 Found 62 hits Enz. Inhib. hit(s) with all data for entry = 50017787
Found 62 hits Enz. Inhib. hit(s) with all data for entry = 50017787 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186457

(1-(4-(5-(2-(4,6-dimethylpyridin-2-ylamino)thiazol-...)Show SMILES COc1cc(C)c(Sc2cnc(Nc3cc(C)cc(C)n3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C25H29N5O3S2/c1-15-10-17(3)27-22(11-15)28-25-26-14-23(35-25)34-21-13-19(20(33-5)12-16(21)2)24(32)30-8-6-29(7-9-30)18(4)31/h10-14H,6-9H2,1-5H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186484

(1-(4-(5-(2-(4,6-dimethylpyridin-2-ylamino)thiazol-...)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cc(Sc2cnc(Nc3cc(C)cc(C)n3)s2)ccc1O Show InChI InChI=1S/C23H25N5O3S2/c1-14-10-15(2)25-20(11-14)26-23-24-13-21(33-23)32-17-4-5-19(30)18(12-17)22(31)28-8-6-27(7-9-28)16(3)29/h4-5,10-13,30H,6-9H2,1-3H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186487

(CHEMBL209881 | N-(5-(4-methoxy-2-methyl-5-(morphol...)Show SMILES CNCc1ccc([nH]1)C(=O)Nc1ncc(Sc2cc(C(=O)N3CCOCC3)c(OC)cc2C)s1 Show InChI InChI=1S/C23H27N5O4S2/c1-14-10-18(31-3)16(22(30)28-6-8-32-9-7-28)11-19(14)33-20-13-25-23(34-20)27-21(29)17-5-4-15(26-17)12-24-2/h4-5,10-11,13,24,26H,6-9,12H2,1-3H3,(H,25,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186478
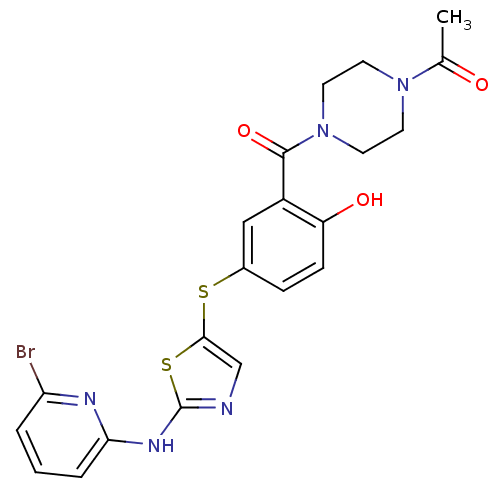
(1-(4-(5-(2-(6-bromopyridin-2-ylamino)thiazol-5-ylt...)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cc(Sc2cnc(Nc3cccc(Br)n3)s2)ccc1O Show InChI InChI=1S/C21H20BrN5O3S2/c1-13(28)26-7-9-27(10-8-26)20(30)15-11-14(5-6-16(15)29)31-19-12-23-21(32-19)25-18-4-2-3-17(22)24-18/h2-6,11-12,29H,7-10H2,1H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186459

(CHEMBL211842 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)C3CC3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C22H26N4O4S2/c1-13-10-17(30-3)16(21(29)26-8-6-25(7-9-26)14(2)27)11-18(13)31-19-12-23-22(32-19)24-20(28)15-4-5-15/h10-12,15H,4-9H2,1-3H3,(H,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186456
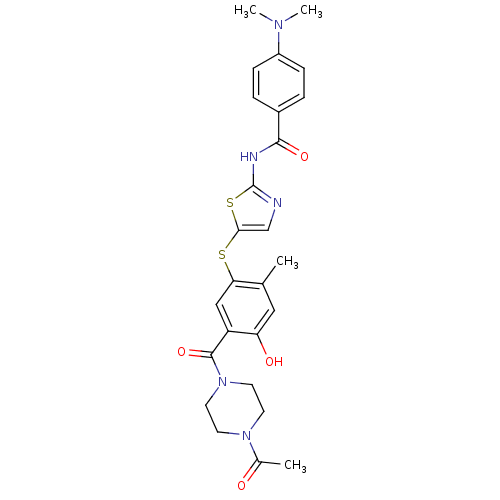
(CHEMBL211355 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2cc(C(=O)N3CCN(CC3)C(C)=O)c(O)cc2C)s1 Show InChI InChI=1S/C26H29N5O4S2/c1-16-13-21(33)20(25(35)31-11-9-30(10-12-31)17(2)32)14-22(16)36-23-15-27-26(37-23)28-24(34)18-5-7-19(8-6-18)29(3)4/h5-8,13-15,33H,9-12H2,1-4H3,(H,27,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186461

