

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307515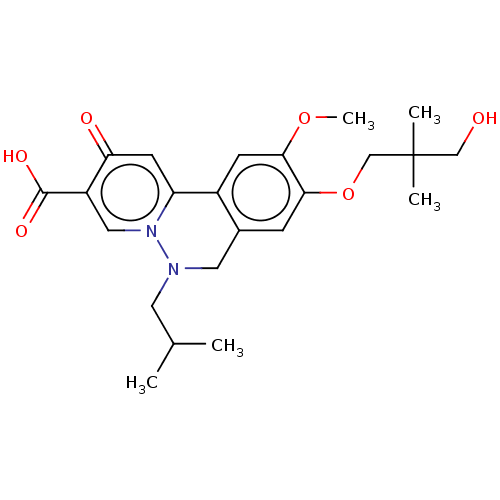 (US10150740, Example 11) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HepG2.2.15 cells (Acs et al. Proc Natl Acad Sci USA, 84, (1987), 4641-4), a constitutively HBV-expressing cell line were cultured in DMEM+Glutamax-I ... | US Patent US10150740 (2018) BindingDB Entry DOI: 10.7270/Q2RN39WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307512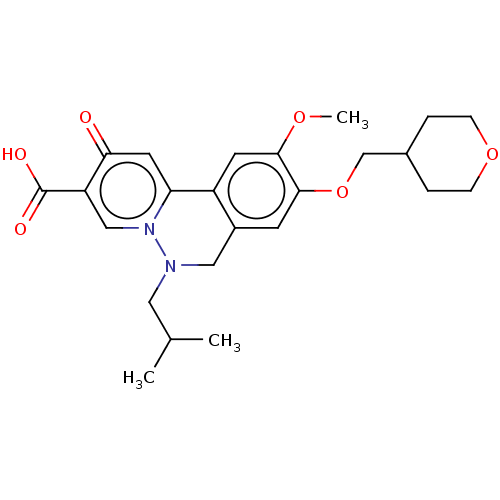 (US10150740, Example 8) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HepG2.2.15 cells (Acs et al. Proc Natl Acad Sci USA, 84, (1987), 4641-4), a constitutively HBV-expressing cell line were cultured in DMEM+Glutamax-I ... | US Patent US10150740 (2018) BindingDB Entry DOI: 10.7270/Q2RN39WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50158837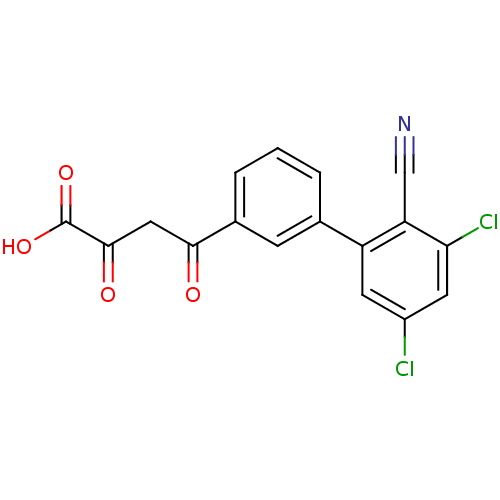 (4-(3'',5''-Dichloro-2''-cyano-biphenyl-3-yl)-2,4-d...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus polymerase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307518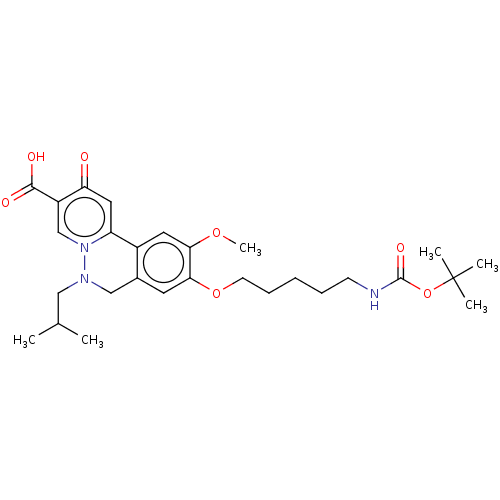 (US10150740, Example 14) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HepG2.2.15 cells (Acs et al. Proc Natl Acad Sci USA, 84, (1987), 4641-4), a constitutively HBV-expressing cell line were cultured in DMEM+Glutamax-I ... | US Patent US10150740 (2018) BindingDB Entry DOI: 10.7270/Q2RN39WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307510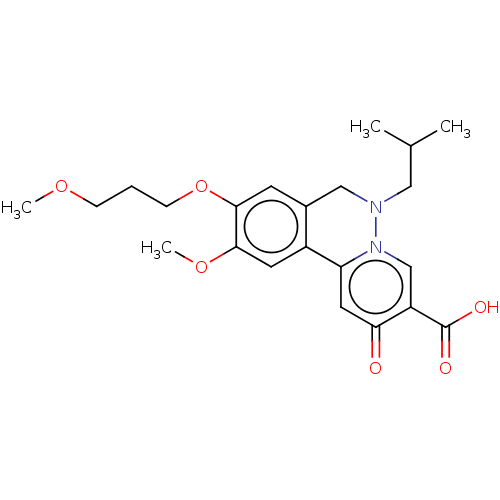 (US10150740, Example 6) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HepG2.2.15 cells (Acs et al. Proc Natl Acad Sci USA, 84, (1987), 4641-4), a constitutively HBV-expressing cell line were cultured in DMEM+Glutamax-I ... | US Patent US10150740 (2018) BindingDB Entry DOI: 10.7270/Q2RN39WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50125376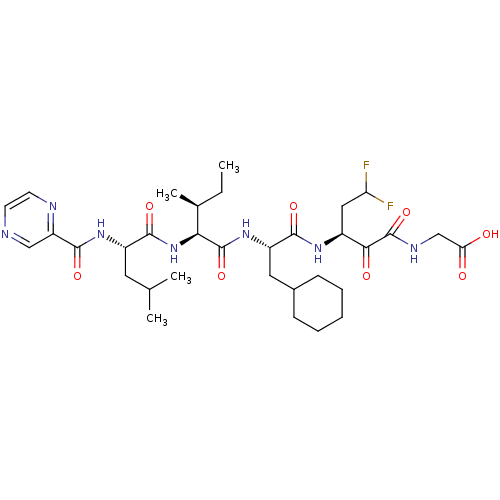 (CHEMBL275290 | {(S)-3-[(S)-3-Cyclohexyl-2-((2S,3S)...