 Found 223 hits with Last Name = 'yan-neale' and Initial = 'y'
Found 223 hits with Last Name = 'yan-neale' and Initial = 'y' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50185443
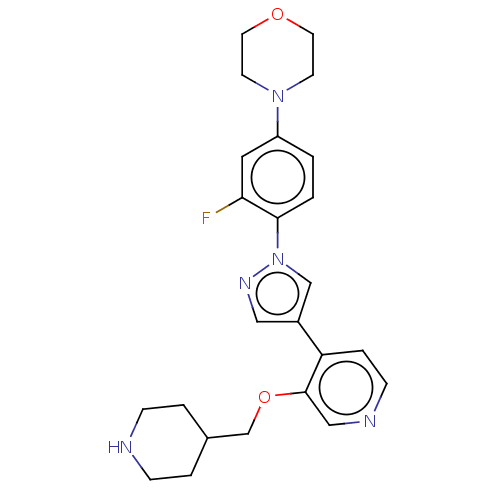
(CHEMBL3824328)Show SMILES Fc1cc(ccc1-n1cc(cn1)-c1ccncc1OCC1CCNCC1)N1CCOCC1 Show InChI InChI=1S/C24H28FN5O2/c25-22-13-20(29-9-11-31-12-10-29)1-2-23(22)30-16-19(14-28-30)21-5-8-27-15-24(21)32-17-18-3-6-26-7-4-18/h1-2,5,8,13-16,18,26H,3-4,6-7,9-12,17H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... |
J Med Chem 59: 4711-23 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00052
BindingDB Entry DOI: 10.7270/Q2MK6FTG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350831
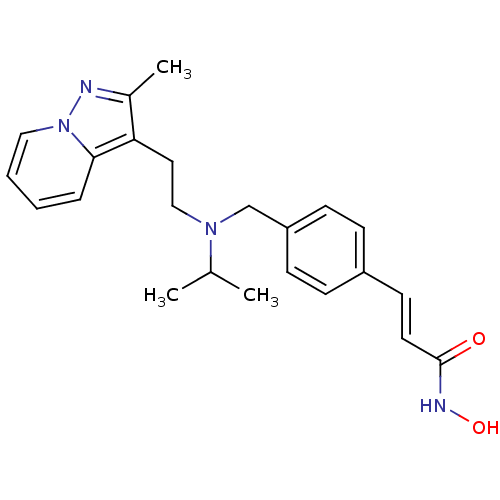
(CHEMBL1819273)Show SMILES CC(C)N(CCc1c(C)nn2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H28N4O2/c1-17(2)26(15-13-21-18(3)24-27-14-5-4-6-22(21)27)16-20-9-7-19(8-10-20)11-12-23(28)25-29/h4-12,14,17,29H,13,15-16H2,1-3H3,(H,25,28)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350832
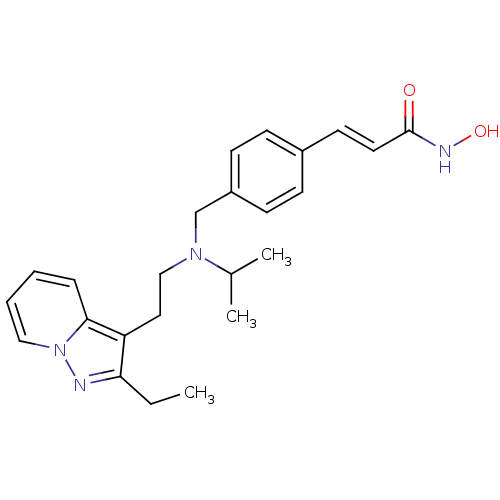
(CHEMBL1819274)Show SMILES CCc1nn2ccccc2c1CCN(Cc1ccc(\C=C\C(=O)NO)cc1)C(C)C Show InChI InChI=1S/C24H30N4O2/c1-4-22-21(23-7-5-6-15-28(23)25-22)14-16-27(18(2)3)17-20-10-8-19(9-11-20)12-13-24(29)26-30/h5-13,15,18,30H,4,14,16-17H2,1-3H3,(H,26,29)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50185439
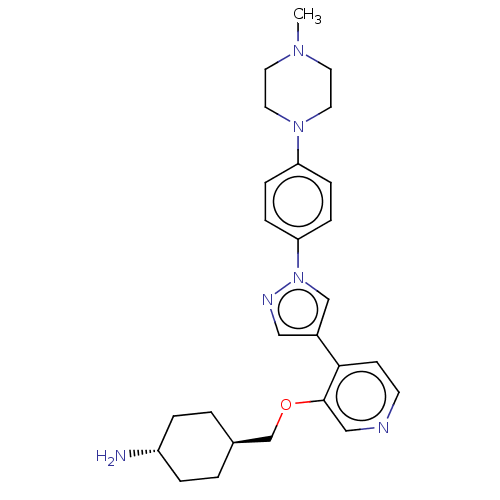
(CHEMBL3823975)Show SMILES CN1CCN(CC1)c1ccc(cc1)-n1cc(cn1)-c1ccncc1OC[C@H]1CC[C@H](N)CC1 |r,wU:29.33,wD:26.29,(15.54,-1.65,;14.31,-1.69,;13.51,-.37,;11.97,-.41,;11.23,-1.77,;12.04,-3.08,;13.58,-3.04,;9.69,-1.81,;8.89,-.5,;7.35,-.54,;6.62,-1.89,;7.42,-3.21,;8.96,-3.17,;5.09,-2.05,;4.06,-.91,;2.67,-1.54,;2.81,-3.06,;4.31,-3.38,;1.33,-.77,;1.33,.77,;,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;-0,-3.08,;-1.34,-3.85,;-1.34,-5.39,;-2.68,-6.16,;-2.68,-7.7,;-1.34,-8.47,;-1.34,-9.7,;-.01,-7.7,;-.01,-6.16,)| Show InChI InChI=1S/C26H34N6O/c1-30-12-14-31(15-13-30)23-6-8-24(9-7-23)32-18-21(16-29-32)25-10-11-28-17-26(25)33-19-20-2-4-22(27)5-3-20/h6-11,16-18,20,22H,2-5,12-15,19,27H2,1H3/t20-,22- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... |
J Med Chem 59: 4711-23 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00052
BindingDB Entry DOI: 10.7270/Q2MK6FTG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350818
