

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50520331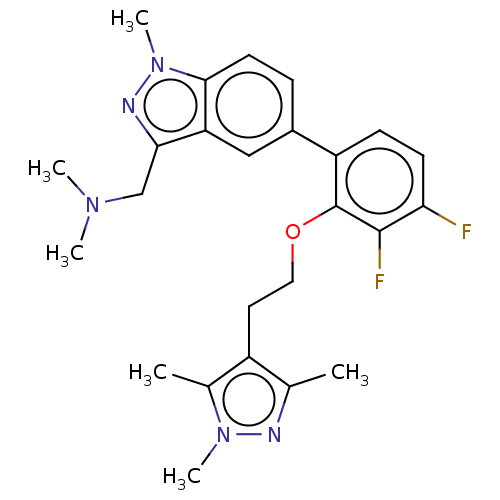 (CHEMBL4445137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Frontier Medicines Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase assessed as reduction in CoASH production by fluorogenic detection based assay | J Med Chem 63: 4430-4444 (2020) Article DOI: 10.1021/acs.jmedchem.9b01581 BindingDB Entry DOI: 10.7270/Q2ZG6WM7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50520331 (CHEMBL4445137) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human NMT1 by fluorescence method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00242 BindingDB Entry DOI: 10.7270/Q2KD22HB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM185107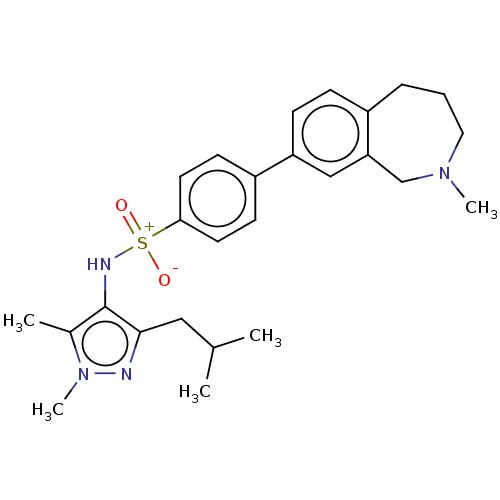 (US9156811, DDD100868) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184848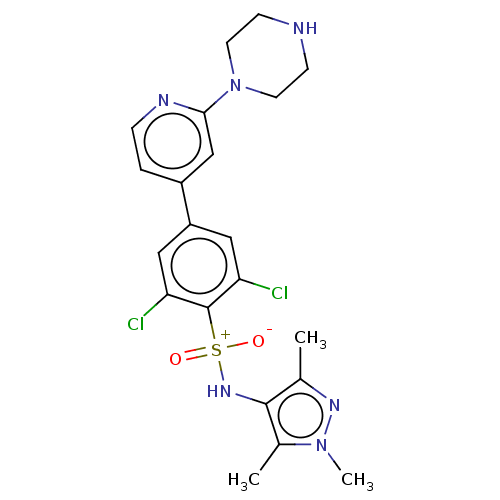 (US9156811, DDD85646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184902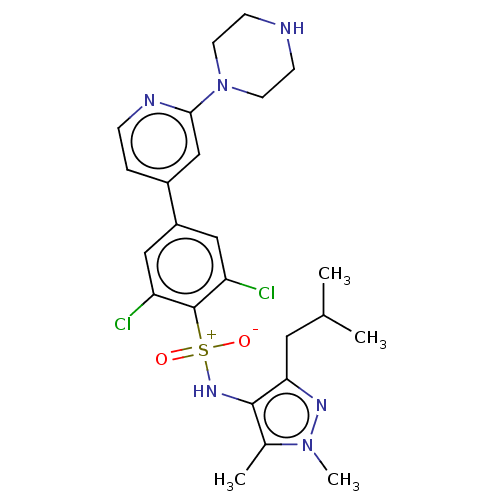 (US9156811, DDD86481) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184977 (US9156811, DDD88646) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50364113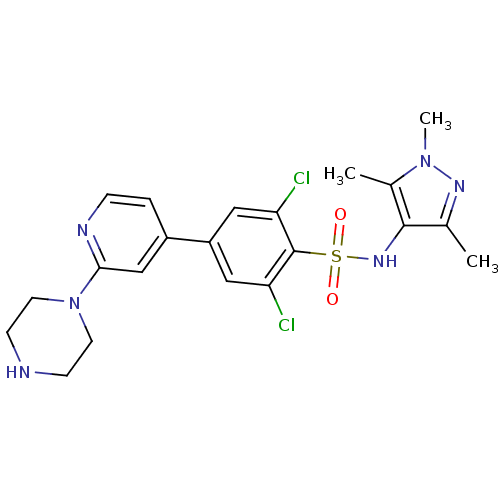 (CHEMBL1230468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 using [3H]myristoyl-CoA and GCGGSKVKPQPPQAK(biotin)-amide as substrate preincubated for 5 mins prior sub... | J Med Chem 55: 140-52 (2012) Article DOI: 10.1021/jm201091t BindingDB Entry DOI: 10.7270/Q25Q4WK9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50364113 (CHEMBL1230468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184854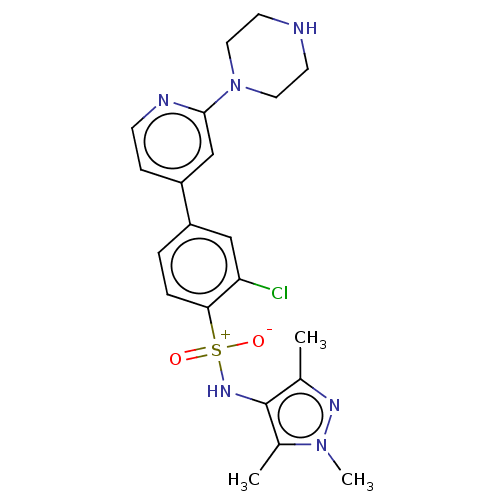 (US9156811, DDD86206) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033616 (CHEMBL3358119) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033485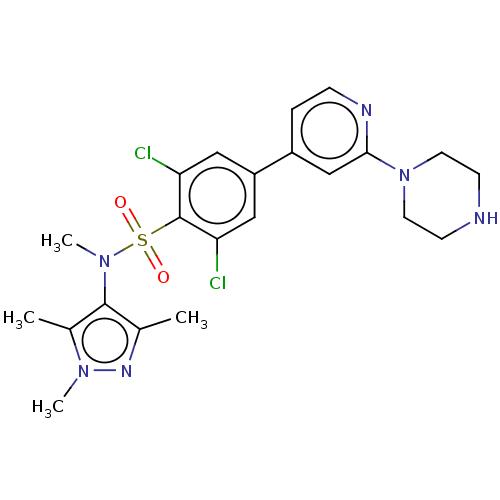 (CHEMBL3357702) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033480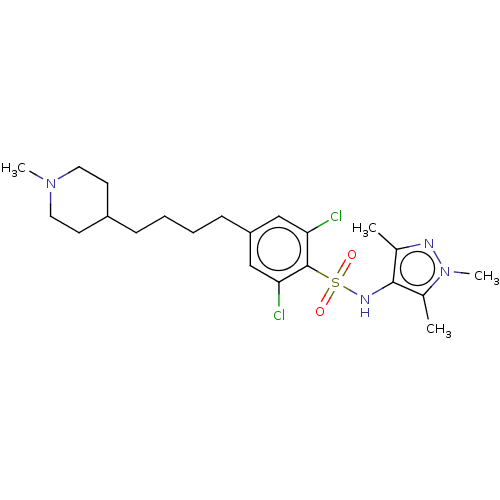 (CHEMBL3357697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033418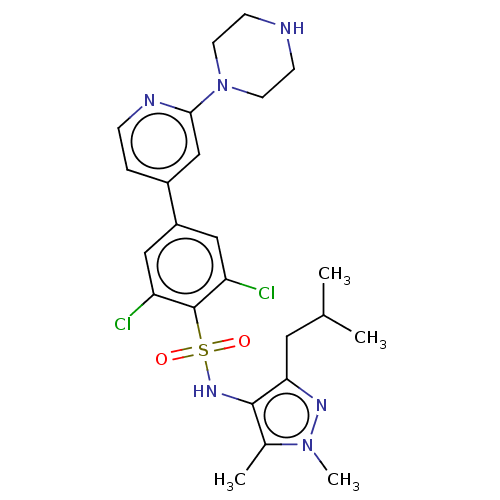 (CHEMBL3357685) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM185101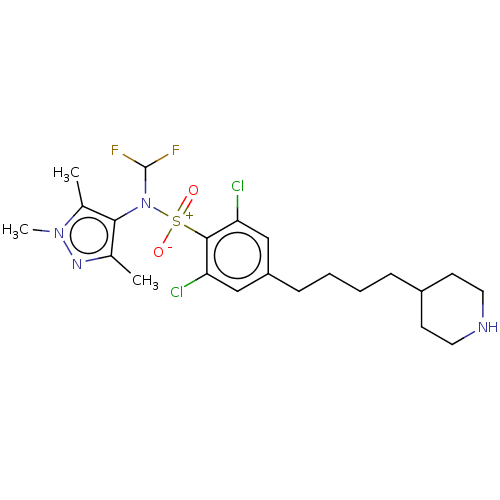 (US9156811, DDD100807) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM185092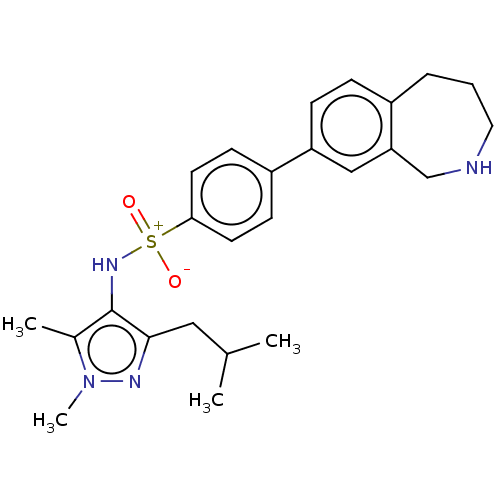 (US9156811, DDD100798) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184978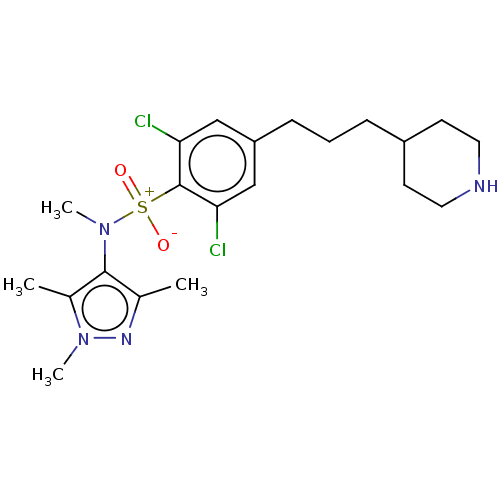 (US9156811, DDD90022) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50364113 (CHEMBL1230468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation/luminescence counting method | J Med Chem 60: 9790-9806 (2017) Article DOI: 10.1021/acs.jmedchem.7b01255 BindingDB Entry DOI: 10.7270/Q25141NZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033618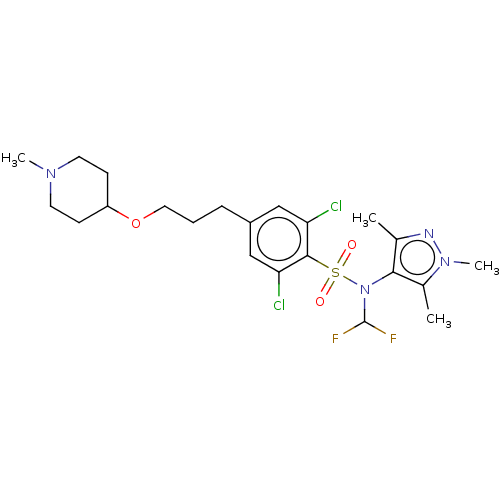 (CHEMBL3358121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033617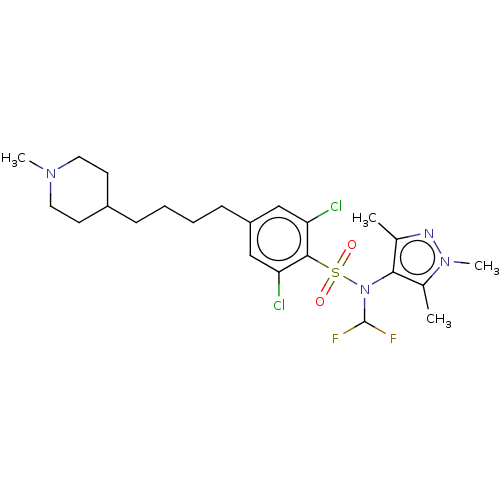 (CHEMBL3358120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation/luminescence counting method | J Med Chem 60: 9790-9806 (2017) Article DOI: 10.1021/acs.jmedchem.7b01255 BindingDB Entry DOI: 10.7270/Q25141NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM185083 (US9156811, DDD100169) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033482 (CHEMBL3357699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033617 (CHEMBL3358120) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184943 (US9156811, DDD88193) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184988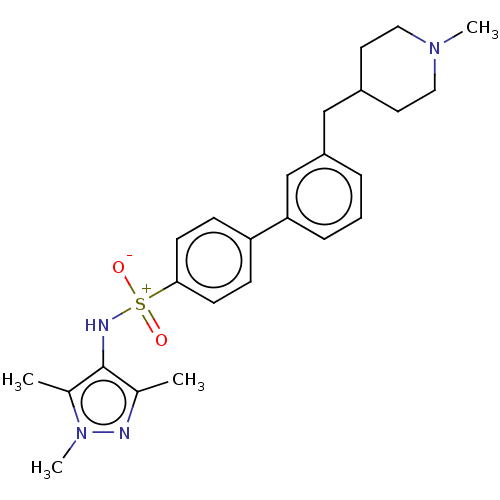 (US9156811, DDD90107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184992 (US9156811, DDD90118) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM185061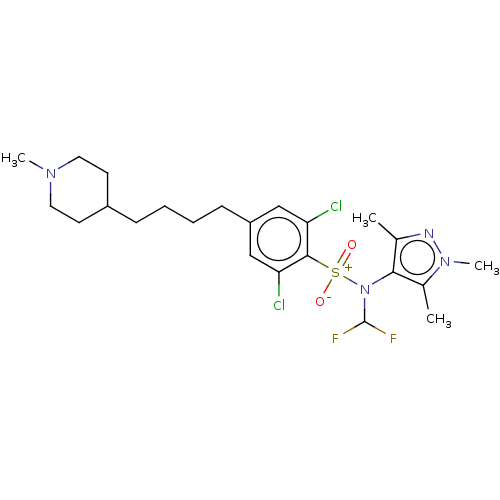 (US9156811, DDD100144) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184937 (US9156811, DDD88186) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM185108 (US9156811, DDD100869) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50364135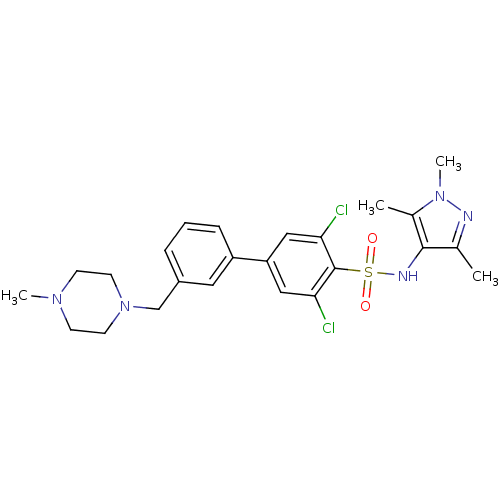 (CHEMBL1951295) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 using [3H]myristoyl-CoA and GCGGSKVKPQPPQAK(biotin)-amide as substrate preincubated for 5 mins prior sub... | J Med Chem 55: 140-52 (2012) Article DOI: 10.1021/jm201091t BindingDB Entry DOI: 10.7270/Q25Q4WK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184965 (US9156811, DDD88558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184860 (US9156811, DDD86213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM185078 (US9156811, DDD100164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033619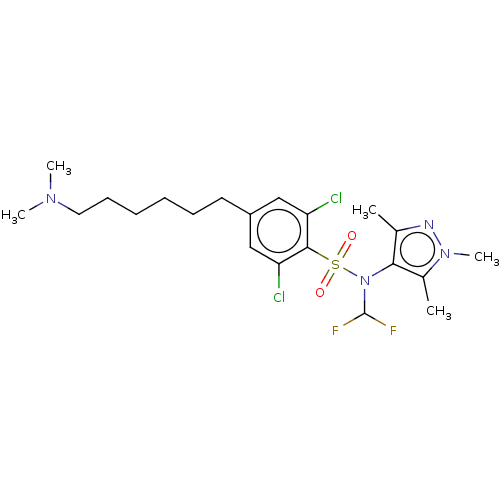 (CHEMBL3358122) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM185080 (US9156811, DDD100166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033478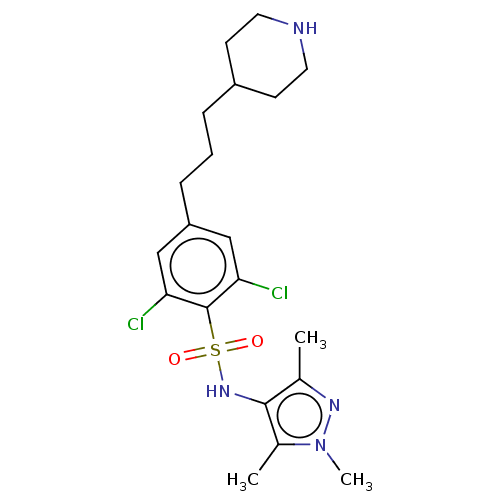 (CHEMBL3357695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM185023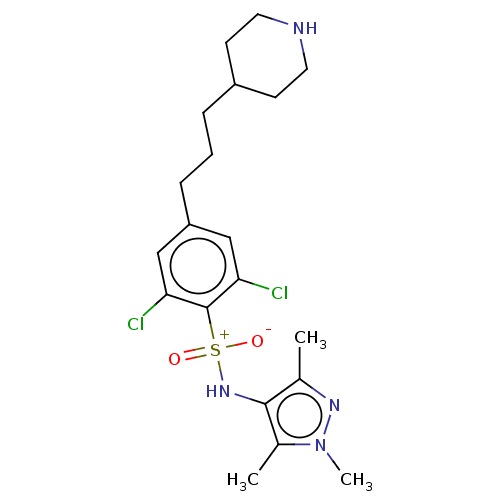 (US9156811, DDD99759) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184903 (US9156811, DDD87748) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184920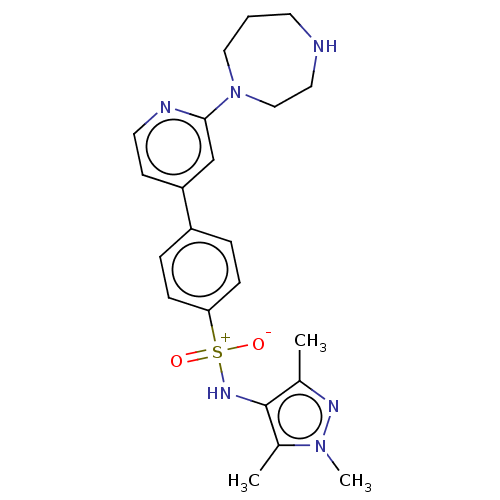 (US9156811, DDD87767) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184859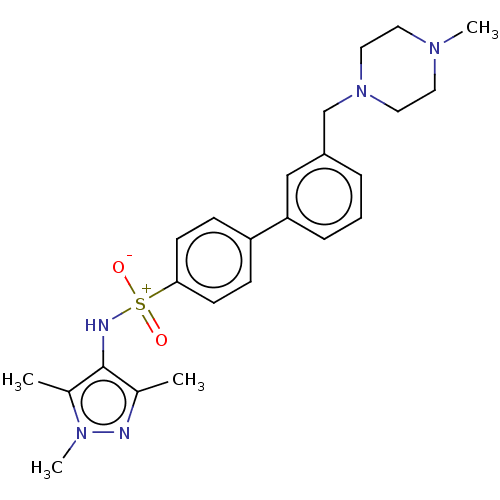 (US9156811, DDD86212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM185054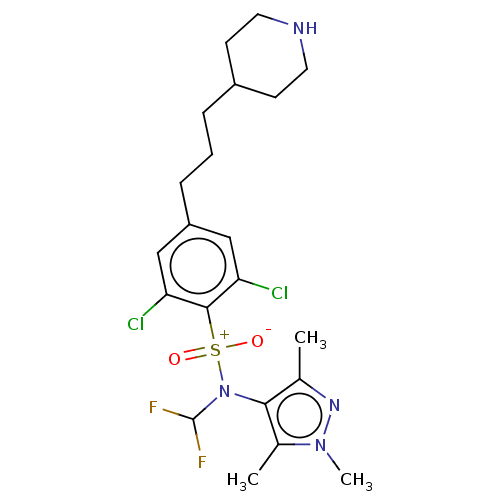 (US9156811, DDD100096) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033414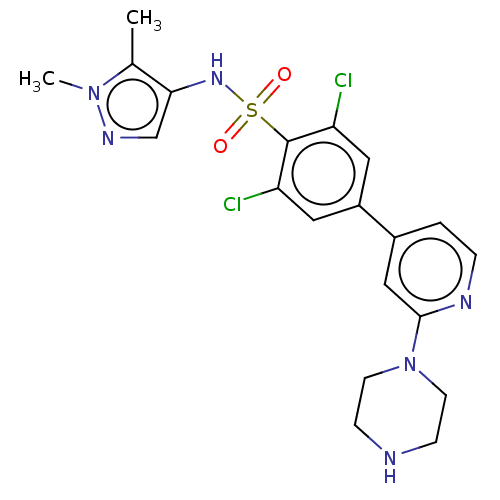 (CHEMBL3357681) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033487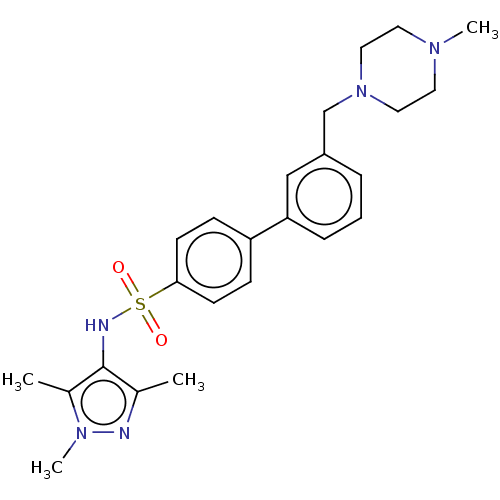 (CHEMBL3357704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM185050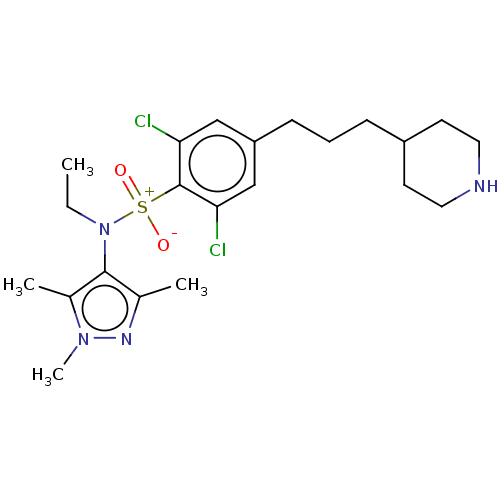 (US9156811, DDD100091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184923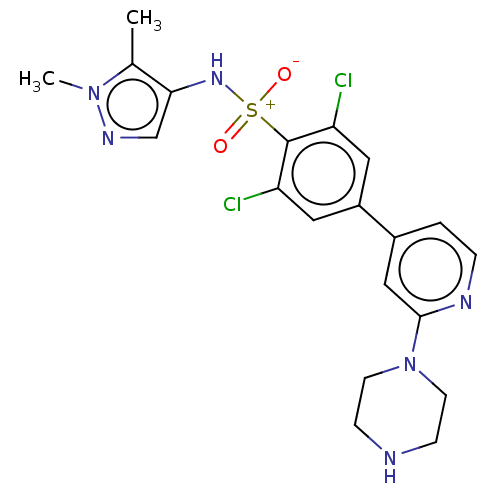 (US9156811, DDD87993) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184939 (US9156811, DDD88188) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184985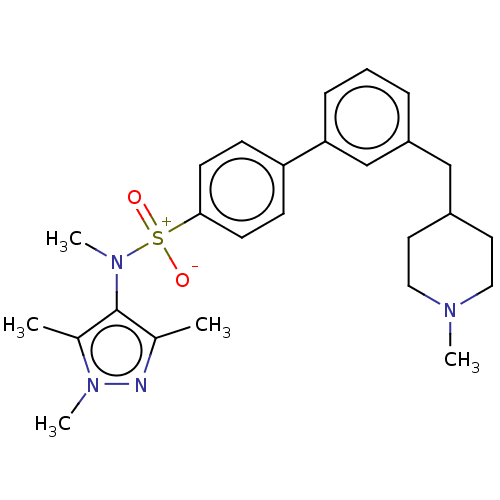 (US9156811, DDD90091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50033614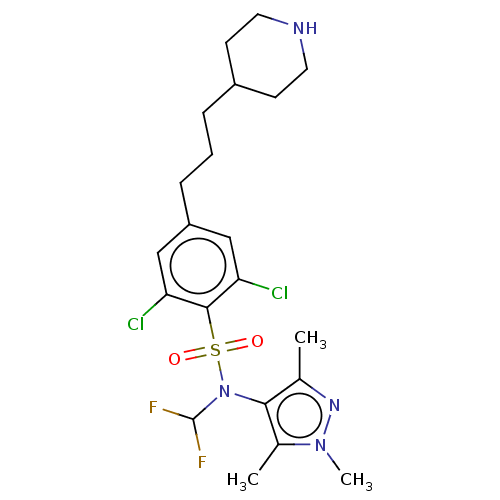 (CHEMBL3358117) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human N-myristoyltransferase 1 assessed as transfer of [3H]-myristic acid to a biotinylated substrate peptide (GCGGSKVKPQPPQAK(biotin)-... | J Med Chem 57: 9855-69 (2014) Article DOI: 10.1021/jm500809c BindingDB Entry DOI: 