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against HCV NS3 protease was determined | Bioorg Med Chem Lett 13: 1111-4 (2003) BindingDB Entry DOI: 10.7270/Q2HQ3Z8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM35553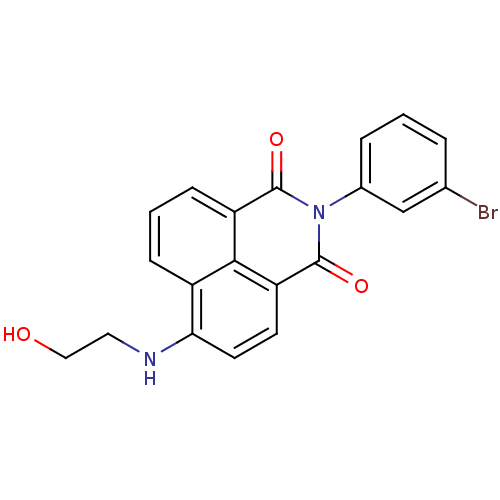 (1H-benzo[de]isoquinoline-1,3(2H)-dione, 1) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL Rome | Assay Description Primer-dependent assays were performed using the homopolymeric template/primer. Compounds, polymerase and template RNA were incubated at RT for 25 mi... | J Med Chem 52: 5217-27 (2009) Article DOI: 10.1021/jm900517t BindingDB Entry DOI: 10.7270/Q2X928P4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50158839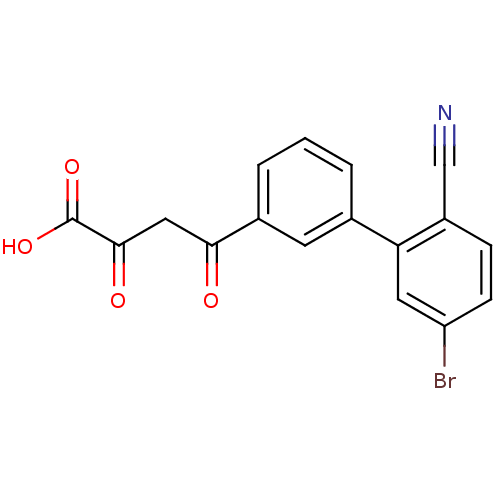 (4-(5''-Bromo-2''-cyano-biphenyl-3-yl)-2,4-dioxo-bu...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus polymerase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50158845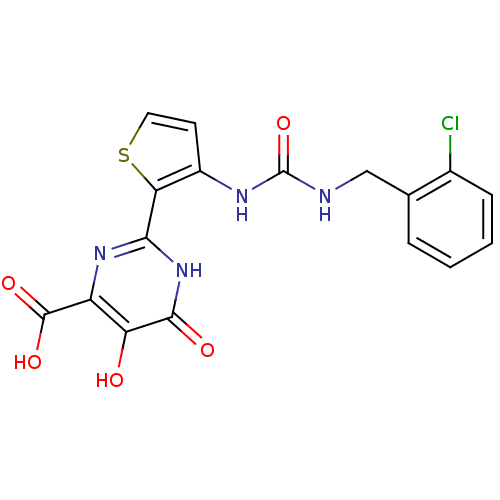 (2-(3-(3-(2-chlorobenzyl)ureido)thiophen-2-yl)-5,6-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus polymerase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50158815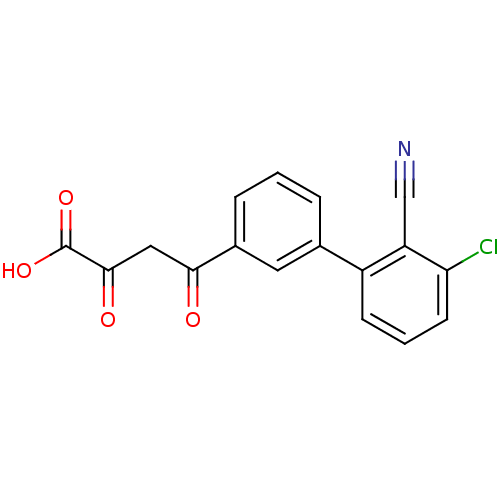 (4-(3''-Chloro-2''-cyano-biphenyl-3-yl)-2,4-dioxo-b...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus polymerase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307517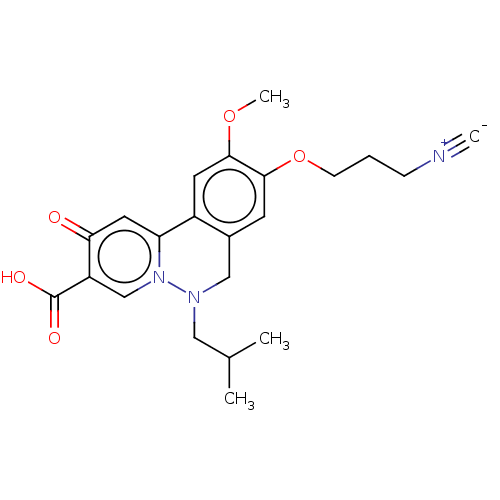 (US10150740, Example 13) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HepG2.2.15 cells (Acs et al. Proc Natl Acad Sci USA, 84, (1987), 4641-4), a constitutively HBV-expressing cell line were cultured in DMEM+Glutamax-I ... | US Patent US10150740 (2018) BindingDB Entry DOI: 10.7270/Q2RN39WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307511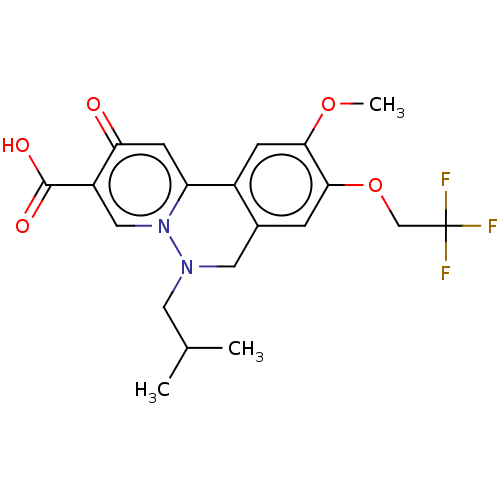 (US10150740, Example 7) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HepG2.2.15 cells (Acs et al. Proc Natl Acad Sci USA, 84, (1987), 4641-4), a constitutively HBV-expressing cell line were cultured in DMEM+Glutamax-I ... | US Patent US10150740 (2018) BindingDB Entry DOI: 10.7270/Q2RN39WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307514 (US10150740, Example 10) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HepG2.2.15 cells (Acs et al. Proc Natl Acad Sci USA, 84, (1987), 4641-4), a constitutively HBV-expressing cell line were cultured in DMEM+Glutamax-I ... | US Patent US10150740 (2018) BindingDB Entry DOI: 10.7270/Q2RN39WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50125388 (CHEMBL13256 | {(S)-3-[(S)-3-Cyclohexyl-2-((2S,3S)-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against HCV NS3 protease was determined | Bioorg Med Chem Lett 13: 1111-4 (2003) BindingDB Entry DOI: 10.7270/Q2HQ3Z8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307509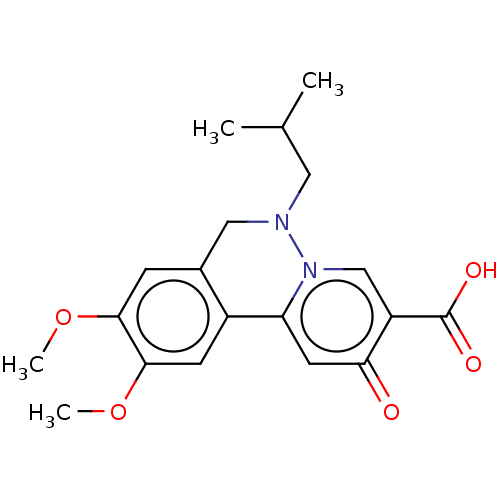 (US10150740, Example 5) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HepG2.2.15 cells (Acs et al. Proc Natl Acad Sci USA, 84, (1987), 4641-4), a constitutively HBV-expressing cell line were cultured in DMEM+Glutamax-I ... | US Patent US10150740 (2018) BindingDB Entry DOI: 10.7270/Q2RN39WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110153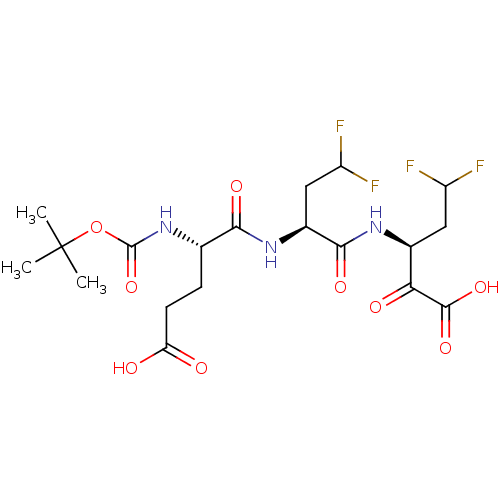 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-4-car...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110146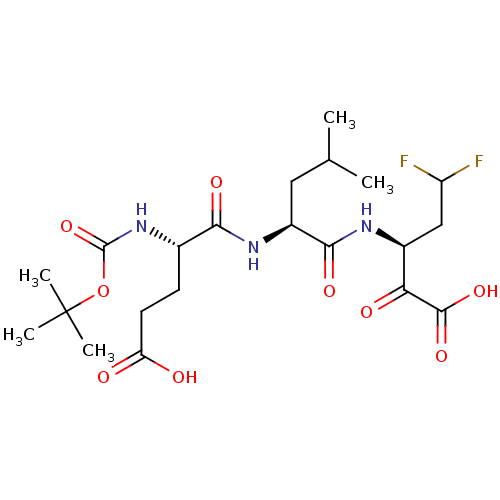 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-4-car...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50158822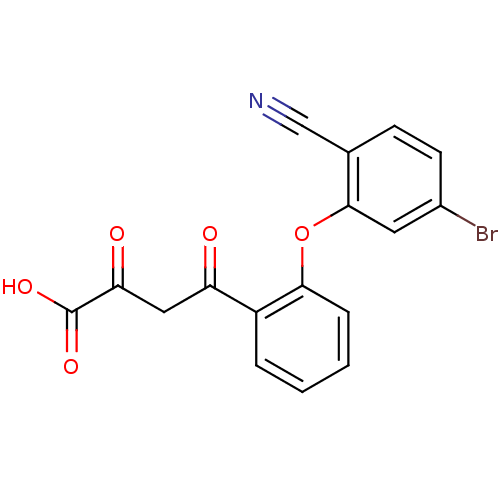 (4-[2-(5-Bromo-2-cyano-phenoxy)-phenyl]-2,4-dioxo-b...