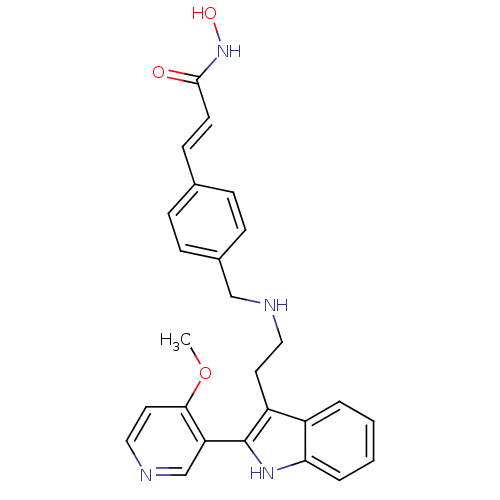
(CHEMBL1819257)Show SMILES COc1ccncc1-c1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C26H26N4O3/c1-33-24-13-15-28-17-22(24)26-21(20-4-2-3-5-23(20)29-26)12-14-27-16-19-8-6-18(7-9-19)10-11-25(31)30-32/h2-11,13,15,17,27,29,32H,12,14,16H2,1H3,(H,30,31)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350827
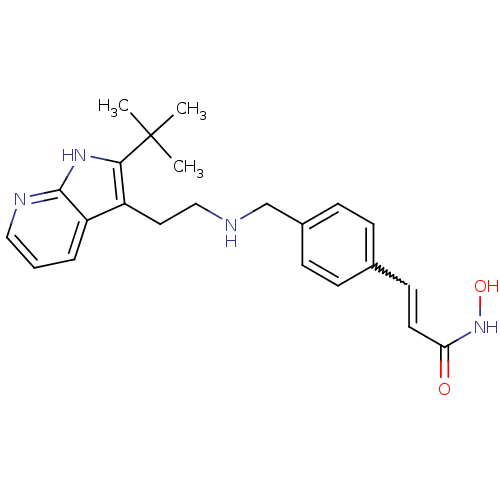
(CHEMBL1819267)Show SMILES CC(C)(C)c1[nH]c2ncccc2c1CCNCc1ccc(C=CC(=O)NO)cc1 |w:21.22| Show InChI InChI=1S/C23H28N4O2/c1-23(2,3)21-18(19-5-4-13-25-22(19)26-21)12-14-24-15-17-8-6-16(7-9-17)10-11-20(28)27-29/h4-11,13,24,29H,12,14-15H2,1-3H3,(H,25,26)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50185433
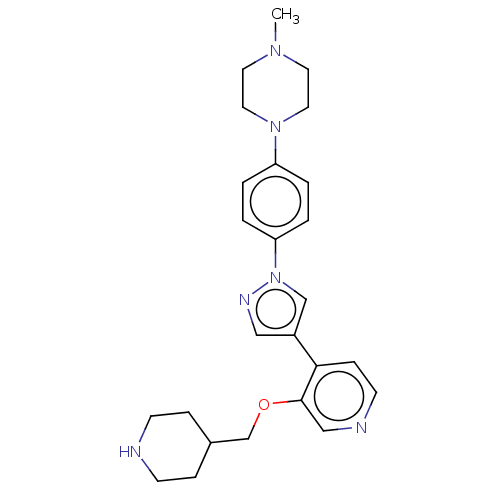
(CHEMBL3824068)Show SMILES CN1CCN(CC1)c1ccc(cc1)-n1cc(cn1)-c1ccncc1OCC1CCNCC1 Show InChI InChI=1S/C25H32N6O/c1-29-12-14-30(15-13-29)22-2-4-23(5-3-22)31-18-21(16-28-31)24-8-11-27-17-25(24)32-19-20-6-9-26-10-7-20/h2-5,8,11,16-18,20,26H,6-7,9-10,12-15,19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to MELK (unknown origin) expressed in Escherichia coli by SPR analysis |
J Med Chem 59: 4711-23 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00052
BindingDB Entry DOI: 10.7270/Q2MK6FTG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350835
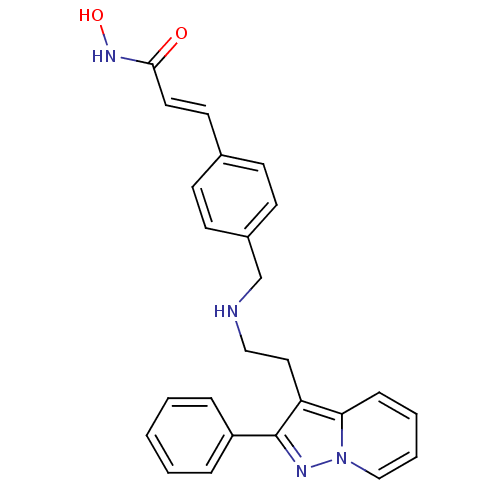
(CHEMBL1819272)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c(nn3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C25H24N4O2/c30-24(28-31)14-13-19-9-11-20(12-10-19)18-26-16-15-22-23-8-4-5-17-29(23)27-25(22)21-6-2-1-3-7-21/h1-14,17,26,31H,15-16,18H2,(H,28,30)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50185433

(CHEMBL3824068)Show SMILES CN1CCN(CC1)c1ccc(cc1)-n1cc(cn1)-c1ccncc1OCC1CCNCC1 Show InChI InChI=1S/C25H32N6O/c1-29-12-14-30(15-13-29)22-2-4-23(5-3-22)31-18-21(16-28-31)24-8-11-27-17-25(24)32-19-20-6-9-26-10-7-20/h2-5,8,11,16-18,20,26H,6-7,9-10,12-15,19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... |
J Med Chem 59: 4711-23 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00052
BindingDB Entry DOI: 10.7270/Q2MK6FTG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350820
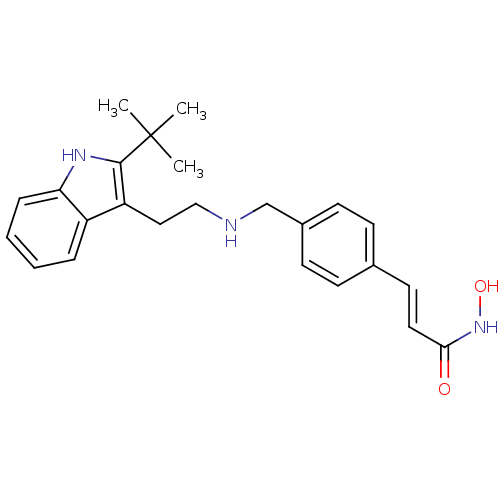
(CHEMBL1819260)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C24H29N3O2/c1-24(2,3)23-20(19-6-4-5-7-21(19)26-23)14-15-25-16-18-10-8-17(9-11-18)12-13-22(28)27-29/h4-13,25-26,29H,14-16H2,1-3H3,(H,27,28)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314628
