

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073270 (CHEMBL333781 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50457437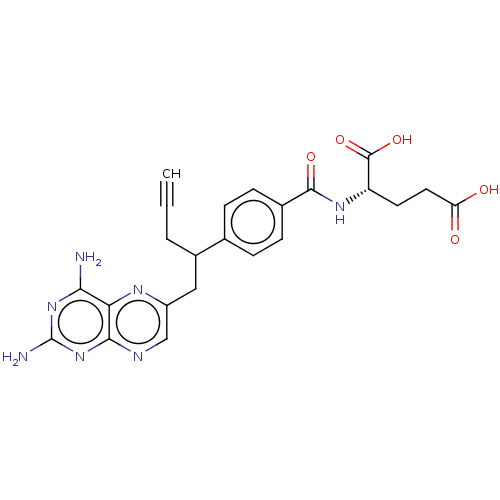 (CHEBI:71223 | Folotyn | PDX | Pralatrexate) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113218 BindingDB Entry DOI: 10.7270/Q2PN99PG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073266 (CHEMBL119898 | {2-[(1S,3S,4S)-1-Benzyl-4-((2S,3S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073253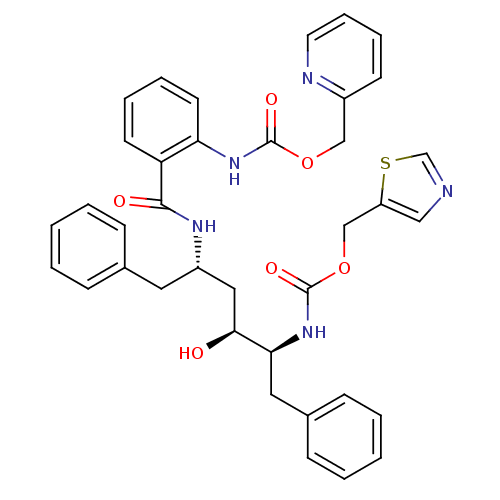 (CHEMBL278935 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073250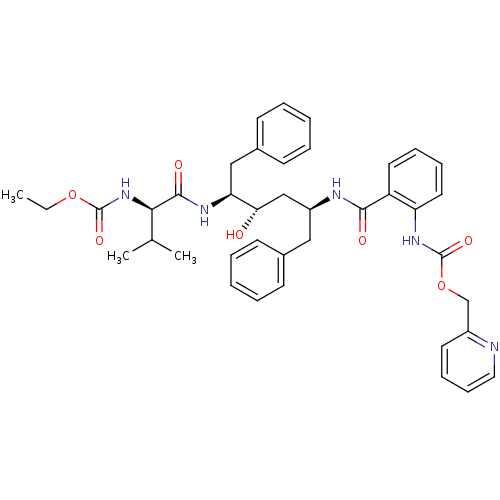 (CHEMBL333420 | {2-[(1S,3S,4S)-1-Benzyl-4-((R)-2-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073269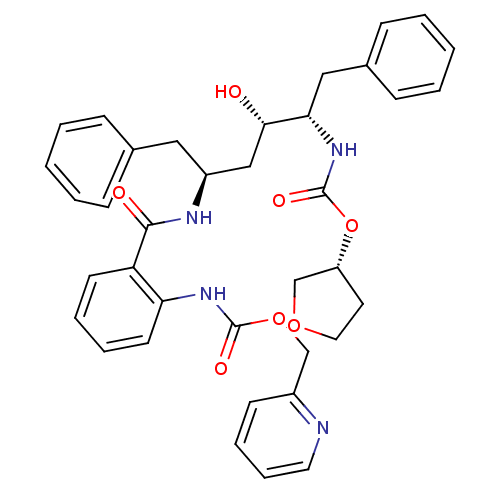 ((2-{(1S,3S,4S)-1-Benzyl-3-hydroxy-5-phenyl-4-[(R)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073252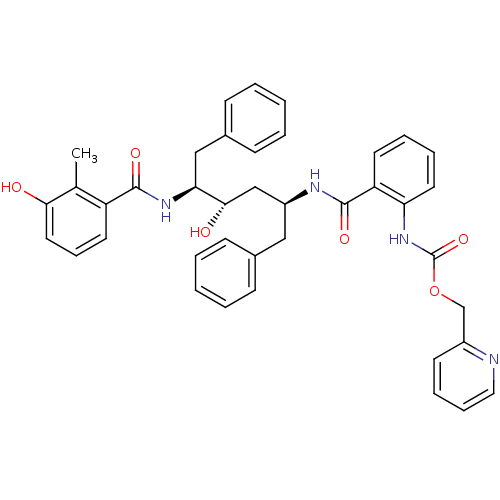 (CHEMBL324157 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073254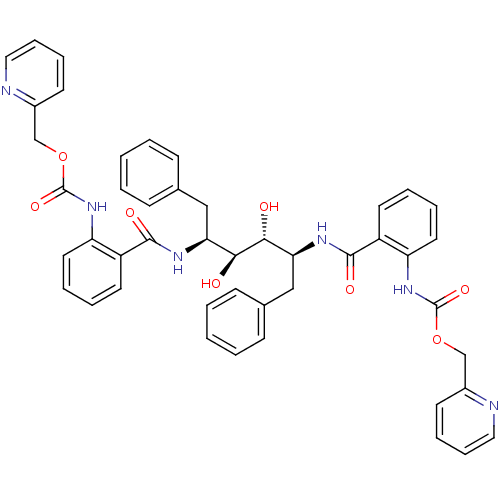 (Anthranilamide derivative | CHEMBL408110) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285701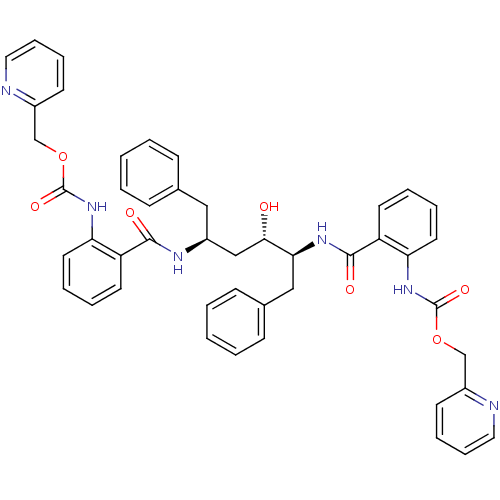 (Anthranilamide derivative | CHEMBL313767) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073265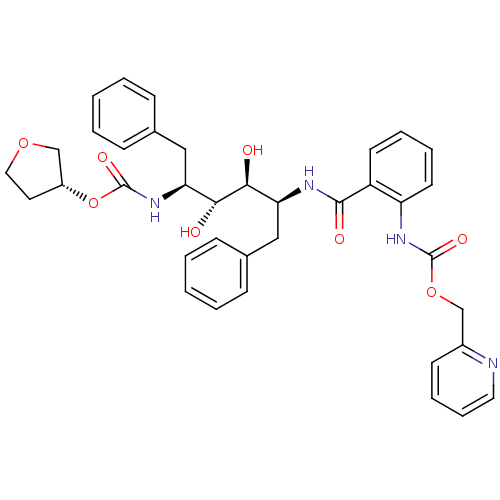 ((2-{(1S,2S,3R,4S)-1-Benzyl-2,3-dihydroxy-5-phenyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073254 (Anthranilamide derivative | CHEMBL408110) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073268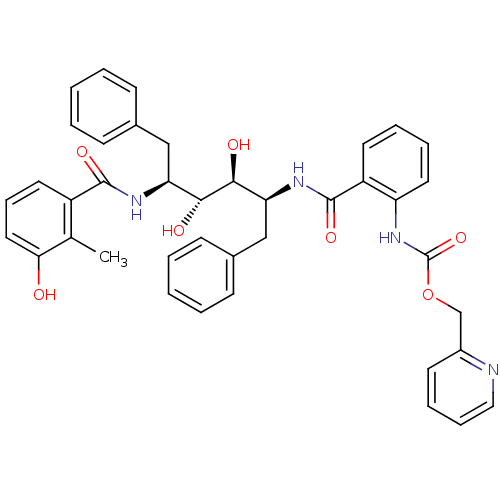 (CHEMBL331294 | {2-[(1S,2S,3R,4S)-1-Benzyl-2,3-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073263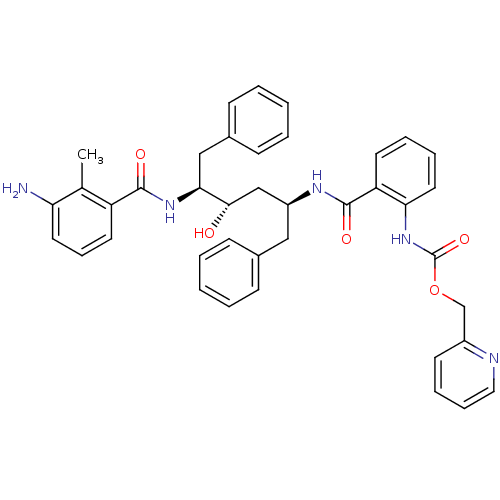 (CHEMBL117629 | {2-[(1S,3S,4S)-4-(3-Amino-2-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073244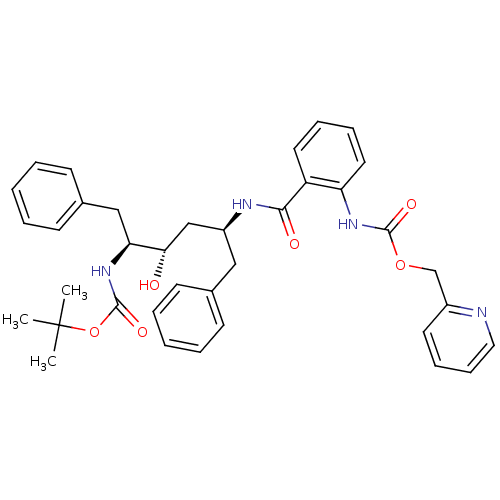 (CHEMBL332825 | [2-((1S,3S,4S)-1-Benzyl-4-tert-buto...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073262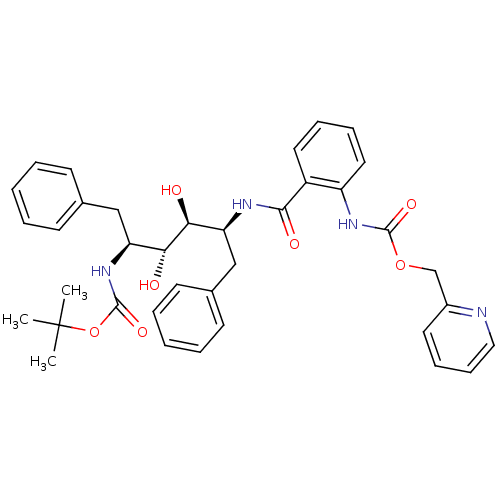 (CHEMBL333290 | [2-((1S,2S,3R,4S)-1-Benzyl-4-tert-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at recombinant human H3 receptor expressed in CHO-K1 cell membranes incubated for 60 mins by [35S]GTPgammaS binding assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112667 BindingDB Entry DOI: 10.7270/Q2HD80BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285698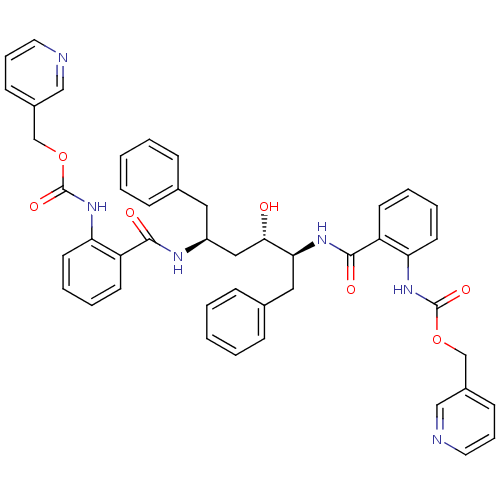 (Anthranilamide derivative | CHEMBL315500) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase 17B (Homo sapiens (Human)) | BDBM50166121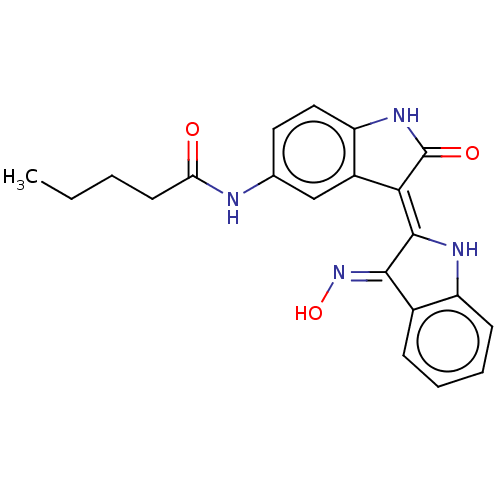 (CHEMBL3797480) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Chemical Technology Curated by ChEMBL | Assay Description Competitive inhibition of DRAK2 (unknown origin) using MRLC3 peptide as substrate incubated for 2 hrs by Lineweaver-Burk plot analysis in presence of... | Bioorg Med Chem Lett 26: 2719-23 (2016) Article DOI: 10.1016/j.bmcl.2016.03.111 BindingDB Entry DOI: 10.7270/Q2N29ZTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM50073587 (CHEMBL3408947 | US10358436, Example A185 | US20230...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Competitive inhibition of PLK-4 (unknown origin) | Eur J Med Chem 95: 35-40 (2015) Article DOI: 10.1016/j.ejmech.2015.03.020 BindingDB Entry DOI: 10.7270/Q2NG4SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073248 (CHEMBL119745 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073243 ((2-{(1S,2S,3R,4S)-1-Benzyl-2,3-dihydroxy-4-[(isoxa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073249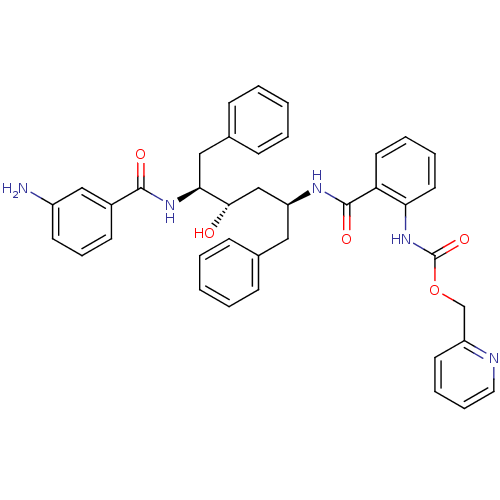 (CHEMBL331199 | {2-[(1S,3S,4S)-4-(3-Amino-benzoylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285700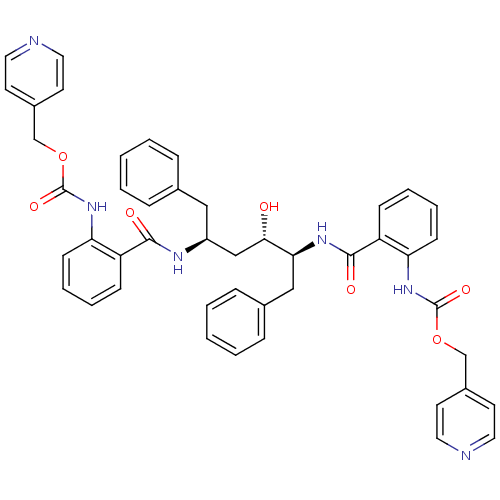 (Anthranilamide derivative | CHEMBL315742) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073259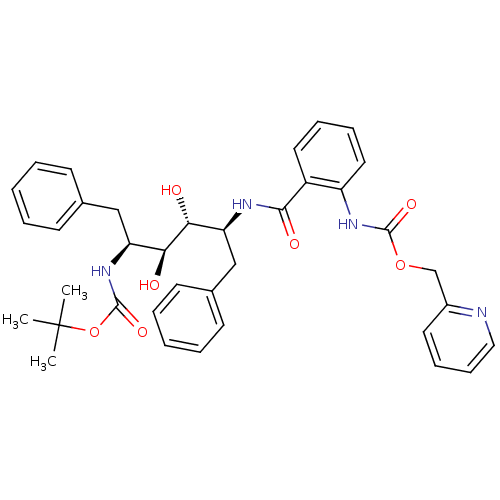 (CHEMBL333407 | [2-((1S,2R,3S,4S)-1-Benzyl-4-tert-b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073261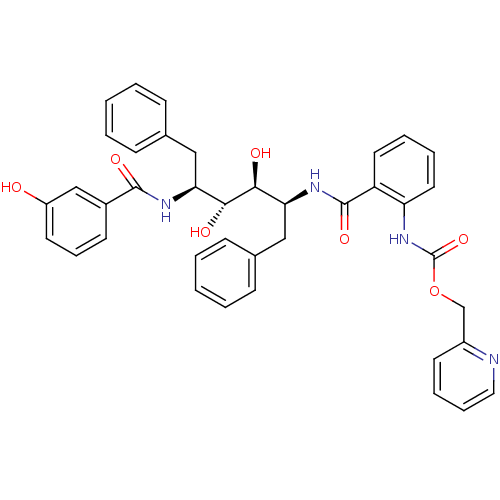 (CHEMBL116482 | {2-[(1S,2S,3R,4S)-1-Benzyl-2,3-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50269559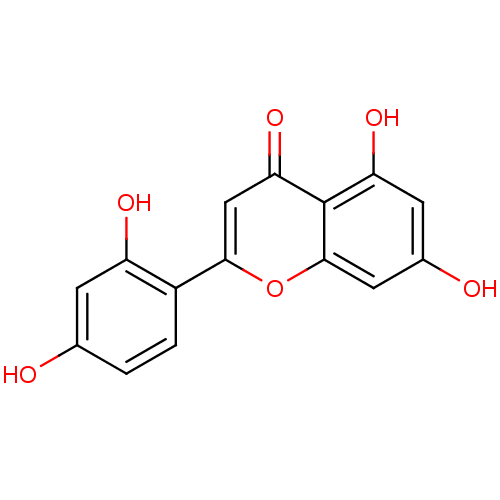 (2-(2,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.610 | -53.5 | n/a | n/a | n/a | n/a | n/a | n/a | 30 |
Gyeongsang National University | Assay Description Mushroom tyrosinase using either L-DOPA or L-tyrosine as substrate. In spectrophotometric experiments, enzyme activity was monitored by dopachrome f... | J Enzyme Inhib Med Chem 23: 922-30 (2008) Article DOI: 10.1080/14756360701810207 BindingDB Entry DOI: 10.7270/Q2P849G3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073251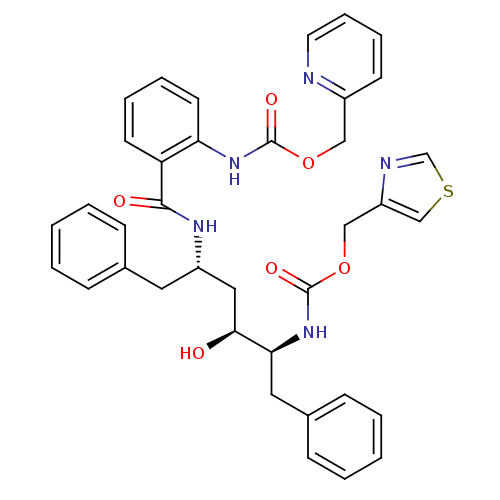 (CHEMBL118396 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073247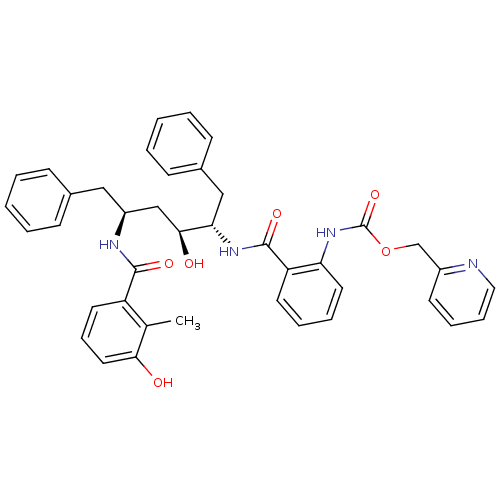 (CHEMBL118513 | {2-[(1S,2S,4S)-1-Benzyl-2-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284919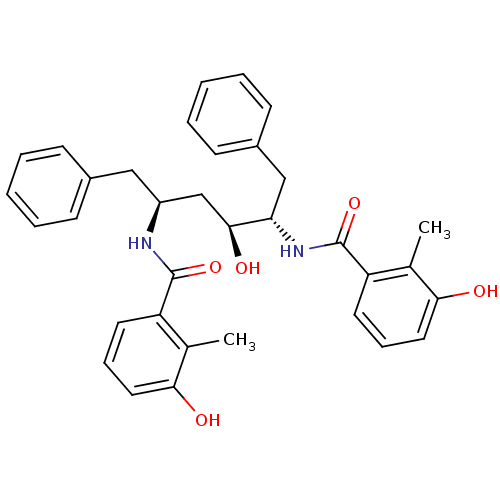 (1N-[1-benzyl-2-hydroxy-4-(3-hydroxy-2-methylphenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV protease | Bioorg Med Chem Lett 5: 1707-1712 (1995) Article DOI: 10.1016/0960-894X(95)00289-6 BindingDB Entry DOI: 10.7270/Q2WD40H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073246 (CHEMBL333392 | {2-[(1S,3S,4S)-1-Benzyl-3-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073256 (CHEMBL122006 | [2-((1S,2S,4S)-1-Benzyl-4-tert-buto...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284922 (1N-[1-benzyl-2,3-dihydroxy-4-(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV protease | Bioorg Med Chem Lett 5: 1707-1712 (1995) Article DOI: 10.1016/0960-894X(95)00289-6 BindingDB Entry DOI: 10.7270/Q2WD40H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285696 (Anthranilamide derivative | CHEMBL85640) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073267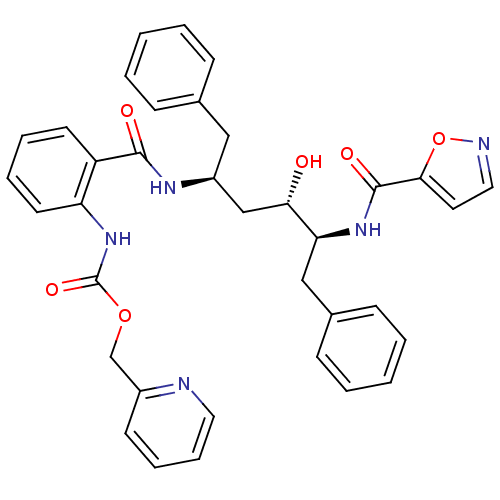 ((2-{(1S,3S,4S)-1-Benzyl-3-hydroxy-4-[(isoxazole-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285703 (Anthranilamide derivative | CHEMBL315928) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Homo sapiens (Human)) | BDBM50027656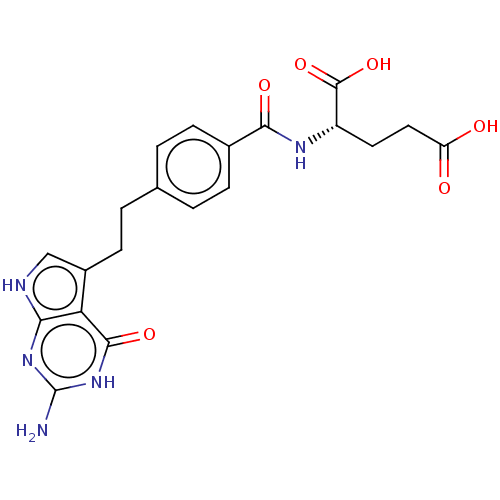 (CHEBI:63616 | LY-2315 | LY-231514 | PEMETREXED | U...