(CHEMBL209163 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)c3ccc[nH]3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25N5O4S2/c1-14-11-18(32-3)16(22(31)28-9-7-27(8-10-28)15(2)29)12-19(14)33-20-13-25-23(34-20)26-21(30)17-5-4-6-24-17/h4-6,11-13,24H,7-10H2,1-3H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186474
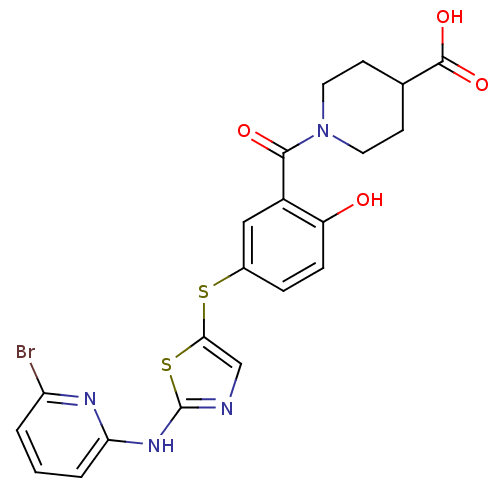
(1-(5-(2-(6-bromopyridin-2-ylamino)thiazol-5-ylthio...)Show SMILES OC(=O)C1CCN(CC1)C(=O)c1cc(Sc2cnc(Nc3cccc(Br)n3)s2)ccc1O Show InChI InChI=1S/C21H19BrN4O4S2/c22-16-2-1-3-17(24-16)25-21-23-11-18(32-21)31-13-4-5-15(27)14(10-13)19(28)26-8-6-12(7-9-26)20(29)30/h1-5,10-12,27H,6-9H2,(H,29,30)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186480
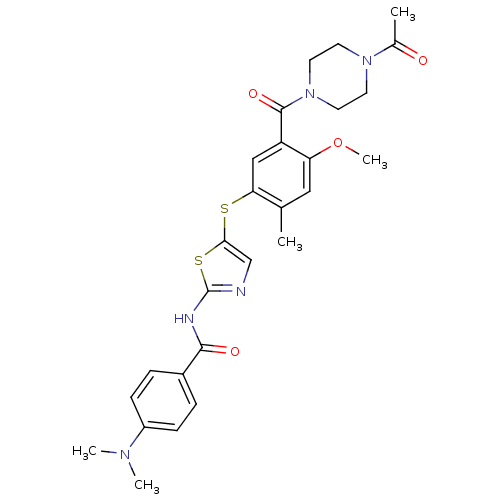
(CHEMBL379114 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)c3ccc(cc3)N(C)C)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C27H31N5O4S2/c1-17-14-22(36-5)21(26(35)32-12-10-31(11-13-32)18(2)33)15-23(17)37-24-16-28-27(38-24)29-25(34)19-6-8-20(9-7-19)30(3)4/h6-9,14-16H,10-13H2,1-5H3,(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186460
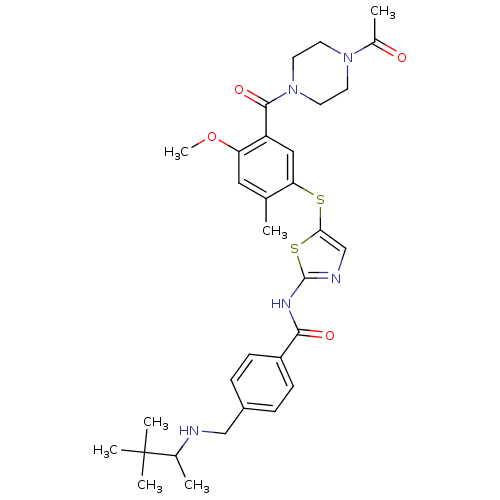
(CHEMBL209148 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)c3ccc(CNC(C)C(C)(C)C)cc3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C32H41N5O4S2/c1-20-16-26(41-7)25(30(40)37-14-12-36(13-15-37)22(3)38)17-27(20)42-28-19-34-31(43-28)35-29(39)24-10-8-23(9-11-24)18-33-21(2)32(4,5)6/h8-11,16-17,19,21,33H,12-15,18H2,1-7H3,(H,34,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186479