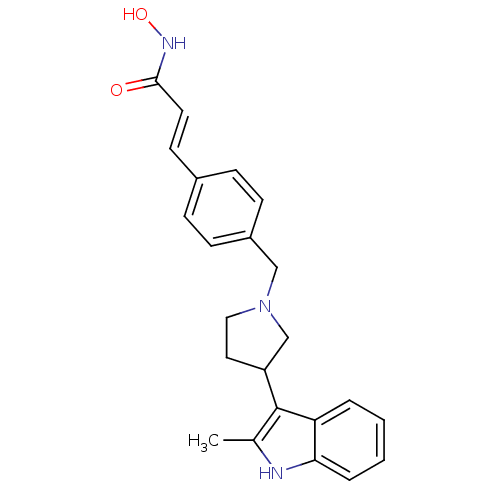
((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pyrr...)Show SMILES Cc1[nH]c2ccccc2c1C1CCN(Cc2ccc(\C=C\C(=O)NO)cc2)C1 Show InChI InChI=1S/C23H25N3O2/c1-16-23(20-4-2-3-5-21(20)24-16)19-12-13-26(15-19)14-18-8-6-17(7-9-18)10-11-22(27)25-28/h2-11,19,24,28H,12-15H2,1H3,(H,25,27)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350833
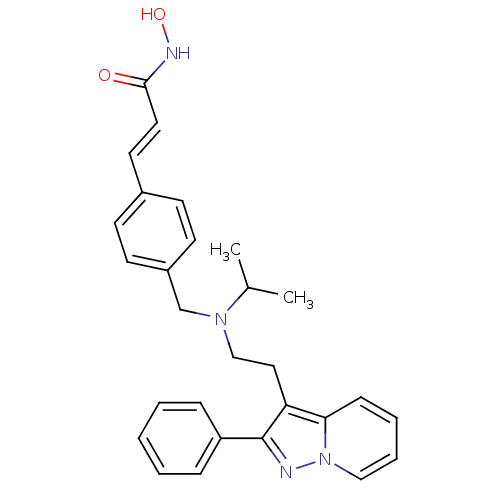
(CHEMBL1819275)Show SMILES CC(C)N(CCc1c(nn2ccccc12)-c1ccccc1)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C28H30N4O2/c1-21(2)31(20-23-13-11-22(12-14-23)15-16-27(33)30-34)19-17-25-26-10-6-7-18-32(26)29-28(25)24-8-4-3-5-9-24/h3-16,18,21,34H,17,19-20H2,1-2H3,(H,30,33)/b16-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350830
(CHEMBL1819270)Show InChI InChI=1S/C20H22N4O2/c1-15-18(19-4-2-3-13-24(19)22-15)11-12-21-14-17-7-5-16(6-8-17)9-10-20(25)23-26/h2-10,13,21,26H,11-12,14H2,1H3,(H,23,25)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314637

((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2)C1 Show InChI InChI=1S/C27H33N3O2/c1-27(2,3)26-25(22-8-4-5-9-23(22)28-26)21-7-6-16-30(18-21)17-20-12-10-19(11-13-20)14-15-24(31)29-32/h4-5,8-15,21,28,32H,6-7,16-18H2,1-3H3,(H,29,31)/b15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314630

((E)-N-Hydroxy-3-(4-{1-[2-(2-methyl-1H-indol-3-yl)e...)Show SMILES Cc1[nH]c2ccccc2c1CCN1CCCC1c1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C24H27N3O2/c1-17-20(21-5-2-3-6-22(21)25-17)14-16-27-15-4-7-23(27)19-11-8-18(9-12-19)10-13-24(28)26-29/h2-3,5-6,8-13,23,25,29H,4,7,14-16H2,1H3,(H,26,28)/b13-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350821
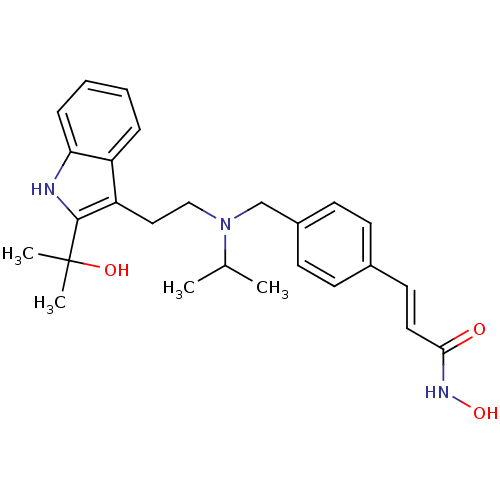
(CHEMBL1819261)Show SMILES CC(C)N(CCc1c([nH]c2ccccc12)C(C)(C)O)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C26H33N3O3/c1-18(2)29(17-20-11-9-19(10-12-20)13-14-24(30)28-32)16-15-22-21-7-5-6-8-23(21)27-25(22)26(3,4)31/h5-14,18,27,31-32H,15-17H2,1-4H3,(H,28,30)/b14-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50185436
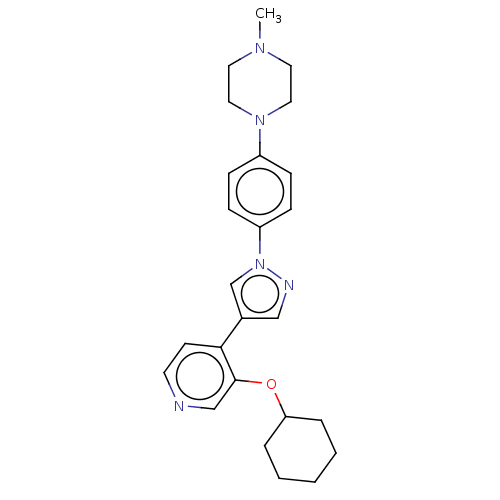
(CHEMBL3823874)Show SMILES CN1CCN(CC1)c1ccc(cc1)-n1cc(cn1)-c1ccncc1OC1CCCCC1 Show InChI InChI=1S/C25H31N5O/c1-28-13-15-29(16-14-28)21-7-9-22(10-8-21)30-19-20(17-27-30)24-11-12-26-18-25(24)31-23-5-3-2-4-6-23/h7-12,17-19,23H,2-6,13-16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... |
