

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461430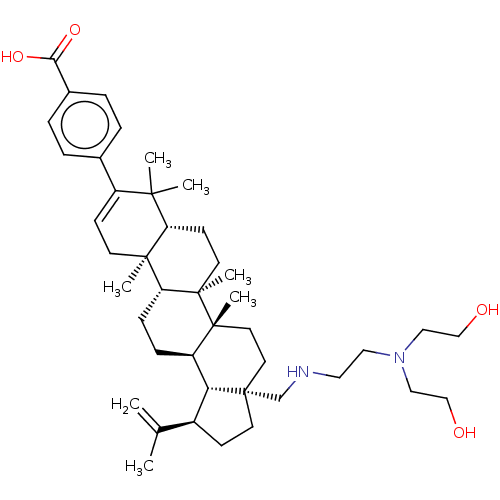 (CHEMBL4226862) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461421 (CHEMBL3828596) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461442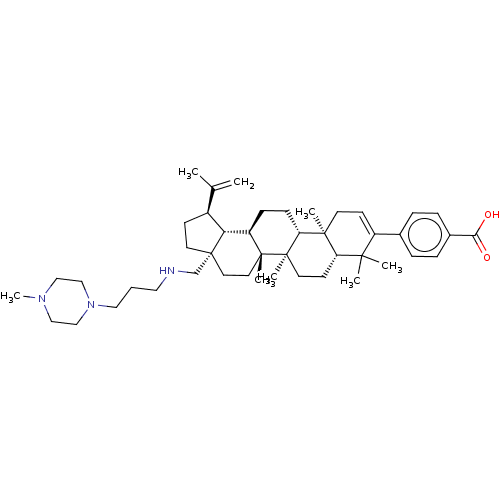 (CHEMBL4225363) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461417 (CHEMBL4225028) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461426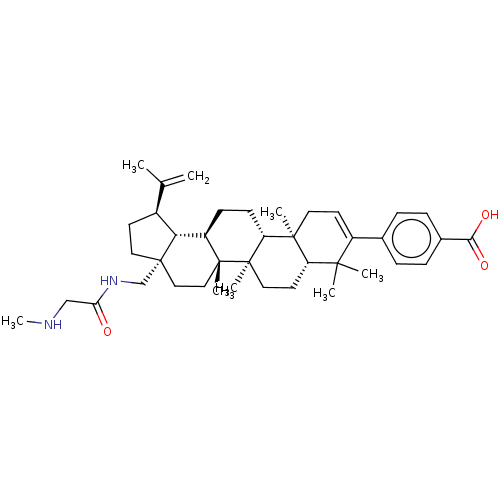 (CHEMBL4226018) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461422 (CHEMBL3827026) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S/V362I double mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347150 (US10202404, Compound A-21) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27614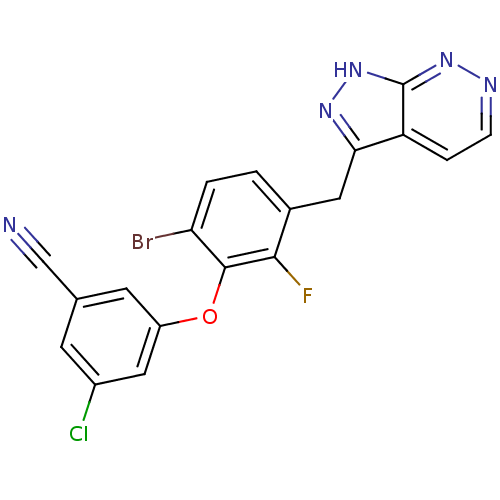 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB Article PubMed | n/a | n/a | 3 | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27612 (3-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-b]pyridin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461422 (CHEMBL3827026) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27617 (5-(6-bromo-2-fluoro-3-{1H-pyrazolo[3,4-c]pyridazin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27616 (3-chloro-5-(6-chloro-2-fluoro-3-{1H-pyrazolo[3,4-c...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | 8.0 | 30 |