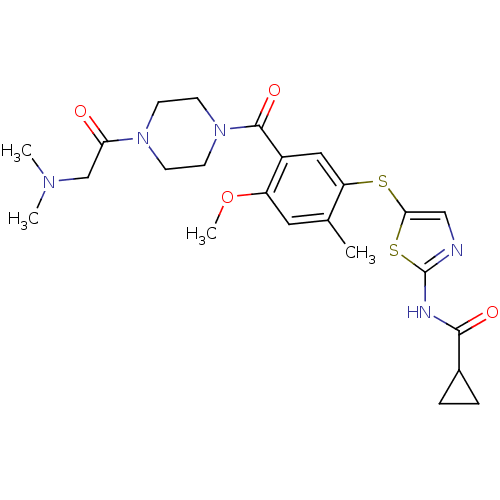
(CHEMBL208901 | N-(5-(5-(1-(2-(dimethylamino)acetyl...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)C3CC3)s2)cc1C(=O)N1CCN(CC1)C(=O)CN(C)C Show InChI InChI=1S/C24H31N5O4S2/c1-15-11-18(33-4)17(23(32)29-9-7-28(8-10-29)20(30)14-27(2)3)12-19(15)34-21-13-25-24(35-21)26-22(31)16-5-6-16/h11-13,16H,5-10,14H2,1-4H3,(H,25,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186470
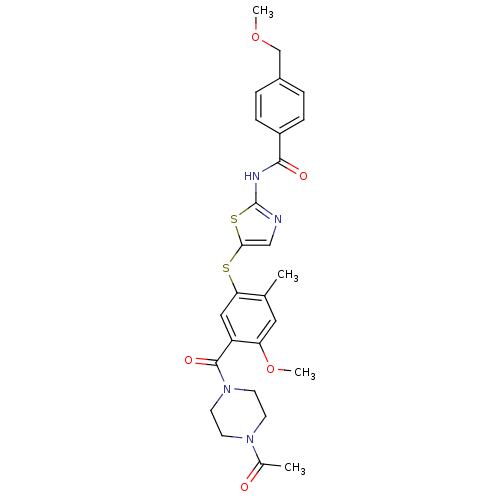
(CHEMBL211291 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COCc1ccc(cc1)C(=O)Nc1ncc(Sc2cc(C(=O)N3CCN(CC3)C(C)=O)c(OC)cc2C)s1 Show InChI InChI=1S/C27H30N4O5S2/c1-17-13-22(36-4)21(26(34)31-11-9-30(10-12-31)18(2)32)14-23(17)37-24-15-28-27(38-24)29-25(33)20-7-5-19(6-8-20)16-35-3/h5-8,13-15H,9-12,16H2,1-4H3,(H,28,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186496
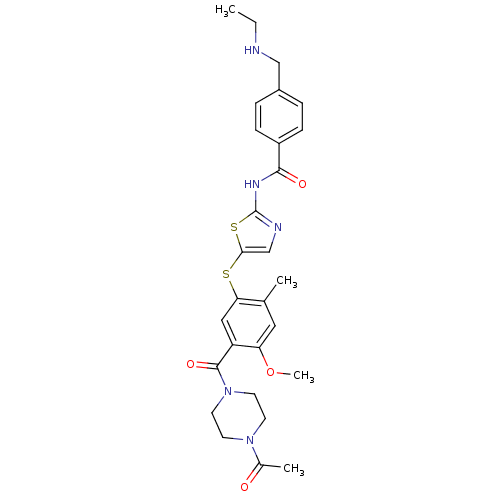
(CHEMBL210985 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES CCNCc1ccc(cc1)C(=O)Nc1ncc(Sc2cc(C(=O)N3CCN(CC3)C(C)=O)c(OC)cc2C)s1 Show InChI InChI=1S/C28H33N5O4S2/c1-5-29-16-20-6-8-21(9-7-20)26(35)31-28-30-17-25(39-28)38-24-15-22(23(37-4)14-18(24)2)27(36)33-12-10-32(11-13-33)19(3)34/h6-9,14-15,17,29H,5,10-13,16H2,1-4H3,(H,30,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186466