J Med Chem 59: 4711-23 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00052
BindingDB Entry DOI: 10.7270/Q2MK6FTG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350826
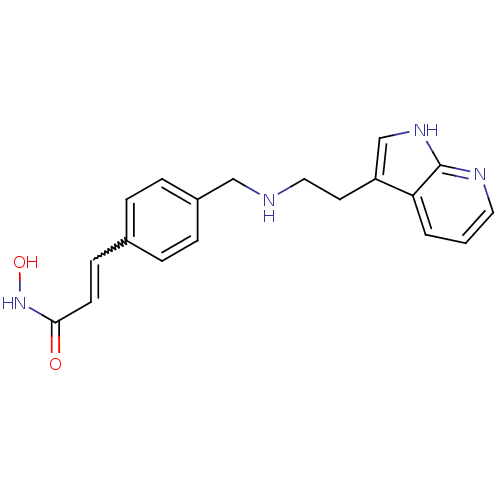
(CHEMBL1819266)Show SMILES ONC(=O)C=Cc1ccc(CNCCc2c[nH]c3ncccc23)cc1 |w:5.5| Show InChI InChI=1S/C19H20N4O2/c24-18(23-25)8-7-14-3-5-15(6-4-14)12-20-11-9-16-13-22-19-17(16)2-1-10-21-19/h1-8,10,13,20,25H,9,11-12H2,(H,21,22)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50185433

(CHEMBL3824068)Show SMILES CN1CCN(CC1)c1ccc(cc1)-n1cc(cn1)-c1ccncc1OCC1CCNCC1 Show InChI InChI=1S/C25H32N6O/c1-29-12-14-30(15-13-29)22-2-4-23(5-3-22)31-18-21(16-28-31)24-8-11-27-17-25(24)32-19-20-6-9-26-10-7-20/h2-5,8,11,16-18,20,26H,6-7,9-10,12-15,19H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of full-length MELK (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence of 2... |
J Med Chem 59: 4711-23 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00052
BindingDB Entry DOI: 10.7270/Q2MK6FTG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314627
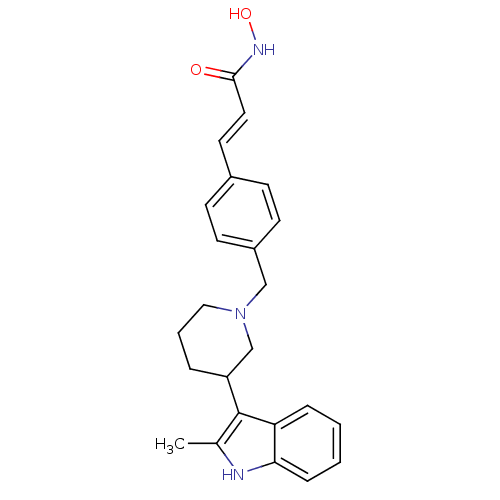
((E)-N-Hydroxy-3-{4-[3-(2-methyl-1H-indol-3-yl)pipe...)Show SMILES Cc1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2)C1 Show InChI InChI=1S/C24H27N3O2/c1-17-24(21-6-2-3-7-22(21)25-17)20-5-4-14-27(16-20)15-19-10-8-18(9-11-19)12-13-23(28)26-29/h2-3,6-13,20,25,29H,4-5,14-16H2,1H3,(H,26,28)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350829
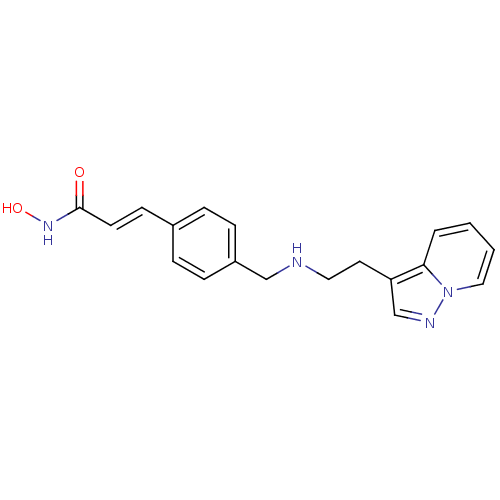
(CHEMBL1819269)Show InChI InChI=1S/C19H20N4O2/c24-19(22-25)9-8-15-4-6-16(7-5-15)13-20-11-10-17-14-21-23-12-2-1-3-18(17)23/h1-9,12,14,20,25H,10-11,13H2,(H,22,24)/b9-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50185437
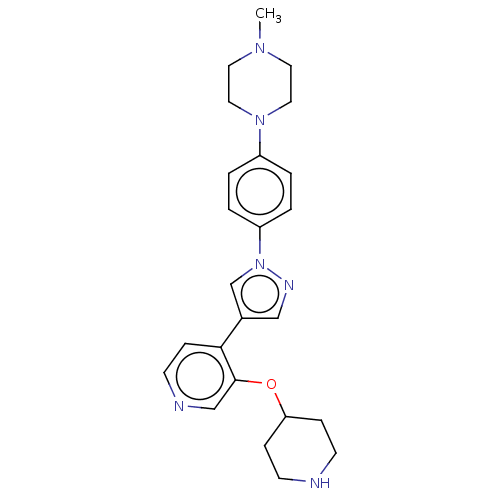
(CHEMBL3822465)Show SMILES CN1CCN(CC1)c1ccc(cc1)-n1cc(cn1)-c1ccncc1OC1CCNCC1 Show InChI InChI=1S/C24H30N6O/c1-28-12-14-29(15-13-28)20-2-4-21(5-3-20)30-18-19(16-27-30)23-8-11-26-17-24(23)31-22-6-9-25-10-7-22/h2-5,8,11,16-18,22,25H,6-7,9-10,12-15H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... |
J Med Chem 59: 4711-23 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00052
BindingDB Entry DOI: 10.7270/Q2MK6FTG |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50185434