Roche Palo Alto LLC | Assay Description IC50s were obtained from the inhibition of the RNA-dependent DNA polymerase activity of the HIV-1 reverse transcriptase enzyme using a primer extensi... | J Med Chem 51: 7449-58 (2008) Article DOI: 10.1021/jm800527x BindingDB Entry DOI: 10.7270/Q2TQ5ZV0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347144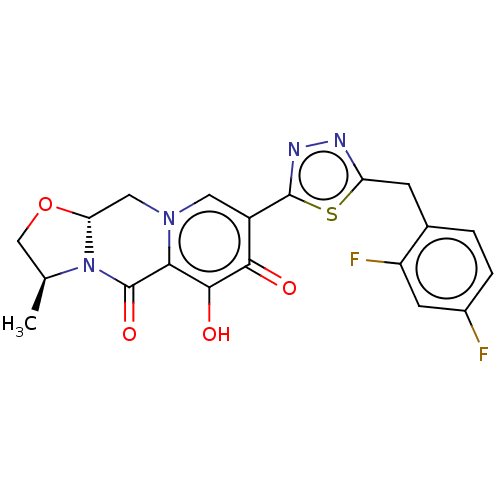 (US10202404, Compound A-15) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347231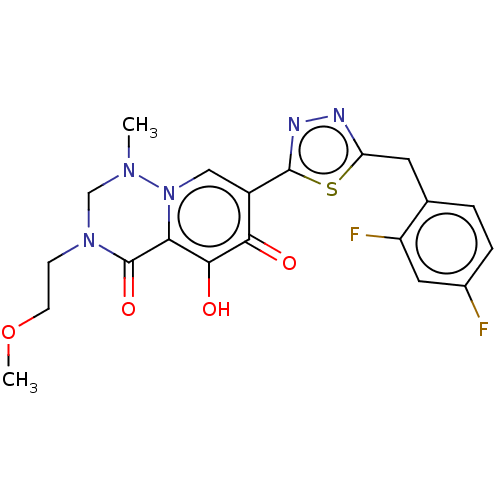 (US10202404, Compound C-3) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM580990 ((4S,7S,9aS)-N-[(1R,2R)-2-{4-[(1R,2R)-1-[(4S,7S,9aS...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
TBA | Assay Description Jurkat HIV-luciferase clones were maintained in RPMI medium 1640 (Gibco by Life Technologies) containing 10% (vol/vol) fetal bovine serum (SAFC/Sigma... | Citation and Details BindingDB Entry DOI: 10.7270/Q2FT8QW9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347248 (US10202404, Compound C-23) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347143 (US10202404, Compound A-14) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347247 (US10202404, Compound C-22) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461441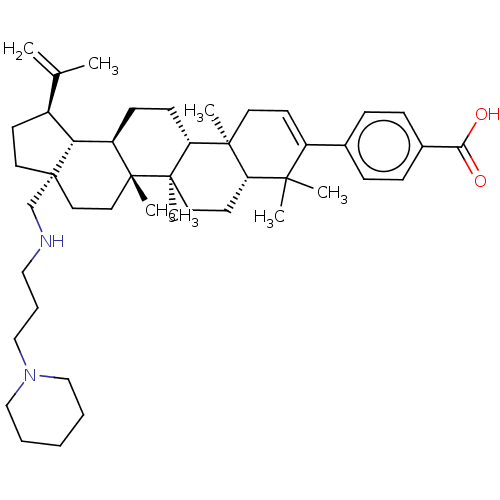 (CHEMBL4228854) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461445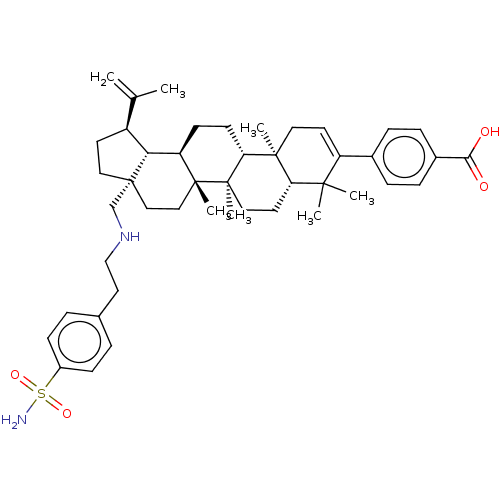 (CHEMBL4225004) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347230 (US10202404, Compound C-2) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347164 (US10202404, Compound A-36) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461435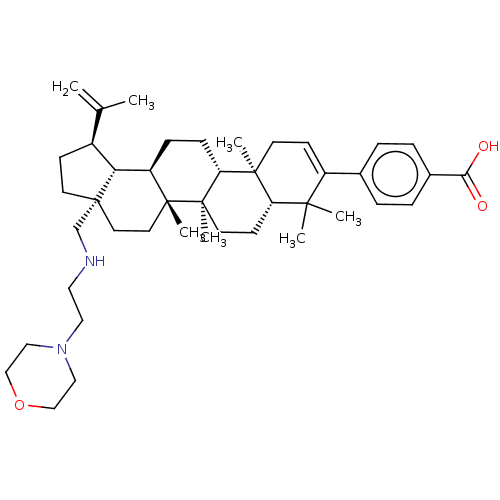 (CHEMBL4224769) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347251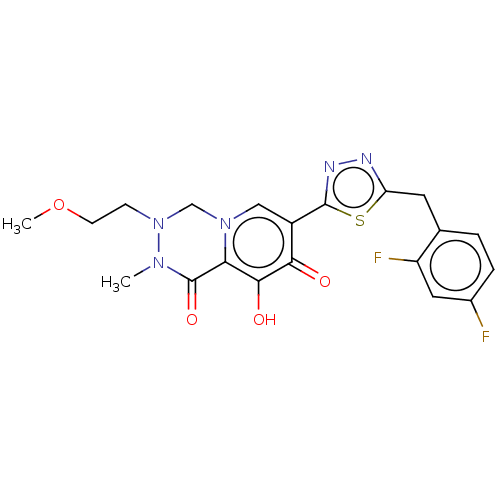 (US10202404, Compound C-26) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347246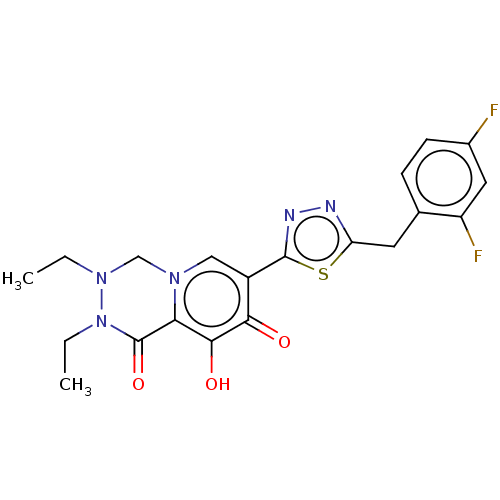 (US10202404, Compound C-21) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347181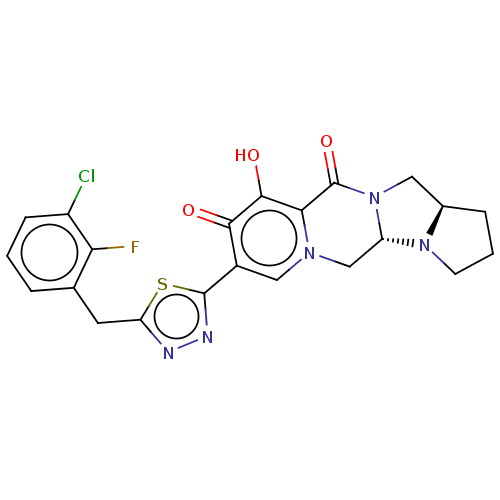 (US10202404, Compound A-54) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347167 (US10202404, Compound A-39) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347176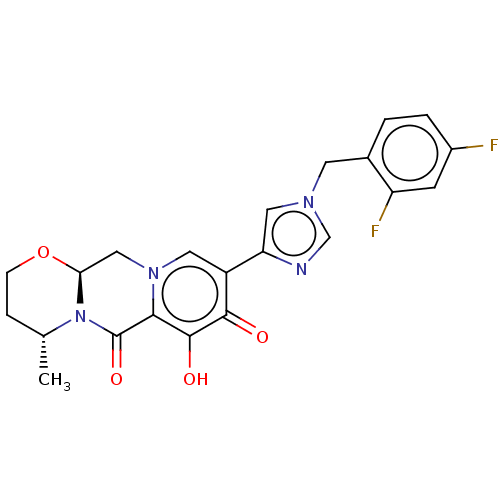 (US10202404, Compound A-49 | US10202404, Compound A...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347189 (US10202404, Compound A-62) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347151 (US10202404, Compound A-22) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347184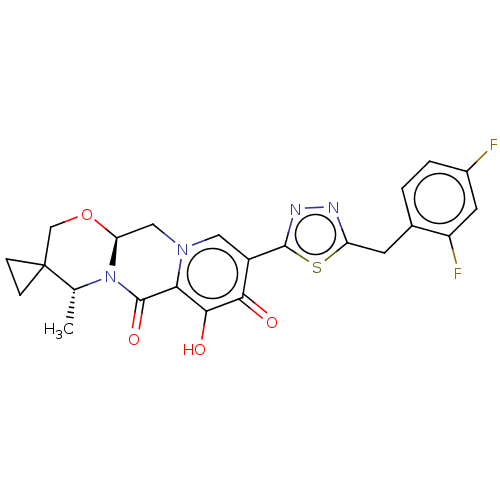 (US10202404, Compound A-57) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347182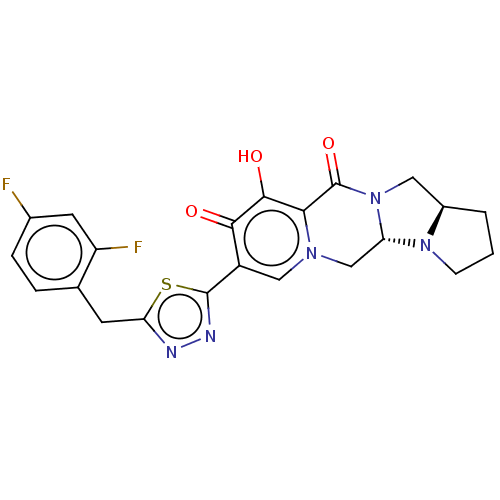 (US10202404, Compound A-55) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461416 (CHEMBL4225279) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461422 (CHEMBL3827026) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S/T371A double mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347245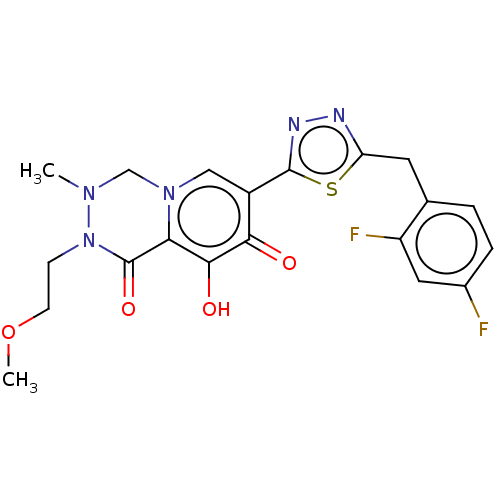 (US10202404, Compound C-20) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347241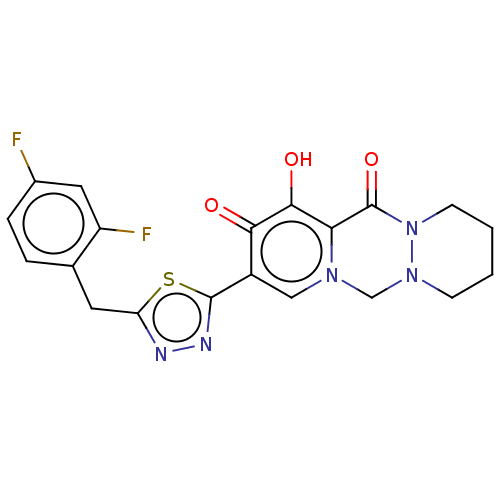 (US10202404, Compound C-15) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347102 (US10202404, Compound A-6) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347187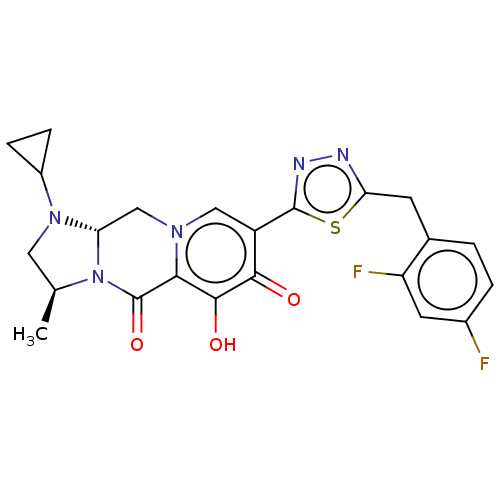 (US10202404, Compound A-60) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347190 (US10202404, Compound A-63) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347216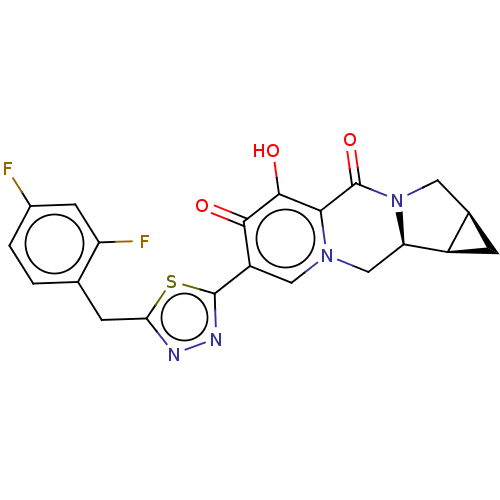 (US10202404, Compound B-9) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461422 (CHEMBL3827026) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| hek293 luciferase (Human immunodeficiency virus type 1 group M subtyp...) | BDBM578558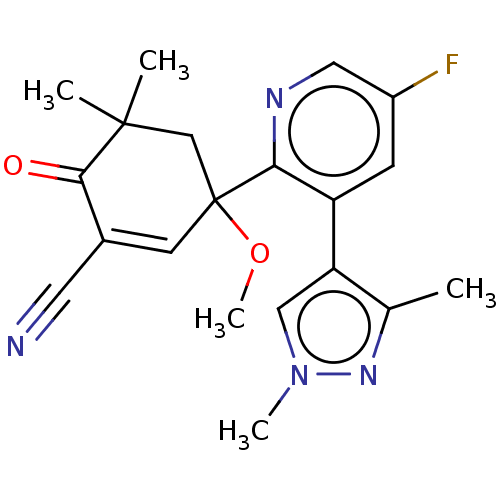 (3-[3-(1,3- dimethylpyrazol- 4-yl)-5-fluoro-2- pyri...