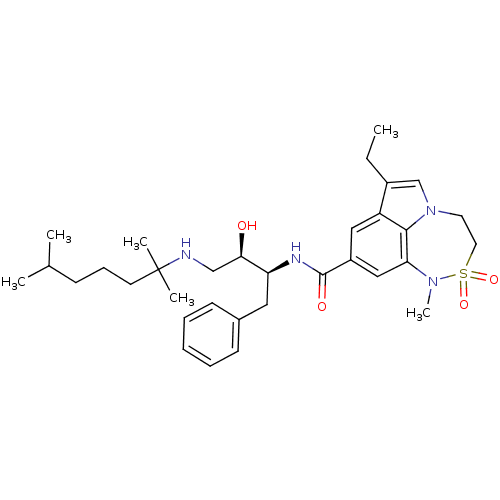 (BMCL193669 Compound 24 | N-[(2S,3R)-4-[(2,6-dimeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | J Med Chem 51: 3313-7 (2008) Article DOI: 10.1021/jm800138h BindingDB Entry DOI: 10.7270/Q2XS5SQR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26774 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-N-[(2S,3R)-3-hydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26776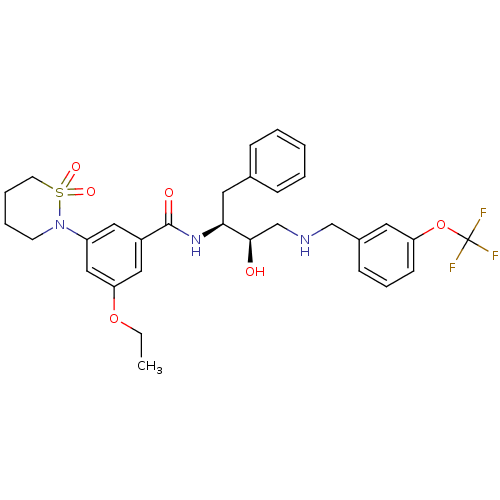 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-ethoxy-N-[(2S,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26777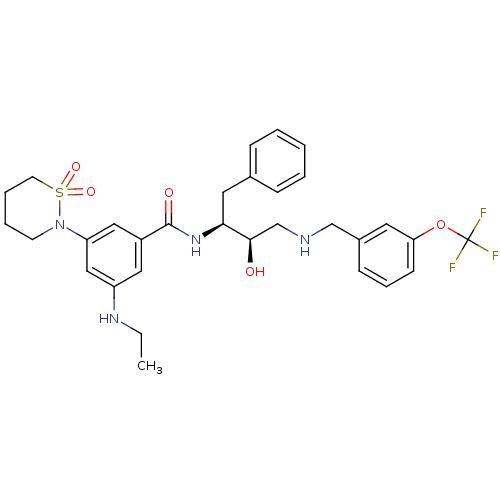 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26782 (N-[(2S,3R)-1-(3,5-difluorophenyl)-3-hydroxy-4-{[(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26506 (BMCL193669 Compound 24 | N-[(2S,3R)-4-[(2,6-dimeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM50282632 (3-((3aR,10aR)-2-Methyl-2,3,3a,4,10,10a-hexahydro-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta 1 | Bioorg Med Chem Lett 4: 1303-1308 (1994) Article DOI: 10.1016/S0960-894X(01)80349-8 BindingDB Entry DOI: 10.7270/Q27H1JJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C gamma type (Rattus norvegicus) | BDBM50282632 (3-((3aR,10aR)-2-Methyl-2,3,3a,4,10,10a-hexahydro-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Protein kinase C gamma (PKC) | Bioorg Med Chem Lett 4: 1303-1308 (1994) Article DOI: 10.1016/S0960-894X(01)80349-8 BindingDB Entry DOI: 10.7270/Q27H1JJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM338336 ((1S,5R,6S)-3-[2,5-Difluoro-4- ((3S,6S)-3-methyl-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26787 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-N-[(2S,3R)-4-{[(1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50322886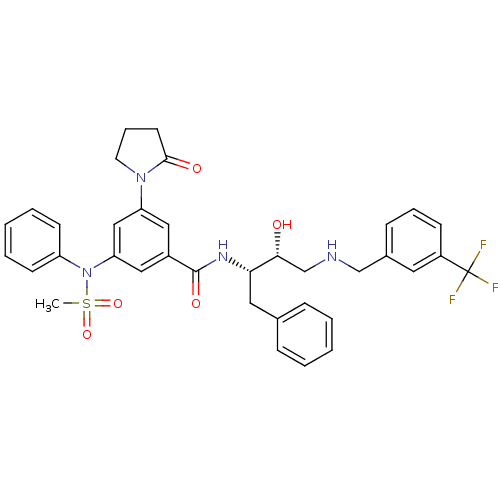 (CHEMBL1210363 | N-((2S,3R)-3-hydroxy-1-phenyl-4-(3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Inhibition of BACE1 | Bioorg Med Chem Lett 20: 4639-44 (2010) Article DOI: 10.1016/j.bmcl.2010.05.111 BindingDB Entry DOI: 10.7270/Q2RB75K7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM29770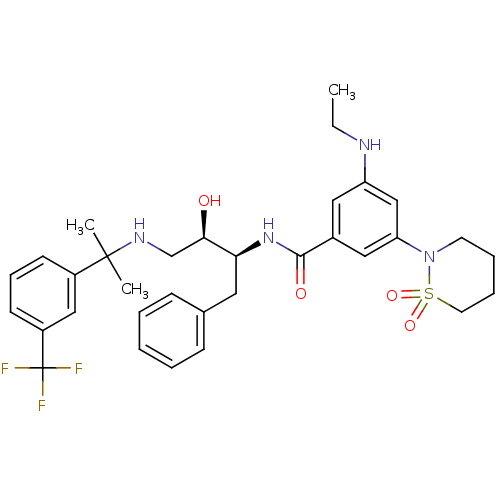 (hydroxyethylamine derivative, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3664-8 (2009) Article DOI: 10.1016/j.bmcl.2009.03.165 BindingDB Entry DOI: 10.7270/Q2F18X23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26773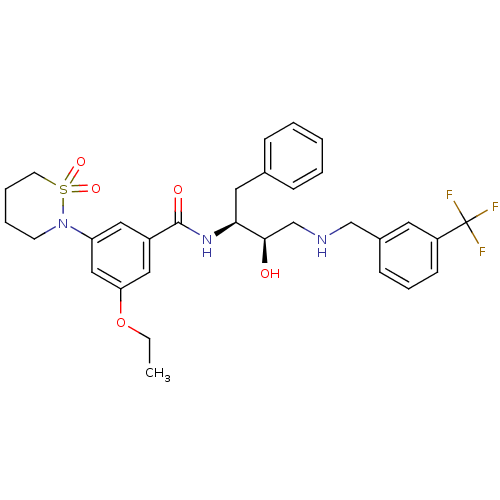 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-ethoxy-N-[(2S,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 18: 1022-6 (2008) Article DOI: 10.1016/j.bmcl.2007.12.020 BindingDB Entry DOI: 10.7270/Q2MC8XBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C beta type (Rattus norvegicus (rat)) | BDBM50282632 (3-((3aR,10aR)-2-Methyl-2,3,3a,4,10,10a-hexahydro-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Protein kinase C beta | Bioorg Med Chem Lett 4: 1303-1308 (1994) Article DOI: 10.1016/S0960-894X(01)80349-8 BindingDB Entry DOI: 10.7270/Q27H1JJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM338400 (8-[3-fluoro-4-[[3S,6R)-3-methyl- 1,1-dioxo-6-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM338436 (1-[4-[4-[[(6S)-5,5-dioxo-6-phenyl- 5$1{circumflex ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50132477 (CHEMBL3632716 | US9751873, Example 127) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM338459 ((3S,6S)-2-[[2,5-difluoro-4- [(1R,5S)-6-(1,2,4-tria...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Assays were carried out in 16-microL reaction volumes in black 384 Plus F Proxiplates (Perkin-Elmer 6008269). All assay components except test ligand... | US Patent US9751873 (2017) BindingDB Entry DOI: 10.7270/Q2BC41NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM26502 (3-(1,1-dioxo-1,2-thiazinan-2-yl)-5-(ethylamino)-N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 22 |
GSK | Assay Description Enzyme activity and inhibition were assayed using a fluorescent FAM-[SEVNLDAEFK]-TAMRA substrate. Control reactions with no enzyme were included in e... | Bioorg Med Chem Lett 19: 3669-73 (2009) Article DOI: 10.1016/j.bmcl.2009.03.150 BindingDB Entry DOI: 10.7270/Q29885BR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 946 total ) | Next | Last >> |