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113218 BindingDB Entry DOI: 10.7270/Q2PN99PG | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073264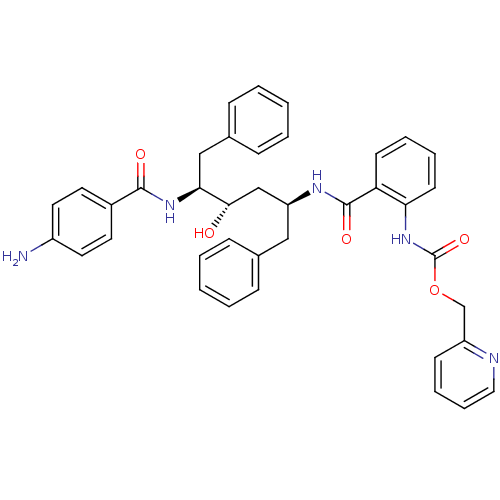 (CHEMBL118531 | {2-[(1S,3S,4S)-4-(4-Amino-benzoylam...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073257 (CHEMBL325499 | {2-[(1S,2R,3S,4S)-1-Benzyl-2,3-dihy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285691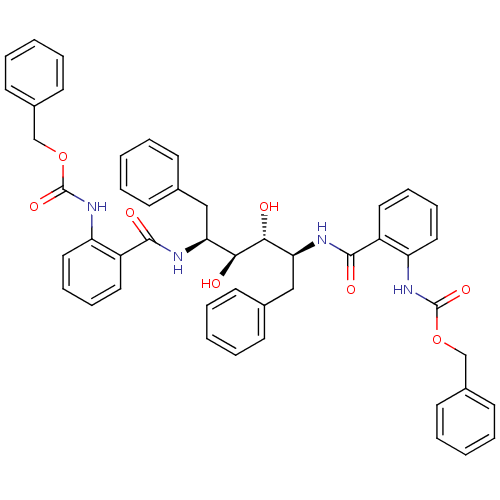 (Anthranilamide derivative | CHEMBL264622) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284921 (1N-[4-(3-amino-2-methylphenylcarboxamido)-1-benzyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV protease | Bioorg Med Chem Lett 5: 1707-1712 (1995) Article DOI: 10.1016/0960-894X(95)00289-6 BindingDB Entry DOI: 10.7270/Q2WD40H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50021655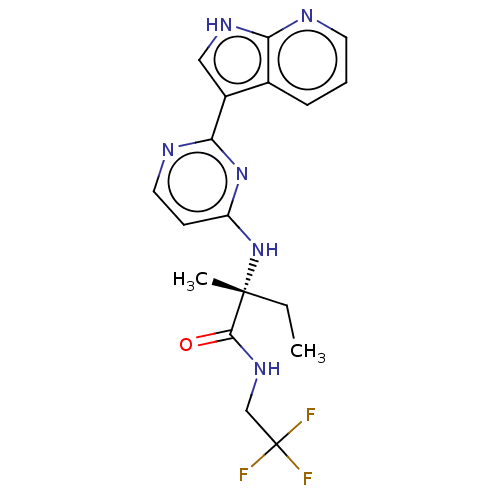 (DECERNOTINIB | US10112907, Example 00017 | US10766...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285695 (Anthranilamide derivative | CHEMBL86263) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of HIV protease using fluorogenic substrate | Bioorg Med Chem Lett 5: 2557-2562 (1995) Article DOI: 10.1016/0960-894X(95)00449-4 BindingDB Entry DOI: 10.7270/Q2RB74KT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK4 (Homo sapiens (Human)) | BDBM25117 (AG-013736 | AXITINIB | N-methyl-2-({3-[(E)-2-(pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University Curated by ChEMBL | Assay Description Inhibition of PLK-4 (unknown origin) | Eur J Med Chem 95: 35-40 (2015) Article DOI: 10.1016/j.ejmech.2015.03.020 BindingDB Entry DOI: 10.7270/Q2NG4SBV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073255 ((2-{(1S,3S,4S)-1-Benzyl-3-hydroxy-5-phenyl-4-[(thi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50070473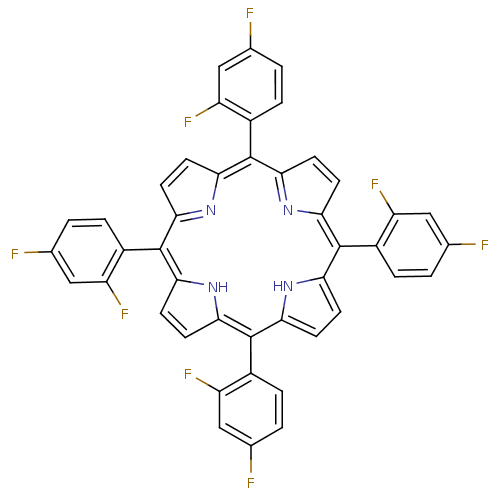 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,4-difluoro-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taejon National University of Technology Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory constant for the electric eel Acetylcholinesterase (AChE) | Bioorg Med Chem Lett 8: 1467-70 (1999) BindingDB Entry DOI: 10.7270/Q21835ND | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50070476 ((1Z,4Z,9Z,15Z)-5,10,15,20-Tetrakis-(2,6-difluoro-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University Curated by ChEMBL | Assay Description Inhibition constant (Ki) against electric eel Acetylcholinesterase as Km/Vmax versus inhibitor concentration replot | Bioorg