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA | Assay Description Purpose of the AssayThe purpose of this assay is to identify small molecule inhibitors of Keap1, preventing Keap1 binding to Nrf2, and thus activatin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GQ720P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461433 (CHEMBL4225441) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347235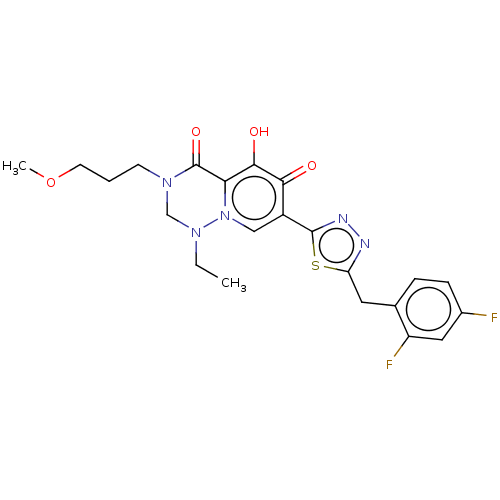 (US10202404, Compound C-7) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| hek293 luciferase (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50502733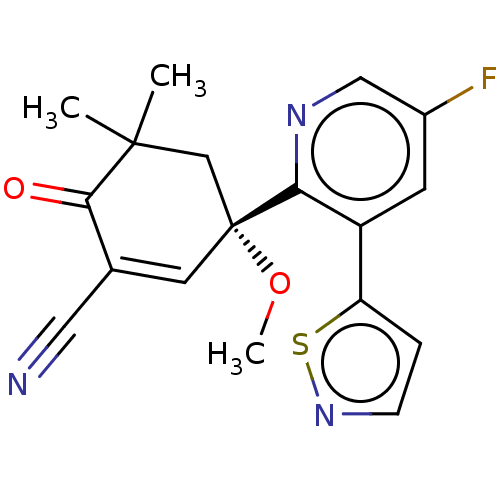 (CHEMBL4446699 | US11479539, Compound 80) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA | Assay Description Purpose of the AssayThe purpose of this assay is to identify small molecule inhibitors of Keap1, preventing Keap1 binding to Nrf2, and thus activatin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GQ720P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| hek293 luciferase (Human immunodeficiency virus type 1 group M subtyp...) | BDBM578561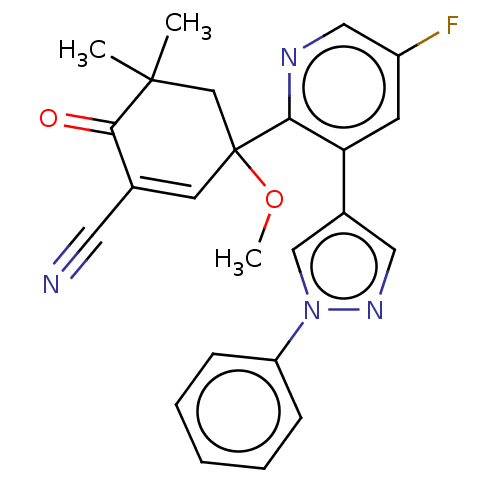 (3-[5-fluoro-3-(1- phenylpyrazol-4- yl)-2-pyridyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA | Assay Description Purpose of the AssayThe purpose of this assay is to identify small molecule inhibitors of Keap1, preventing Keap1 binding to Nrf2, and thus activatin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GQ720P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| hek293 