10.7270/Q2ZC84G2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM50250777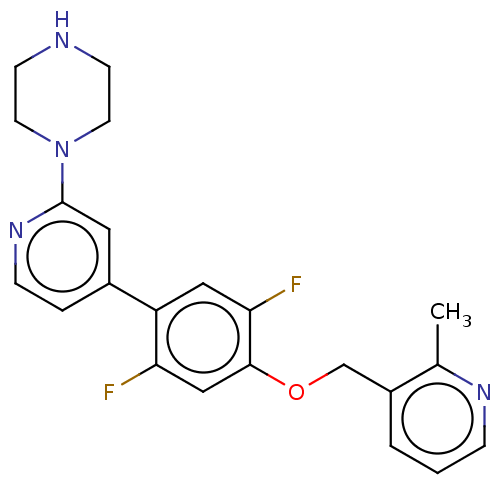 (CHEMBL4080376) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Inhibition of human NMT1 using [3H]-myristoyl-coA/biotinylated CAP5.5 as substrate after 15 mins by scintillation/luminescence counting method | J Med Chem 60: 9790-9806 (2017) Article DOI: 10.1021/acs.jmedchem.7b01255 BindingDB Entry DOI: 10.7270/Q25141NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM185060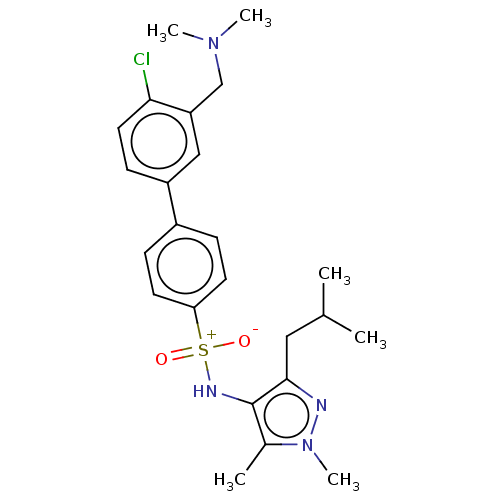 (US9156811, DDD100143) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycylpeptide N-tetradecanoyltransferase 1 (Homo sapiens (Human)) | BDBM184984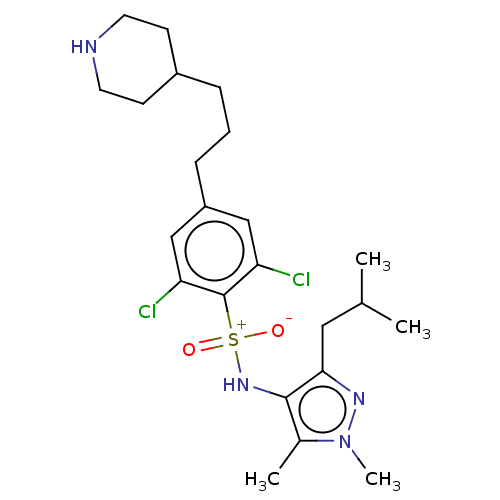 (US9156811, DDD90086) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Univeristy of Dundee US Patent | Assay Description Measurement of the ability of compounds to inhibit the NMT-1 and/or NMT-2 enzyme isoforms of human, trypanosome (T. brucei), leishmanial (L. major) a... | US Patent US9156811 (2015) BindingDB Entry DOI: 10.7270/Q2K35SGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 468 total ) | Next | Last >> |