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus polymerase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110145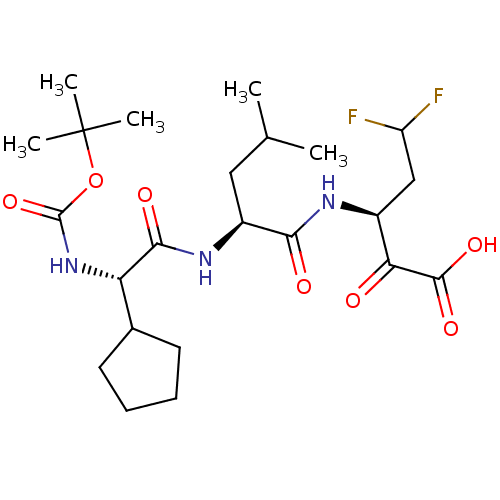 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-2-cyc...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50139666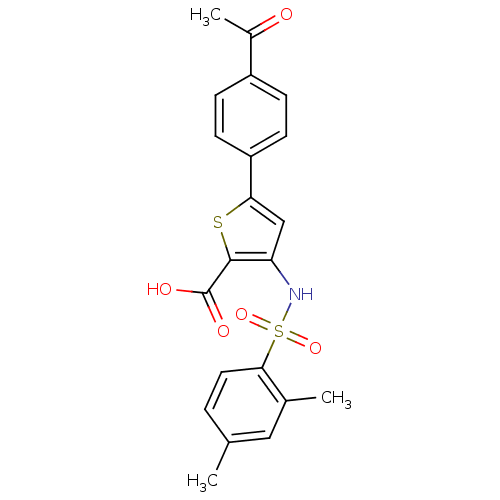 (5-(4-Acetyl-phenyl)-3-(2,4-dimethyl-benzenesulfony...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B polymerase | Bioorg Med Chem Lett 14: 793-6 (2004) BindingDB Entry DOI: 10.7270/Q2PV6JSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50125392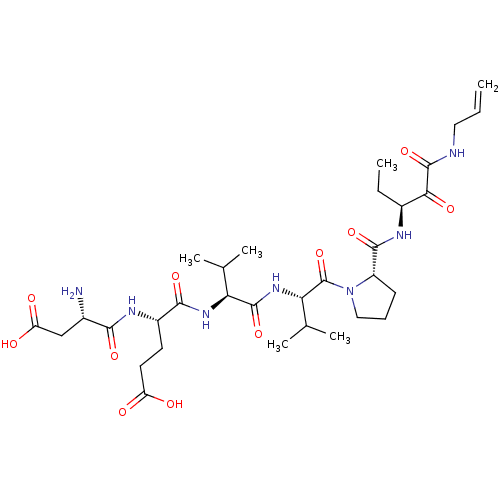 ((S)-4-((S)-1-{(S)-1-[(S)-2-((S)-1-Allylaminooxalyl...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against HCV NS3 protease was determined | Bioorg Med Chem Lett 13: 1111-4 (2003) BindingDB Entry DOI: 10.7270/Q2HQ3Z8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110143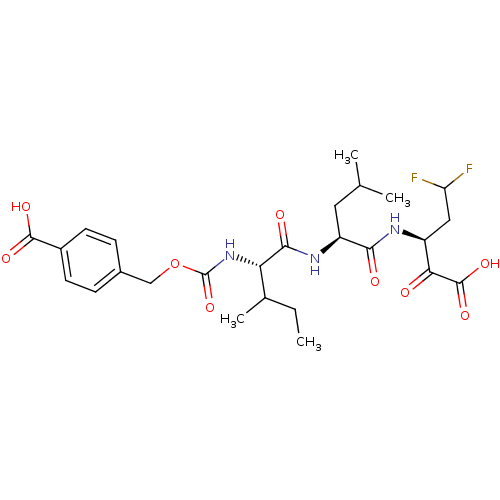 (4-{(S)-1-[(S)-1-((S)-3,3-Difluoro-1-oxalyl-propylc...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110134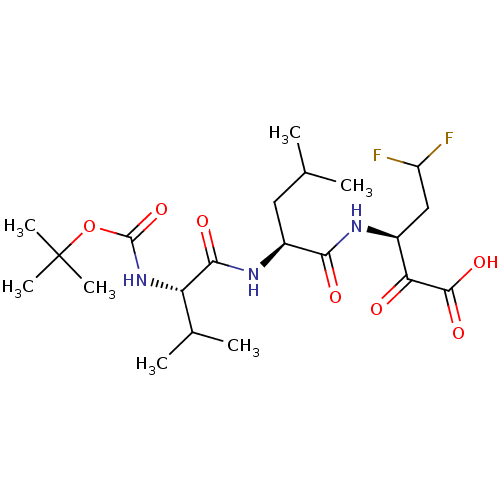 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307519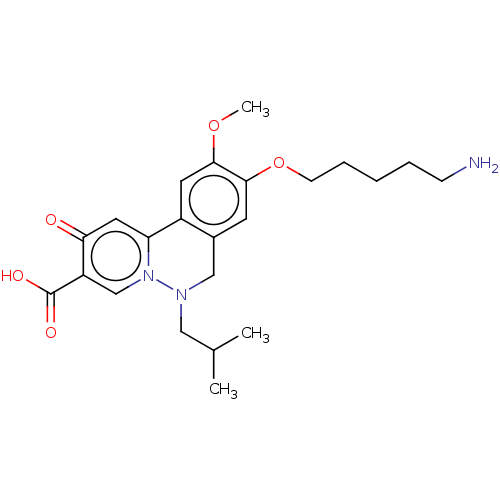 (US10150740, Example 15) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HepG2.2.15 cells (Acs et al. Proc Natl Acad Sci USA, 84, (1987), 4641-4), a constitutively HBV-expressing cell line were cultured in DMEM+Glutamax-I ... | US Patent US10150740 (2018) BindingDB Entry DOI: 10.7270/Q2RN39WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307513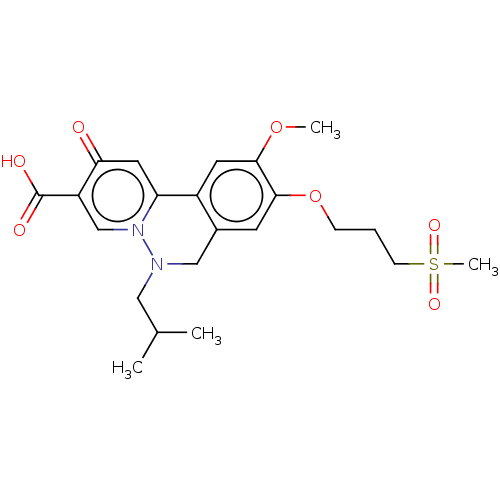 (US10150740, Example 9) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 624 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HepG2.2.15 cells (Acs et al. Proc Natl Acad Sci USA, 84, (1987), 4641-4), a constitutively HBV-expressing cell