(1-(4-(2-hydroxy-5-(2-(pyridin-2-ylamino)thiazol-5-...)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cc(Sc2cnc(Nc3ccccn3)s2)ccc1O Show InChI InChI=1S/C21H21N5O3S2/c1-14(27)25-8-10-26(11-9-25)20(29)16-12-15(5-6-17(16)28)30-19-13-23-21(31-19)24-18-4-2-3-7-22-18/h2-7,12-13,28H,8-11H2,1H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186472

(CHEMBL209004 | N-(5-(3-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2ccc(O)c(c2)C(=O)N2CCN(CC2)C(C)=O)s1 Show InChI InChI=1S/C25H27N5O4S2/c1-16(31)29-10-12-30(13-11-29)24(34)20-14-19(8-9-21(20)32)35-22-15-26-25(36-22)27-23(33)17-4-6-18(7-5-17)28(2)3/h4-9,14-15,32H,10-13H2,1-3H3,(H,26,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186467
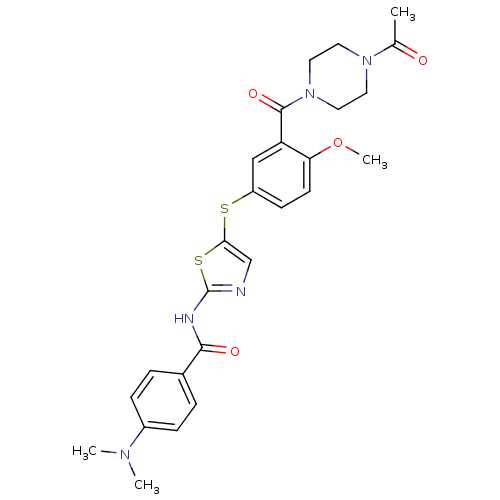
(CHEMBL210528 | N-(5-(3-(1-acetylpiperazine-4-carbo...)Show SMILES COc1ccc(Sc2cnc(NC(=O)c3ccc(cc3)N(C)C)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C26H29N5O4S2/c1-17(32)30-11-13-31(14-12-30)25(34)21-15-20(9-10-22(21)35-4)36-23-16-27-26(37-23)28-24(33)18-5-7-19(8-6-18)29(2)3/h5-10,15-16H,11-14H2,1-4H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186488
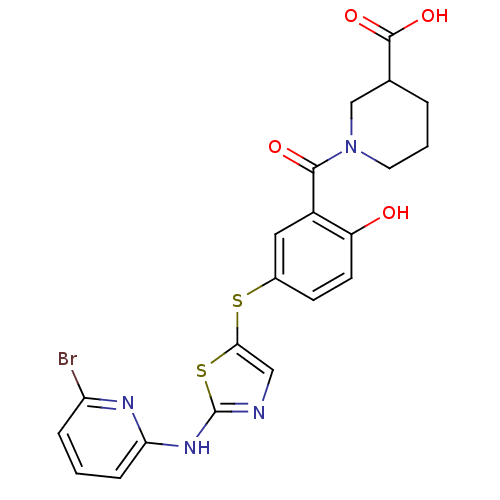
(1-(5-(2-(6-bromopyridin-2-ylamino)thiazol-5-ylthio...)Show SMILES OC(=O)C1CCCN(C1)C(=O)c1cc(Sc2cnc(Nc3cccc(Br)n3)s2)ccc1O Show InChI InChI=1S/C21H19BrN4O4S2/c22-16-4-1-5-17(24-16)25-21-23-10-18(32-21)31-13-6-7-15(27)14(9-13)19(28)26-8-2-3-12(11-26)20(29)30/h1,4-7,9-10,12,27H,2-3,8,11H2,(H,29,30)(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186477
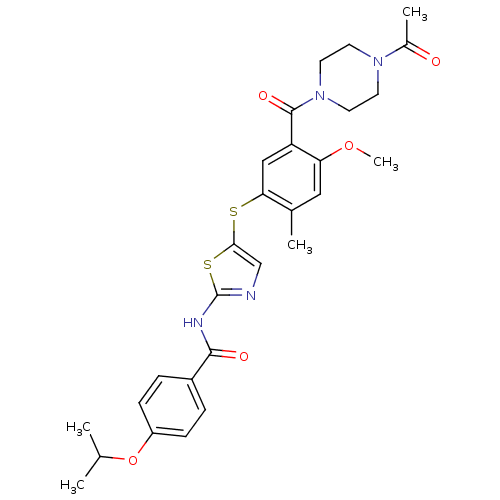
(CHEMBL380058 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)c3ccc(OC(C)C)cc3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C28H32N4O5S2/c1-17(2)37-21-8-6-20(7-9-21)26(34)30-28-29-16-25(39-28)38-24-15-22(23(36-5)14-18(24)3)27(35)32-12-10-31(11-13-32)19(4)33/h6-9,14-17H,10-13H2,1-5H3,(H,29,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186468
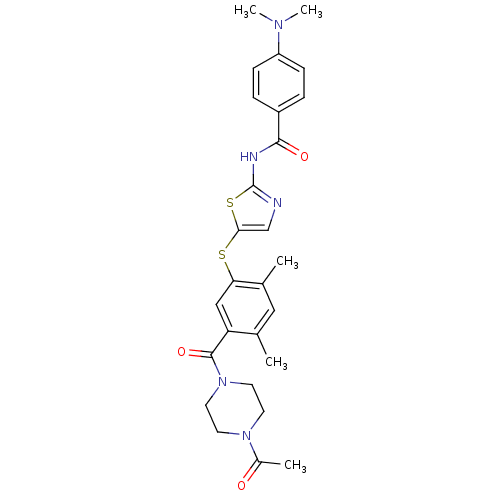
(CHEMBL206905 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2cc(C(=O)N3CCN(CC3)C(C)=O)c(C)cc2C)s1 Show InChI InChI=1S/C27H31N5O3S2/c1-17-14-18(2)23(15-22(17)26(35)32-12-10-31(11-13-32)19(3)33)36-24-16-28-27(37-24)29-25(34)20-6-8-21(9-7-20)30(4)5/h6-9,14-16H,10-13H2,1-5H3,(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186486

(1-(5-(2-(6-bromopyridin-2-ylamino)thiazol-5-ylthio...)Show SMILES CS(=O)(=O)NC(=O)C1CCCN(C1)C(=O)c1cc(Sc2cnc(Nc3cccc(Br)n3)s2)ccc1O Show InChI InChI=1S/C22H22BrN5O5S3/c1-36(32,33)27-20(30)13-4-3-9-28(12-13)21(31)15-10-14(7-8-16(15)29)34-19-11-24-22(35-19)26-18-6-2-5-17(23)25-18/h2,5-8,10-11,13,29H,3-4,9,12H2,1H3,(H,27,30)(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186495
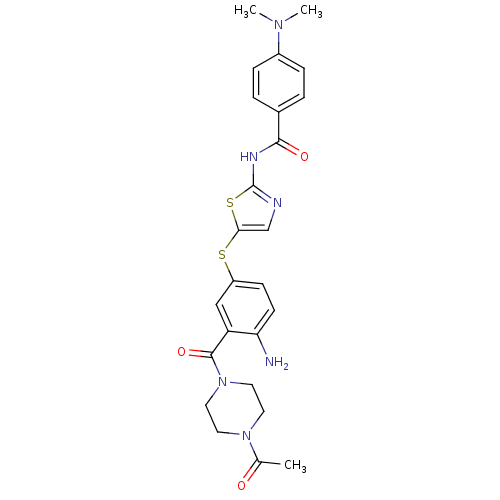
(CHEMBL210243 | N-(5-(3-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2ccc(N)c(c2)C(=O)N2CCN(CC2)C(C)=O)s1 Show InChI InChI=1S/C25H28N6O3S2/c1-16(32)30-10-12-31(13-11-30)24(34)20-14-19(8-9-21(20)26)35-22-15-27-25(36-22)28-23(33)17-4-6-18(7-5-17)29(2)3/h4-9,14-15H,10-13,26H2,1-3H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50182833