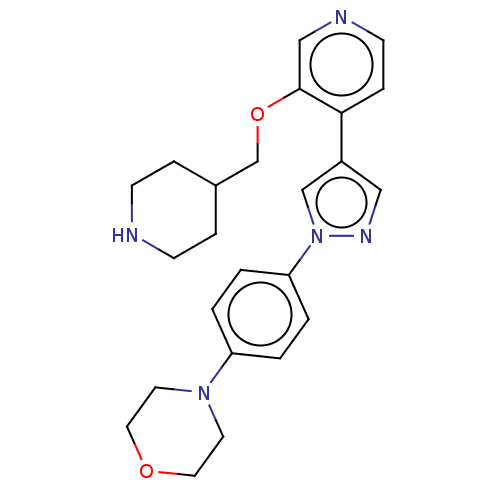
(CHEMBL3823302)Show SMILES C(Oc1cnccc1-c1cnn(c1)-c1ccc(cc1)N1CCOCC1)C1CCNCC1 Show InChI InChI=1S/C24H29N5O2/c1-3-22(4-2-21(1)28-11-13-30-14-12-28)29-17-20(15-27-29)23-7-10-26-16-24(23)31-18-19-5-8-25-9-6-19/h1-4,7,10,15-17,19,25H,5-6,8-9,11-14,18H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... |
J Med Chem 59: 4711-23 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00052
BindingDB Entry DOI: 10.7270/Q2MK6FTG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350838
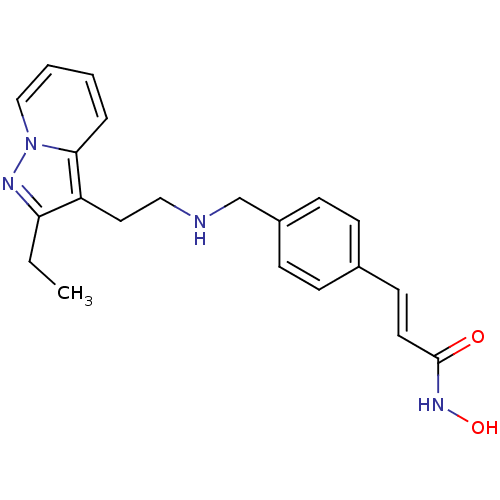
(CHEMBL1819271)Show InChI InChI=1S/C21H24N4O2/c1-2-19-18(20-5-3-4-14-25(20)23-19)12-13-22-15-17-8-6-16(7-9-17)10-11-21(26)24-27/h3-11,14,22,27H,2,12-13,15H2,1H3,(H,24,26)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350824
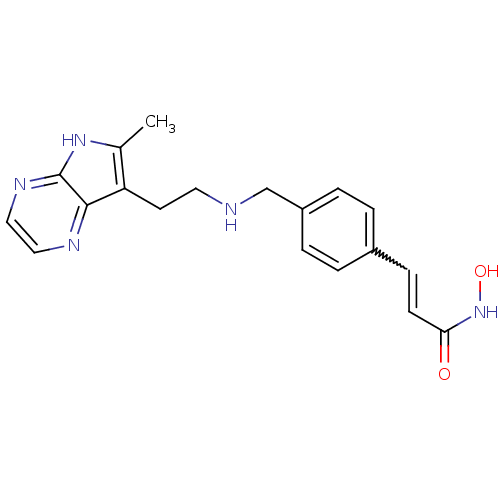
(CHEMBL1819264)Show SMILES Cc1[nH]c2nccnc2c1CCNCc1ccc(C=CC(=O)NO)cc1 |w:18.19| Show InChI InChI=1S/C19H21N5O2/c1-13-16(18-19(23-13)22-11-10-21-18)8-9-20-12-15-4-2-14(3-5-15)6-7-17(25)24-26/h2-7,10-11,20,26H,8-9,12H2,1H3,(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50185435
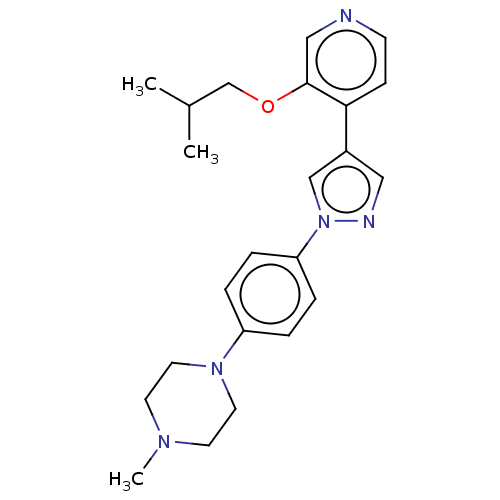
(CHEMBL3823918)Show SMILES CC(C)COc1cnccc1-c1cnn(c1)-c1ccc(cc1)N1CCN(C)CC1 Show InChI InChI=1S/C23H29N5O/c1-18(2)17-29-23-15-24-9-8-22(23)19-14-25-28(16-19)21-6-4-20(5-7-21)27-12-10-26(3)11-13-27/h4-9,14-16,18H,10-13,17H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... |
J Med Chem 59: 4711-23 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00052
BindingDB Entry DOI: 10.7270/Q2MK6FTG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50314642

((E)-3-{4-[(R)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC3 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314640
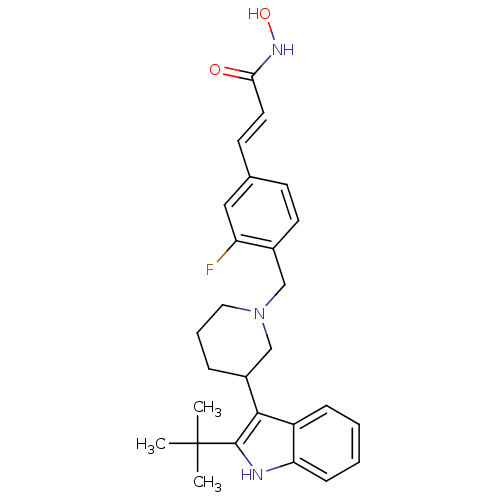
((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350828
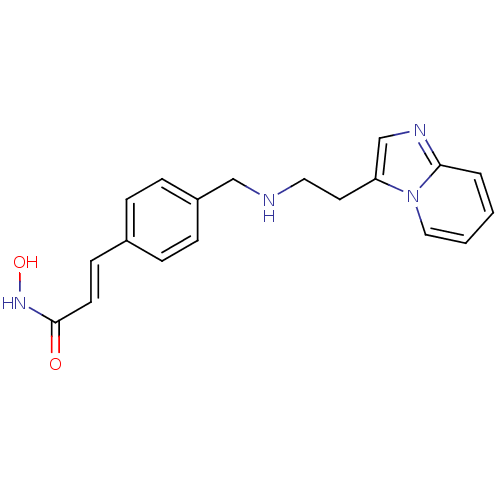
(CHEMBL1819268)Show InChI InChI=1S/C19H20N4O2/c24-19(22-25)9-8-15-4-6-16(7-5-15)13-20-11-10-17-14-21-18-3-1-2-12-23(17)18/h1-9,12,14,20,25H,10-11,13H2,(H,22,24)/b9-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314635

((E)-N-Hydroxy-3-{4-[3-(1H-indol-3-yl)piperidin-1-y...)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c[nH]c3ccccc23)cc1 Show InChI InChI=1S/C23H25N3O2/c27-23(25-28)12-11-17-7-9-18(10-8-17)15-26-13-3-4-19(16-26)21-14-24-22-6-2-1-5-20(21)22/h1-2,5-12,14,19,24,28H,3-4,13,15-16H2,(H,25,27)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350822