line were cultured in DMEM+Glutamax-I ... | US Patent US10150740 (2018) BindingDB Entry DOI: 10.7270/Q2RN39WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM307507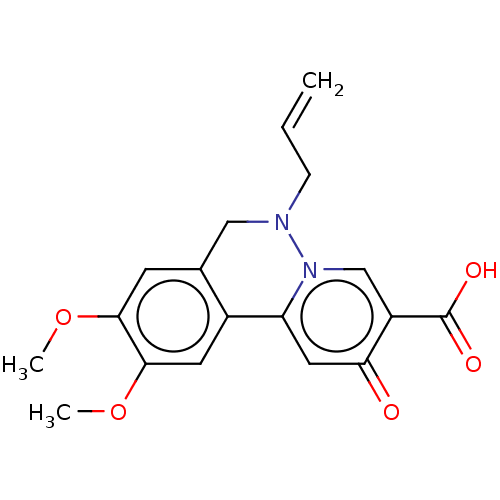 (US10150740, Example 3) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HepG2.2.15 cells (Acs et al. Proc Natl Acad Sci USA, 84, (1987), 4641-4), a constitutively HBV-expressing cell line were cultured in DMEM+Glutamax-I ... | US Patent US10150740 (2018) BindingDB Entry DOI: 10.7270/Q2RN39WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50139669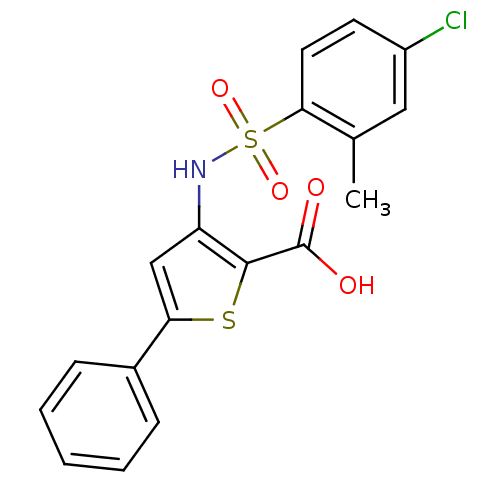 (3-(4-Chloro-2-methyl-benzenesulfonylamino)-5-pheny...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B polymerase | Bioorg Med Chem Lett 14: 793-6 (2004) BindingDB Entry DOI: 10.7270/Q2PV6JSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50410369 (CHEMBL363650) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
IMIM/Universitat Pompeu Fabra Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease (IC50 spans 2.5 log units) | J Med Chem 48: 2687-94 (2005) Article DOI: 10.1021/jm049113+ BindingDB Entry DOI: 10.7270/Q29S1QH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM30406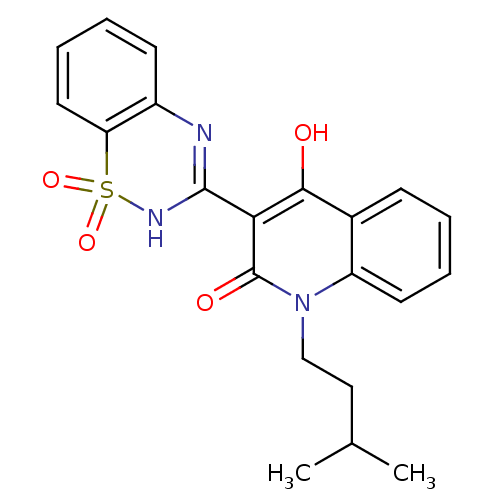 (CHEMBL176058 | benzo[1,2,4]thiadiazine-1,1-dioxide...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus polymerase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50144349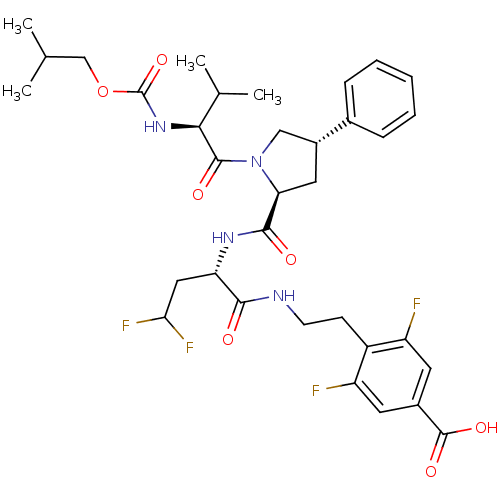 (4-[2-((S)-4,4-Difluoro-2-{[(2S,4S)-1-(2-isobutoxyc...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of Hepatitis C virus NS3 protease in vitro. | Bioorg Med Chem Lett 14: 2151-4 (2004) Article DOI: 10.1016/j.bmcl.2004.02.032 BindingDB Entry DOI: 10.7270/Q2HH6JJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110130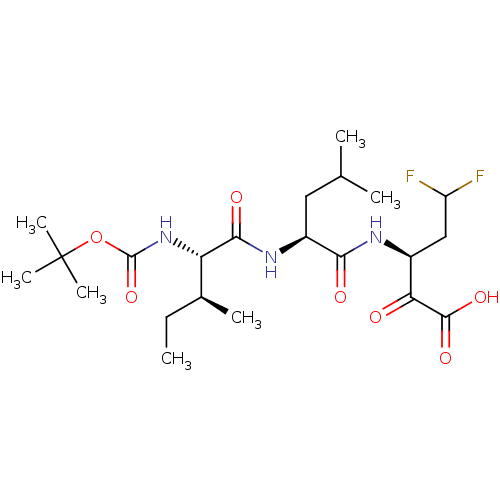 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-3-met...