(CHEMBL382362 | N-(5-(3-(1-acetylpiperazine-4-carbo...)Show SMILES CC(NCc1ccc(cc1)C(=O)Nc1ncc(SCc2cc(C)c(C)c(c2)C(=O)N2CCN(CC2)C(C)=O)s1)C(C)(C)C Show InChI InChI=1S/C33H43N5O3S2/c1-21-16-26(17-28(22(21)2)31(41)38-14-12-37(13-15-38)24(4)39)20-42-29-19-35-32(43-29)36-30(40)27-10-8-25(9-11-27)18-34-23(3)33(5,6)7/h8-11,16-17,19,23,34H,12-15,18,20H2,1-7H3,(H,35,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186498
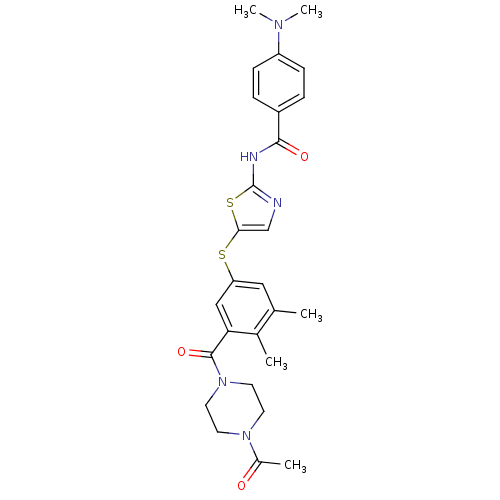
(CHEMBL209400 | N-(5-(3-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2cc(C)c(C)c(c2)C(=O)N2CCN(CC2)C(C)=O)s1 Show InChI InChI=1S/C27H31N5O3S2/c1-17-14-22(15-23(18(17)2)26(35)32-12-10-31(11-13-32)19(3)33)36-24-16-28-27(37-24)29-25(34)20-6-8-21(9-7-20)30(4)5/h6-9,14-16H,10-13H2,1-5H3,(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186497

(CHEMBL211136 | N-(5-(4-acetamido-3-(1-acetylpipera...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2ccc(NC(C)=O)c(c2)C(=O)N2CCN(CC2)C(C)=O)s1 Show InChI InChI=1S/C27H30N6O4S2/c1-17(34)29-23-10-9-21(15-22(23)26(37)33-13-11-32(12-14-33)18(2)35)38-24-16-28-27(39-24)30-25(36)19-5-7-20(8-6-19)31(3)4/h5-10,15-16H,11-14H2,1-4H3,(H,29,34)(H,28,30,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186476

(CHEMBL210932 | N-(5-(5-(1-ethylpiperazine-4-carbon...)Show SMILES CCN1CCN(CC1)C(=O)c1cc(Sc2cnc(NC(=O)C3CC3)s2)c(C)cc1OC Show InChI InChI=1S/C22H28N4O3S2/c1-4-25-7-9-26(10-8-25)21(28)16-12-18(14(2)11-17(16)29-3)30-19-13-23-22(31-19)24-20(27)15-5-6-15/h11-13,15H,4-10H2,1-3H3,(H,23,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186463

(CHEMBL209009 | N-(5-(3-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2ccc(C)c(c2)C(=O)N2CCN(CC2)C(C)=O)s1 Show InChI InChI=1S/C26H29N5O3S2/c1-17-5-10-21(15-22(17)25(34)31-13-11-30(12-14-31)18(2)32)35-23-16-27-26(36-23)28-24(33)19-6-8-20(9-7-19)29(3)4/h5-10,15-16H,11-14H2,1-4H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186469

(2-((5-(5-(1-acetylpiperazine-4-carbonyl)-4-methoxy...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)C3CC3C(O)=O)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H26N4O6S2/c1-12-8-17(33-3)16(21(30)27-6-4-26(5-7-27)13(2)28)10-18(12)34-19-11-24-23(35-19)25-20(29)14-9-15(14)22(31)32/h8,10-11,14-15H,4-7,9H2,1-3H3,(H,31,32)(H,24,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186464

(2-(5-(2-(6-bromopyridin-2-ylamino)thiazol-5-ylthio...)Show SMILES CN(CC(O)=O)C(=O)c1cc(Sc2cnc(Nc3cccc(Br)n3)s2)ccc1O Show InChI InChI=1S/C18H15BrN4O4S2/c1-23(9-15(25)26)17(27)11-7-10(5-6-12(11)24)28-16-8-20-18(29-16)22-14-4-2-3-13(19)21-14/h2-8,24H,9H2,1H3,(H,25,26)(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186492