Med Chem Lett 10: 1435-8 (2000) BindingDB Entry DOI: 10.7270/Q20K27SF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073258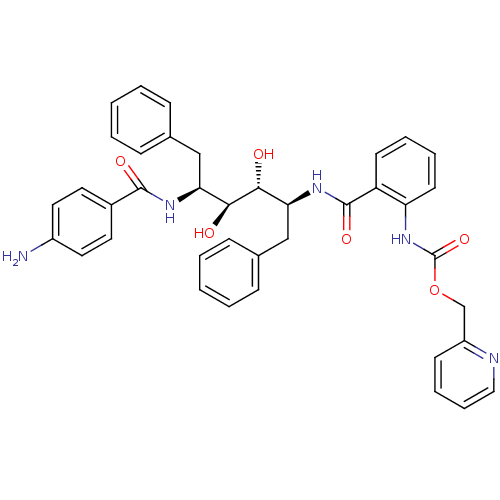 (CHEMBL118221 | {2-[(1S,2R,3S,4S)-4-(4-Amino-benzoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50027656 (CHEBI:63616 | LY-2315 | LY-231514 | PEMETREXED | U...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113218 BindingDB Entry DOI: 10.7270/Q2PN99PG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284916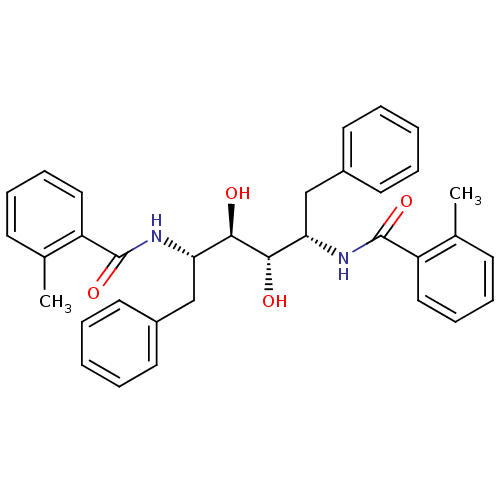 (1N-[1-benzyl-2,3-dihydroxy-4-(2-methylphenylcarbox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HIV protease | Bioorg Med Chem Lett 5: 1707-1712 (1995) Article DOI: 10.1016/0960-894X(95)00289-6 BindingDB Entry DOI: 10.7270/Q2WD40H2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50073245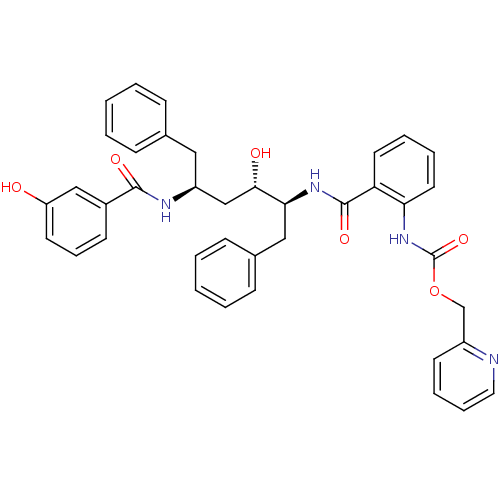 (CHEMBL118362 | {2-[(1S,2S,4S)-1-Benzyl-2-hydroxy-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AIDS Drug Screening and Development Laboratory, SA Curated by ChEMBL | Assay Description Inhibitory activity against HIV protease | Bioorg Med Chem Lett 8: 3537-42 (1999) BindingDB Entry DOI: 10.7270/Q2HM57MB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4609 total ) | Next | Last >> |