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50139657 (3-(2,4-Dimethyl-benzenesulfonylamino)-5-phenyl-thi...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc Curated by ChEMBL | Assay Description In vitro inhibition of Hepatitis C polymerase. | Bioorg Med Chem Lett 14: 797-800 (2004) BindingDB Entry DOI: 10.7270/Q2K35T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50139657 (3-(2,4-Dimethyl-benzenesulfonylamino)-5-phenyl-thi...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B polymerase | Bioorg Med Chem Lett 14: 793-6 (2004) BindingDB Entry DOI: 10.7270/Q2PV6JSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50139682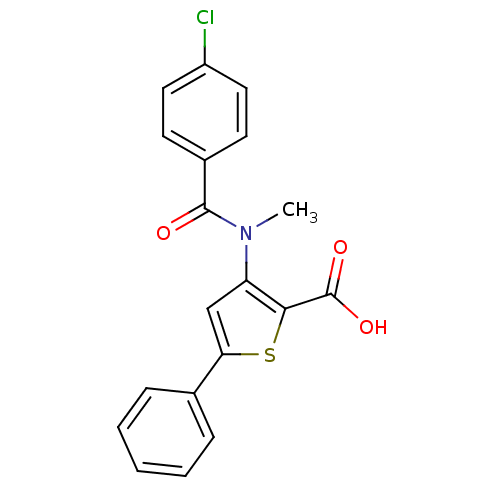 (3-[(4-Chloro-benzoyl)-methyl-amino]-5-phenyl-thiop...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc Curated by ChEMBL | Assay Description In vitro inhibition of Hepatitis C polymerase. | Bioorg Med Chem Lett 14: 797-800 (2004) BindingDB Entry DOI: 10.7270/Q2K35T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50139683 (3-[(2,4-DICHLOROBENZOYL)(ISOPROPYL)AMINO]-5-PHENYL...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc Curated by ChEMBL | Assay Description In vitro inhibition of Hepatitis C polymerase. | Bioorg Med Chem Lett 14: 797-800 (2004) BindingDB Entry DOI: 10.7270/Q2K35T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50125371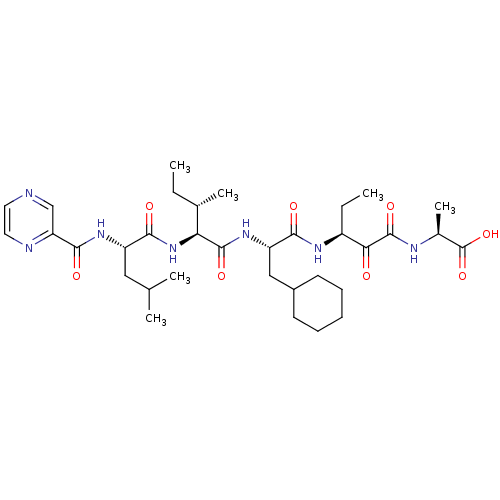 ((S)-2-{(S)-3-[(S)-3-Cyclohexyl-2-((2S,3S)-3-methyl...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against HCV NS3 protease was determined | Bioorg Med Chem Lett 13: 1111-4 (2003) BindingDB Entry DOI: 10.7270/Q2HQ3Z8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110156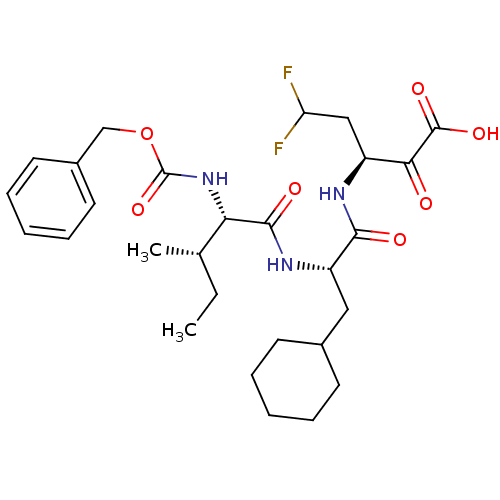 ((S)-3-[(S)-2-((2S,3S)-2-Benzyloxycarbonylamino-3-m...