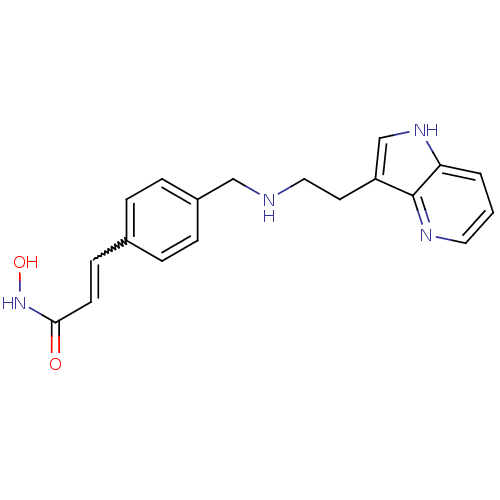
(CHEMBL1819262)Show SMILES ONC(=O)C=Cc1ccc(CNCCc2c[nH]c3cccnc23)cc1 |w:5.5| Show InChI InChI=1S/C19H20N4O2/c24-18(23-25)8-7-14-3-5-15(6-4-14)12-20-11-9-16-13-22-17-2-1-10-21-19(16)17/h1-8,10,13,20,22,25H,9,11-12H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314636
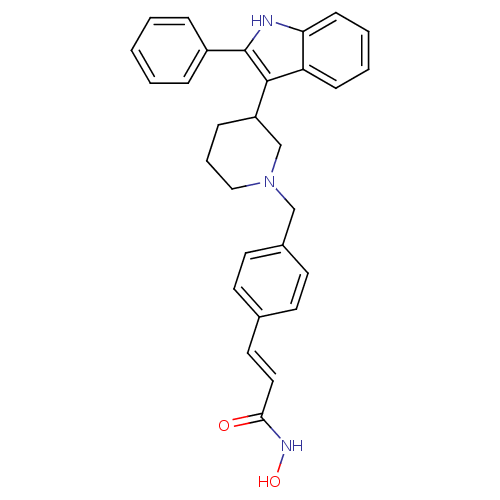
((E)-N-Hydroxy-3-{4-[3-(2-phenyl-1H-indol-3-yl)pipe...)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c([nH]c3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C29H29N3O2/c33-27(31-34)17-16-21-12-14-22(15-13-21)19-32-18-6-9-24(20-32)28-25-10-4-5-11-26(25)30-29(28)23-7-2-1-3-8-23/h1-5,7-8,10-17,24,30,34H,6,9,18-20H2,(H,31,33)/b17-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350817
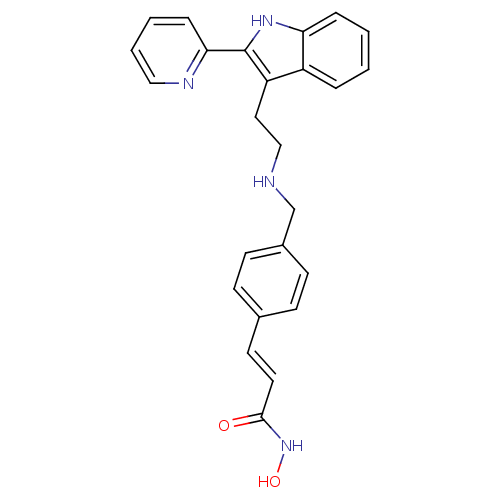
(CHEMBL1819256)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c([nH]c3ccccc23)-c2ccccn2)cc1 Show InChI InChI=1S/C25H24N4O2/c30-24(29-31)13-12-18-8-10-19(11-9-18)17-26-16-14-21-20-5-1-2-6-22(20)28-25(21)23-7-3-4-15-27-23/h1-13,15,26,28,31H,14,16-17H2,(H,29,30)/b13-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350816
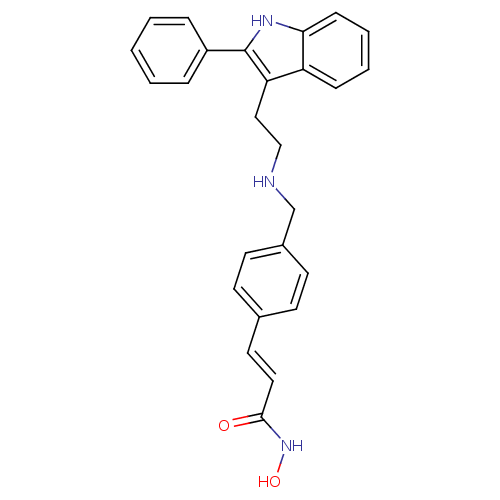
(CHEMBL1819255)Show SMILES ONC(=O)\C=C\c1ccc(CNCCc2c([nH]c3ccccc23)-c2ccccc2)cc1 Show InChI InChI=1S/C26H25N3O2/c30-25(29-31)15-14-19-10-12-20(13-11-19)18-27-17-16-23-22-8-4-5-9-24(22)28-26(23)21-6-2-1-3-7-21/h1-15,27-28,31H,16-18H2,(H,29,30)/b15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350813
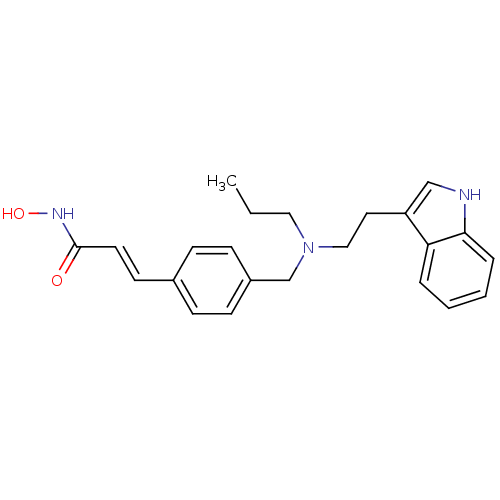
(CHEMBL1819141)Show SMILES CCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H27N3O2/c1-2-14-26(15-13-20-16-24-22-6-4-3-5-21(20)22)17-19-9-7-18(8-10-19)11-12-23(27)25-28/h3-12,16,24,28H,2,13-15,17H2,1H3,(H,25,27)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350810
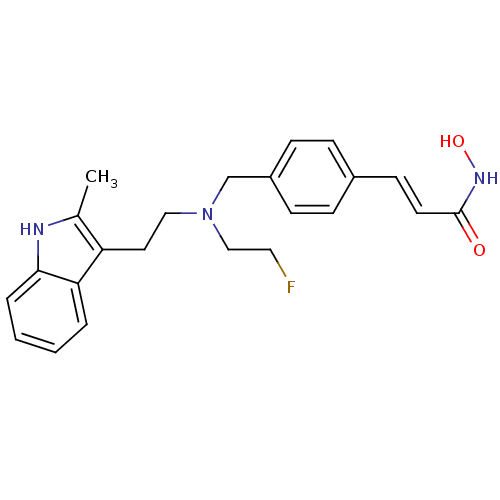
(CHEMBL1819138)Show SMILES Cc1[nH]c2ccccc2c1CCN(CCF)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C23H26FN3O2/c1-17-20(21-4-2-3-5-22(21)25-17)12-14-27(15-13-24)16-19-8-6-18(7-9-19)10-11-23(28)26-29/h2-11,25,29H,12-16H2,1H3,(H,26,28)/b11-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314633