(CHEMBL373513 | N-(5-(3-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2ccc(Cl)c(c2)C(=O)N2CCN(CC2)C(C)=O)s1 Show InChI InChI=1S/C25H26ClN5O3S2/c1-16(32)30-10-12-31(13-11-30)24(34)20-14-19(8-9-21(20)26)35-22-15-27-25(36-22)28-23(33)17-4-6-18(7-5-17)29(2)3/h4-9,14-15H,10-13H2,1-3H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186482
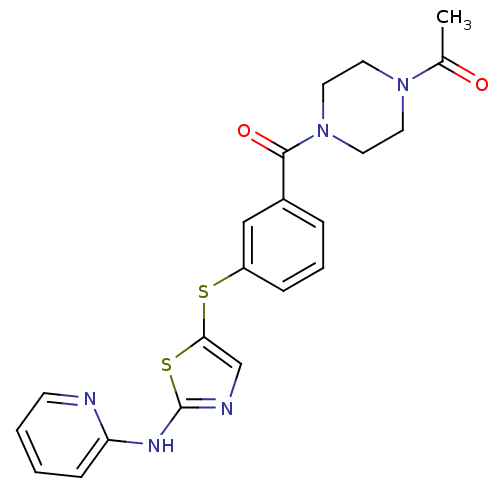
(1-(4-(3-(2-(pyridin-2-ylamino)thiazol-5-ylthio)ben...)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cccc(Sc2cnc(Nc3ccccn3)s2)c1 Show InChI InChI=1S/C21H21N5O2S2/c1-15(27)25-9-11-26(12-10-25)20(28)16-5-4-6-17(13-16)29-19-14-23-21(30-19)24-18-7-2-3-8-22-18/h2-8,13-14H,9-12H2,1H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186458
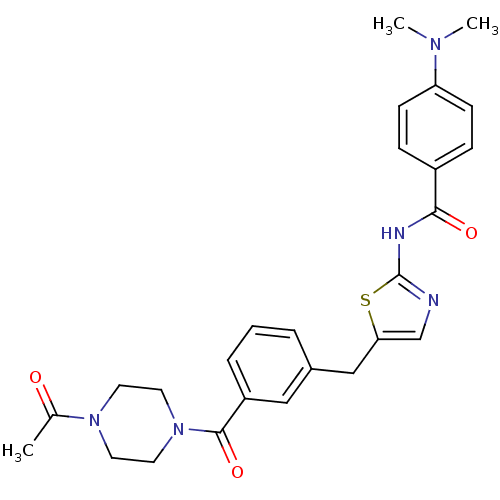
(CHEMBL273966 | N-(5-(3-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Cc2cccc(c2)C(=O)N2CCN(CC2)C(C)=O)s1 Show InChI InChI=1S/C26H29N5O3S/c1-18(32)30-11-13-31(14-12-30)25(34)21-6-4-5-19(15-21)16-23-17-27-26(35-23)28-24(33)20-7-9-22(10-8-20)29(2)3/h4-10,15,17H,11-14,16H2,1-3H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186483
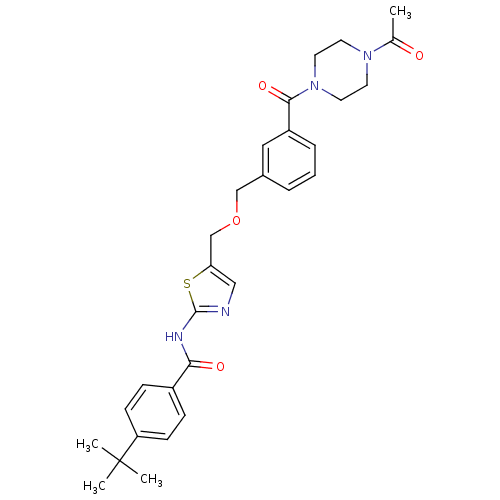
(CHEMBL211021 | N-(5-((3-(1-acetylpiperazine-4-carb...)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cccc(COCc2cnc(NC(=O)c3ccc(cc3)C(C)(C)C)s2)c1 Show InChI InChI=1S/C29H34N4O4S/c1-20(34)32-12-14-33(15-13-32)27(36)23-7-5-6-21(16-23)18-37-19-25-17-30-28(38-25)31-26(35)22-8-10-24(11-9-22)29(2,3)4/h5-11,16-17H,12-15,18-19H2,1-4H3,(H,30,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186473
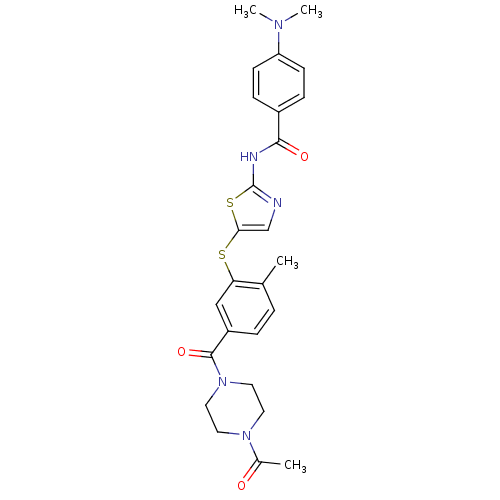
(CHEMBL210735 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2cc(ccc2C)C(=O)N2CCN(CC2)C(C)=O)s1 Show InChI InChI=1S/C26H29N5O3S2/c1-17-5-6-20(25(34)31-13-11-30(12-14-31)18(2)32)15-22(17)35-23-16-27-26(36-23)28-24(33)19-7-9-21(10-8-19)29(3)4/h5-10,15-16H,11-14H2,1-4H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186471
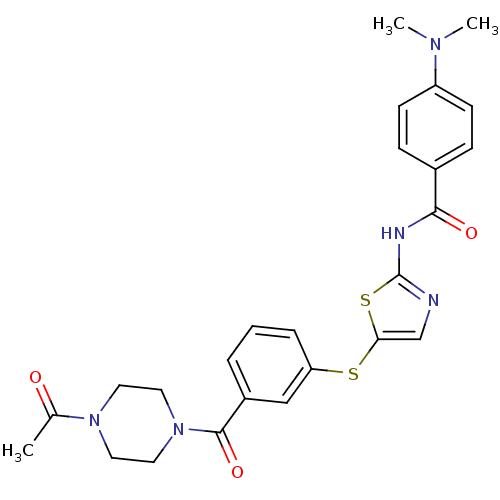
(CHEMBL437495 | N-(5-(3-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2cccc(c2)C(=O)N2CCN(CC2)C(C)=O)s1 Show InChI InChI=1S/C25H27N5O3S2/c1-17(31)29-11-13-30(14-12-29)24(33)19-5-4-6-21(15-19)34-22-16-26-25(35-22)27-23(32)18-7-9-20(10-8-18)28(2)3/h4-10,15-16H,11-14H2,1-3H3,(H,26,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50182839