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50139676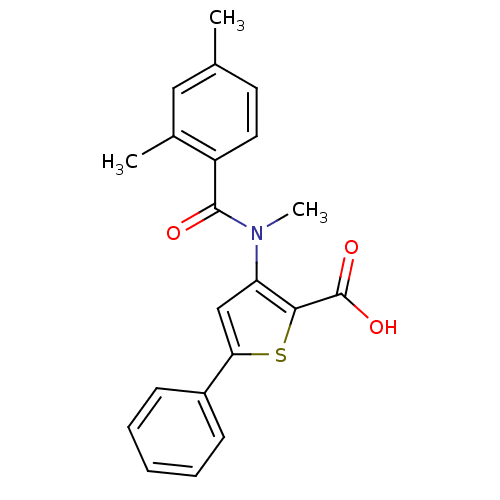 (3-[(2,4-Dimethyl-benzoyl)-methyl-amino]-5-phenyl-t...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc Curated by ChEMBL | Assay Description In vitro inhibition of Hepatitis C polymerase. | Bioorg Med Chem Lett 14: 797-800 (2004) BindingDB Entry DOI: 10.7270/Q2K35T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50139659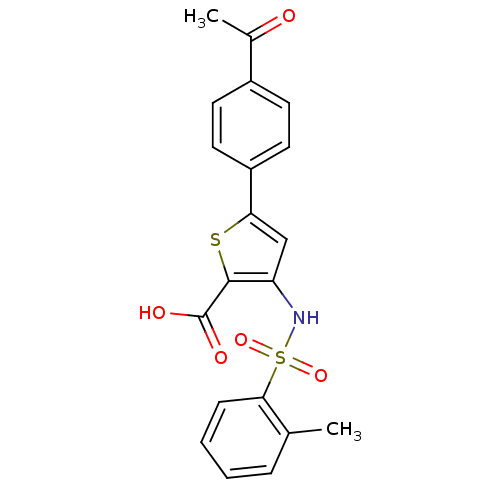 (5-(4-Acetyl-phenyl)-3-(toluene-2-sulfonylamino)-th...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B polymerase | Bioorg Med Chem Lett 14: 793-6 (2004) BindingDB Entry DOI: 10.7270/Q2PV6JSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50125378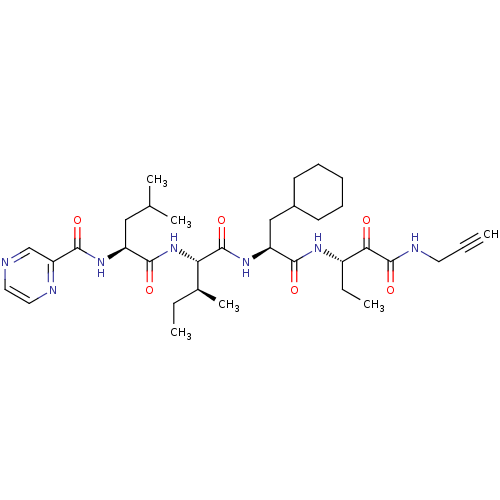 (CHEMBL274547 | Pyrazine-2-carboxylic acid ((S)-1-{...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against HCV NS3 protease was determined | Bioorg Med Chem Lett 13: 1111-4 (2003) BindingDB Entry DOI: 10.7270/Q2HQ3Z8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110148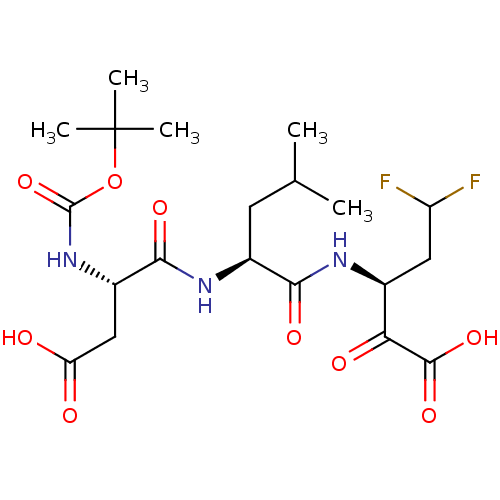 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-3-car...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110149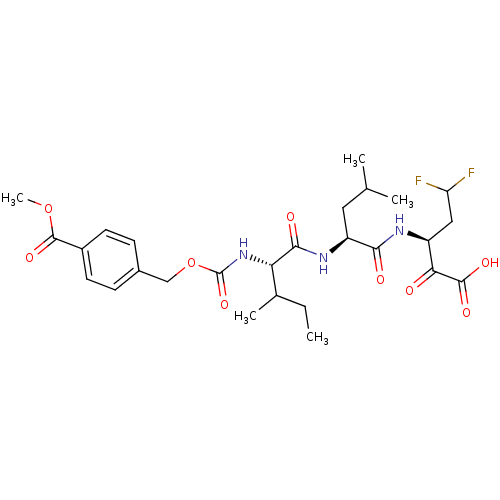 (4-{(S)-1-[(S)-1-((S)-3,3-Difluoro-1-oxalyl-propylc...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110152 ((S)-3-[(S)-2-((2S,3S)-2-Benzyloxycarbonylamino-3-m...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50125385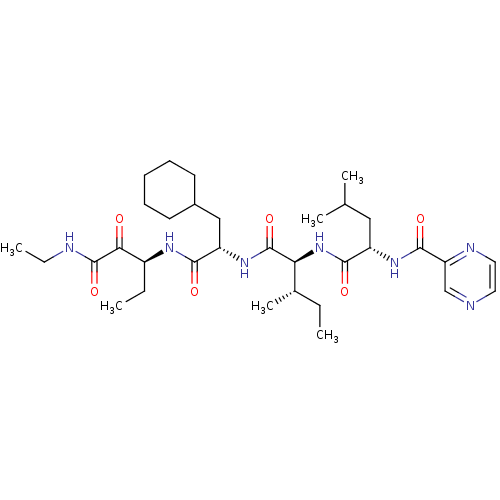 (CHEMBL13370 | Pyrazine-2-carboxylic acid ((S)-1-{(...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against HCV NS3 protease was determined | Bioorg Med Chem Lett 13: 1111-4 (2003) BindingDB Entry DOI: 10.7270/Q2HQ3Z8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50139658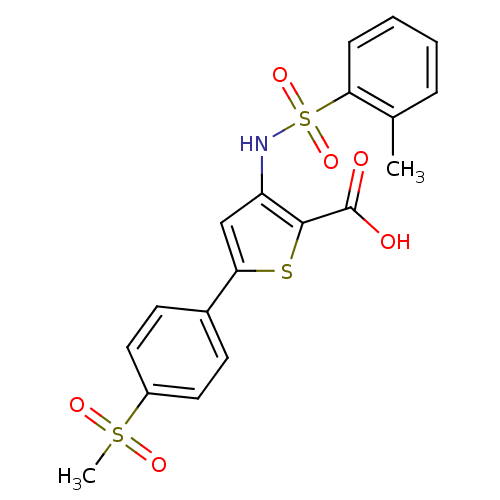 (5-(4-Methanesulfonyl-phenyl)-3-(toluene-2-sulfonyl...