(CHEMBL1093362 | N-hydroxy-3-(2-(2-(2-methyl-1H-ind...)Show SMILES Cc1[nH]c2ccccc2c1CCN1CCc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C23H25N3O2/c1-16-20(21-4-2-3-5-22(21)24-16)11-13-26-12-10-18-8-6-17(14-19(18)15-26)7-9-23(27)25-28/h2-9,14,24,28H,10-13,15H2,1H3,(H,25,27)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Maternal embryonic leucine zipper kinase
(Homo sapiens (Human)) | BDBM50185444
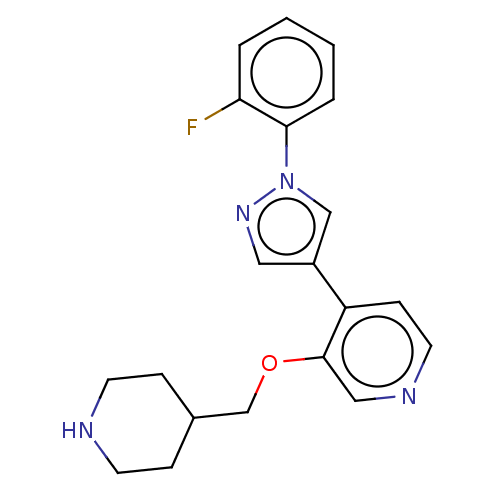
(CHEMBL3822876)Show InChI InChI=1S/C20H21FN4O/c21-18-3-1-2-4-19(18)25-13-16(11-24-25)17-7-10-23-12-20(17)26-14-15-5-8-22-9-6-15/h1-4,7,10-13,15,22H,5-6,8-9,14H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... |
J Med Chem 59: 4711-23 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00052
BindingDB Entry DOI: 10.7270/Q2MK6FTG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314643
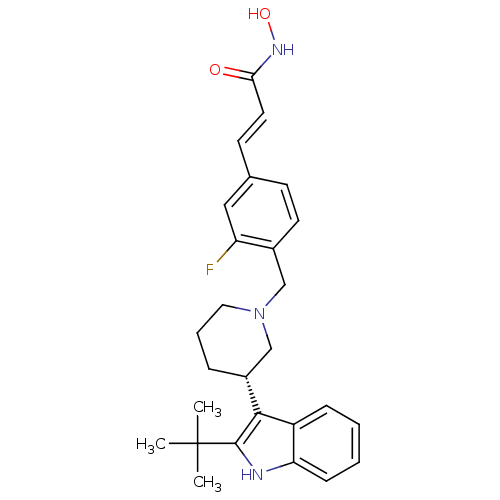
((E)-3-{4-[(S)-3-(2-tert-Butyl-1H-indol-3-yl)piperi...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1[C@@H]1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2F)C1 |r| Show InChI InChI=1S/C27H32FN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM25150
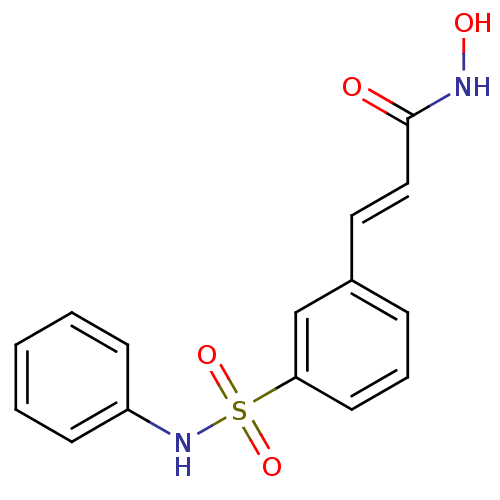
((2E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2...)Show InChI InChI=1S/C15H14N2O4S/c18-15(16-19)10-9-12-5-4-8-14(11-12)22(20,21)17-13-6-2-1-3-7-13/h1-11,17,19H,(H,16,18)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19428

((2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol...)Show SMILES OCCN(CCc1c[nH]c2ccccc12)Cc1ccc(\C=C\C(=O)NO)cc1 Show InChI InChI=1S/C22H25N3O3/c26-14-13-25(12-11-19-15-23-21-4-2-1-3-20(19)21)16-18-7-5-17(6-8-18)9-10-22(27)24-28/h1-10,15,23,26,28H,11-14,16H2,(H,24,27)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50134232
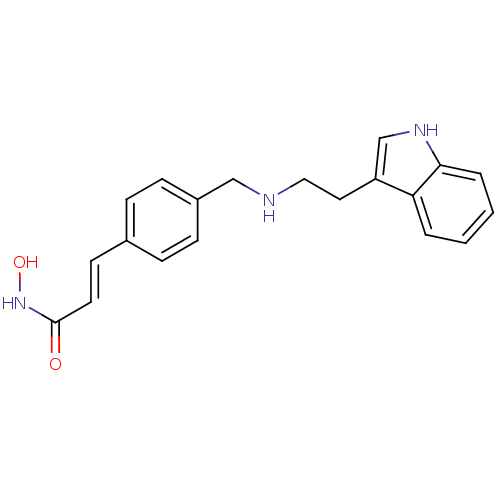
((E)-N-Hydroxy-3-(4-{[2-(1H-indol-3-yl)-ethylamino]...)Show InChI InChI=1S/C20H21N3O2/c24-20(23-25)10-9-15-5-7-16(8-6-15)13-21-12-11-17-14-22-19-4-2-1-3-18(17)19/h1-10,14,21-22,25H,11-13H2,(H,23,24)/b10-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314639

((E)-3-{3-Fluoro-4-[3-(2-phenyl-1H-indol-3-yl)piper...)Show SMILES ONC(=O)\C=C\c1ccc(CN2CCCC(C2)c2c([nH]c3ccccc23)-c2ccccc2)c(F)c1 Show InChI InChI=1S/C29H28FN3O2/c30-25-17-20(13-15-27(34)32-35)12-14-22(25)18-33-16-6-9-23(19-33)28-24-10-4-5-11-26(24)31-29(28)21-7-2-1-3-8-21/h1-5,7-8,10-15,17,23,31,35H,6,9,16,18-19H2,(H,32,34)/b15-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349504