luciferase (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50502732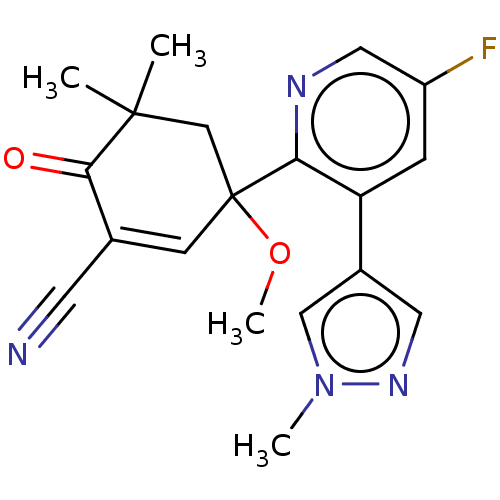 (CHEMBL4557371 | US11479539, Compound 69) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
TBA | Assay Description Purpose of the AssayThe purpose of this assay is to identify small molecule inhibitors of Keap1, preventing Keap1 binding to Nrf2, and thus activatin... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GQ720P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM347252 (US10202404, Compound C-27) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Previously, a series of two-fold dilution on test samples were carried out in a 96-well plate (50 μL/well). Two plates were made for measuring a... | US Patent US10202404 (2019) BindingDB Entry DOI: 10.7270/Q2WQ05WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461415 (CHEMBL4224747) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM50461418 (CHEMBL4228598) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Binding affinity to CA-SP1 P373S mutant in HIV-1 subtype B NL4-3 infected in human MT2 cells assessed as inhibition of viral maturation by measuring ... | Bioorg Med Chem Lett 28: 1550-1557 (2018) Article DOI: 10.1016/j.bmcl.2018.03.067 BindingDB Entry DOI: 10.7270/Q29G5QFN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 538 total ) | Next | Last >> |