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B polymerase | Bioorg Med Chem Lett 14: 793-6 (2004) BindingDB Entry DOI: 10.7270/Q2PV6JSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50139673 (2-[(2,4-Dichloro-benzoyl)-(3-trifluoromethyl-benzy...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc. Curated by ChEMBL | Assay Description Inhibition of HCV NS5B polymerase | Bioorg Med Chem Lett 14: 793-6 (2004) BindingDB Entry DOI: 10.7270/Q2PV6JSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50410376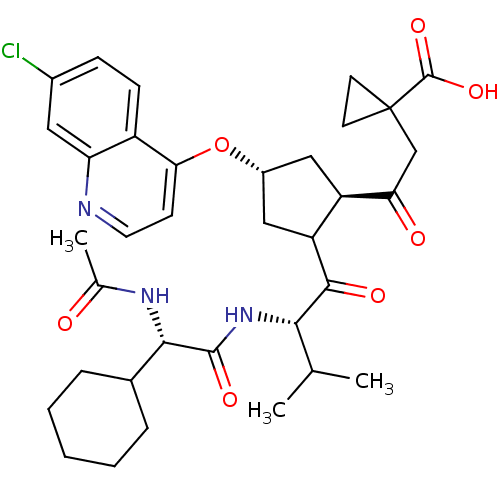 (CHEMBL190689) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IMIM/Universitat Pompeu Fabra Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease (IC50 spans 2.5 log units) | J Med Chem 48: 2687-94 (2005) Article DOI: 10.1021/jm049113+ BindingDB Entry DOI: 10.7270/Q29S1QH4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50139675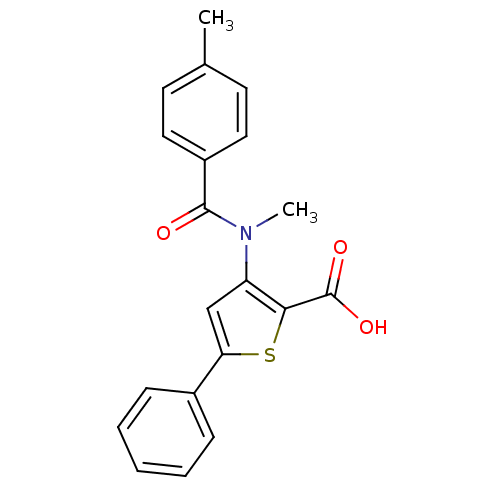 (3-[Methyl-(4-methyl-benzoyl)-amino]-5-phenyl-thiop...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc Curated by ChEMBL | Assay Description In vitro inhibition of Hepatitis C polymerase. | Bioorg Med Chem Lett 14: 797-800 (2004) BindingDB Entry DOI: 10.7270/Q2K35T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50139681 (3-[Ethyl-(4-methyl-benzoyl)-amino]-5-phenyl-thioph...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shire BioChem Inc Curated by ChEMBL | Assay Description In vitro inhibition of Hepatitis C polymerase. | Bioorg Med Chem Lett 14: 797-800 (2004) BindingDB Entry DOI: 10.7270/Q2K35T2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50110129 ((S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-4-car...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition to hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 189 total ) | Next | Last >> |