(CHEMBL205799 | N-(5-(3-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(SCc2cccc(c2)C(=O)N2CCN(CC2)C(C)=O)s1 Show InChI InChI=1S/C26H29N5O3S2/c1-18(32)30-11-13-31(14-12-30)25(34)21-6-4-5-19(15-21)17-35-23-16-27-26(36-23)28-24(33)20-7-9-22(10-8-20)29(2)3/h4-10,15-16H,11-14,17H2,1-3H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50186460

(CHEMBL209148 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)c3ccc(CNC(C)C(C)(C)C)cc3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C32H41N5O4S2/c1-20-16-26(41-7)25(30(40)37-14-12-36(13-15-37)22(3)38)17-27(20)42-28-19-34-31(43-28)35-29(39)24-10-8-23(9-11-24)18-33-21(2)32(4,5)6/h8-11,16-17,19,21,33H,12-15,18H2,1-7H3,(H,34,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Insulin receptor |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50186460

(CHEMBL209148 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)c3ccc(CNC(C)C(C)(C)C)cc3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C32H41N5O4S2/c1-20-16-26(41-7)25(30(40)37-14-12-36(13-15-37)22(3)38)17-27(20)42-28-19-34-31(43-28)35-29(39)24-10-8-23(9-11-24)18-33-21(2)32(4,5)6/h8-11,16-17,19,21,33H,12-15,18H2,1-7H3,(H,34,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Fyn |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186489

(CHEMBL210046 | N-(5-(3-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2cc(N)cc(c2)C(=O)N2CCN(CC2)C(C)=O)s1 Show InChI InChI=1S/C25H28N6O3S2/c1-16(32)30-8-10-31(11-9-30)24(34)18-12-19(26)14-21(13-18)35-22-15-27-25(36-22)28-23(33)17-4-6-20(7-5-17)29(2)3/h4-7,12-15H,8-11,26H2,1-3H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186475
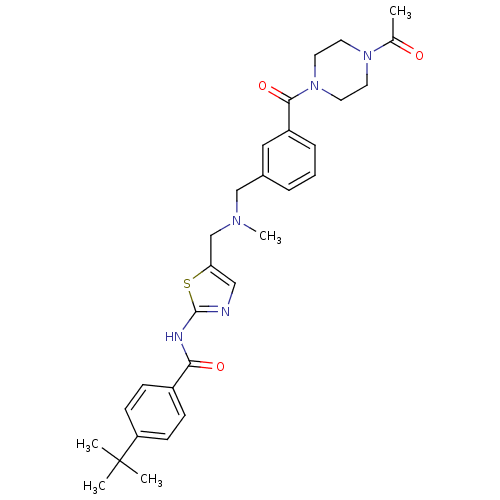
(CHEMBL426685 | N-(5-(((3-(1-acetylpiperazine-4-car...)Show SMILES CN(Cc1cnc(NC(=O)c2ccc(cc2)C(C)(C)C)s1)Cc1cccc(c1)C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C30H37N5O3S/c1-21(36)34-13-15-35(16-14-34)28(38)24-8-6-7-22(17-24)19-33(5)20-26-18-31-29(39-26)32-27(37)23-9-11-25(12-10-23)30(2,3)4/h6-12,17-18H,13-16,19-20H2,1-5H3,(H,31,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50186460