(CHEMBL1808644)Show SMILES ONC(=O)\C=C\c1ccc2CN(CCc3c[nH]c4ccccc34)Cc2c1 Show InChI InChI=1S/C21H21N3O2/c25-21(23-26)8-6-15-5-7-17-13-24(14-18(17)11-15)10-9-16-12-22-20-4-2-1-3-19(16)20/h1-8,11-12,22,26H,9-10,13-14H2,(H,23,25)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50350834
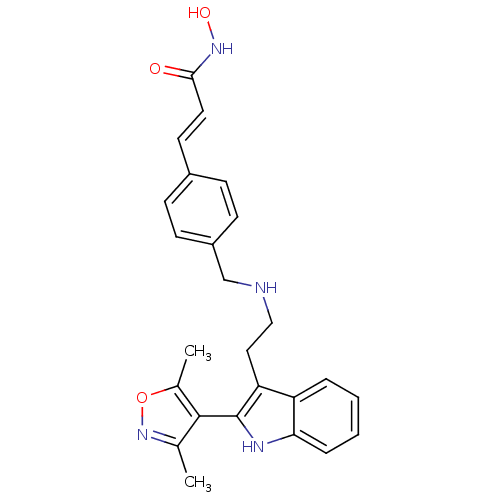
(CHEMBL1819259)Show SMILES Cc1noc(C)c1-c1[nH]c2ccccc2c1CCNCc1ccc(\C=C\C(=O)NO)cc1 |(-6.03,-13.03,;-4.7,-12.25,;-3.29,-12.87,;-2.26,-11.72,;-3.04,-10.39,;-2.42,-8.98,;-4.56,-10.72,;-5.71,-9.69,;-7.22,-10.01,;-8,-8.67,;-9.51,-8.34,;-9.98,-6.87,;-8.94,-5.73,;-7.44,-6.05,;-6.96,-7.52,;-5.55,-8.15,;-4.21,-7.37,;-2.88,-8.14,;-1.54,-7.37,;-.21,-8.14,;1.12,-7.37,;1.12,-5.82,;2.45,-5.05,;3.79,-5.82,;5.12,-5.04,;6.46,-5.81,;7.79,-5.03,;7.78,-3.49,;9.12,-5.8,;10.45,-5.02,;3.79,-7.37,;2.46,-8.14,)| Show InChI InChI=1S/C25H26N4O3/c1-16-24(17(2)32-29-16)25-21(20-5-3-4-6-22(20)27-25)13-14-26-15-19-9-7-18(8-10-19)11-12-23(30)28-31/h3-12,26-27,31H,13-15H2,1-2H3,(H,28,30)/b12-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
J Med Chem 54: 4752-72 (2011)
Article DOI: 10.1021/jm200388e
BindingDB Entry DOI: 10.7270/Q23N24DW |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50314641
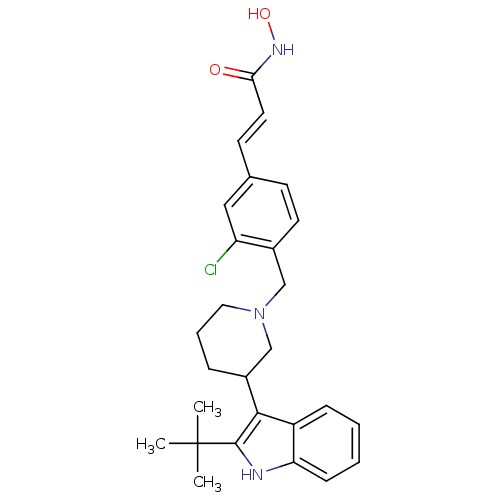
((E)-3-{4-[3-(2-tert-Butyl-1H-indol-3-yl)piperidin-...)Show SMILES CC(C)(C)c1[nH]c2ccccc2c1C1CCCN(Cc2ccc(\C=C\C(=O)NO)cc2Cl)C1 Show InChI InChI=1S/C27H32ClN3O2/c1-27(2,3)26-25(21-8-4-5-9-23(21)29-26)20-7-6-14-31(17-20)16-19-12-10-18(15-22(19)28)11-13-24(32)30-33/h4-5,8-13,15,20,29,33H,6-7,14,16-17H2,1-3H3,(H,30,32)/b13-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged HDAC1 expressed in HEK293 cells by fluorescent assay |
J Med Chem 53: 2952-63 (2010)
Article DOI: 10.1021/jm100007m
BindingDB Entry DOI: 10.7270/Q20V8CZC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50349485

(CHEMBL1808625)Show SMILES Cc1[nH]c2ccccc2c1CC(=O)N1Cc2ccc(\C=C\C(=O)NO)cc2C1 Show InChI InChI=1S/C22H21N3O3/c1-14-19(18-4-2-3-5-20(18)23-14)11-22(27)25-12-16-8-6-15(10-17(16)13-25)7-9-21(26)24-28/h2-10,23,28H,11-13H2,1H3,(H,24,26)/b9-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of purified recombinant HDAC1 |
Bioorg Med Chem Lett 21: 4909-12 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.015
BindingDB Entry DOI: 10.7270/Q2542NZQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data