(CHEMBL209148 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)c3ccc(CNC(C)C(C)(C)C)cc3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C32H41N5O4S2/c1-20-16-26(41-7)25(30(40)37-14-12-36(13-15-37)22(3)38)17-27(20)42-28-19-34-31(43-28)35-29(39)24-10-8-23(9-11-24)18-33-21(2)32(4,5)6/h8-11,16-17,19,21,33H,12-15,18H2,1-7H3,(H,34,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of LCK |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186494
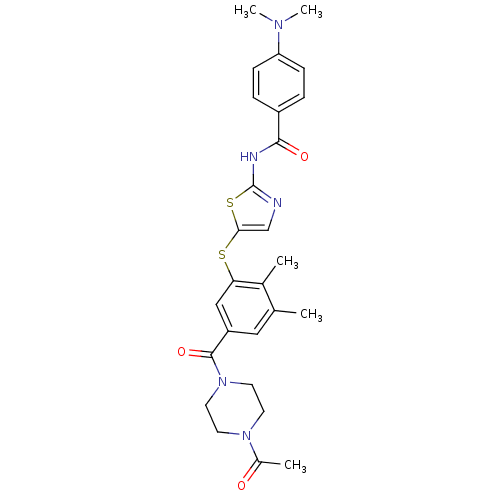
(CHEMBL209056 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2cc(cc(C)c2C)C(=O)N2CCN(CC2)C(C)=O)s1 Show InChI InChI=1S/C27H31N5O3S2/c1-17-14-21(26(35)32-12-10-31(11-13-32)19(3)33)15-23(18(17)2)36-24-16-28-27(37-24)29-25(34)20-6-8-22(9-7-20)30(4)5/h6-9,14-16H,10-13H2,1-5H3,(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50186460

(CHEMBL209148 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)c3ccc(CNC(C)C(C)(C)C)cc3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C32H41N5O4S2/c1-20-16-26(41-7)25(30(40)37-14-12-36(13-15-37)22(3)38)17-27(20)42-28-19-34-31(43-28)35-29(39)24-10-8-23(9-11-24)18-33-21(2)32(4,5)6/h8-11,16-17,19,21,33H,12-15,18H2,1-7H3,(H,34,35,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Btk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186491
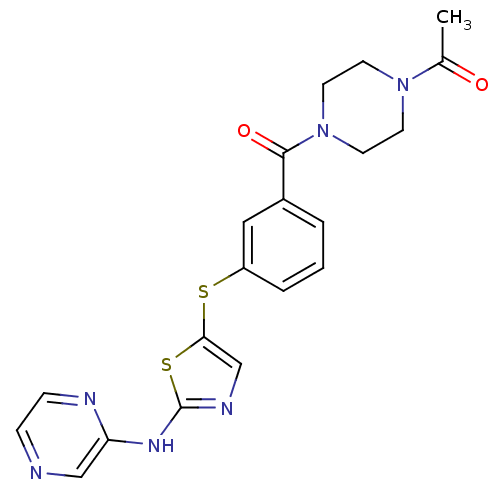
(1-(4-(3-(2-(pyrazin-2-ylamino)thiazol-5-ylthio)ben...)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cccc(Sc2cnc(Nc3cnccn3)s2)c1 Show InChI InChI=1S/C20H20N6O2S2/c1-14(27)25-7-9-26(10-8-25)19(28)15-3-2-4-16(11-15)29-18-13-23-20(30-18)24-17-12-21-5-6-22-17/h2-6,11-13H,7-10H2,1H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186455

(1-(4-(3-(2-(thiazol-2-ylamino)thiazol-5-ylthio)ben...)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cccc(Sc2cnc(Nc3nccs3)s2)c1 Show InChI InChI=1S/C19H19N5O2S3/c1-13(25)23-6-8-24(9-7-23)17(26)14-3-2-4-15(11-14)28-16-12-21-19(29-16)22-18-20-5-10-27-18/h2-5,10-12H,6-9H2,1H3,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase TXK
(Homo sapiens (Human)) | BDBM50186460

(CHEMBL209148 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)c3ccc(CNC(C)C(C)(C)C)cc3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C32H41N5O4S2/c1-20-16-26(41-7)25(30(40)37-14-12-36(13-15-37)22(3)38)17-27(20)42-28-19-34-31(43-28)35-29(39)24-10-8-23(9-11-24)18-33-21(2)32(4,5)6/h8-11,16-17,19,21,33H,12-15,18H2,1-7H3,(H,34,35,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Txk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Tec
(Homo sapiens (Human)) | BDBM50186460

(CHEMBL209148 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)c3ccc(CNC(C)C(C)(C)C)cc3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C32H41N5O4S2/c1-20-16-26(41-7)25(30(40)37-14-12-36(13-15-37)22(3)38)17-27(20)42-28-19-34-31(43-28)35-29(39)24-10-8-23(9-11-24)18-33-21(2)32(4,5)6/h8-11,16-17,19,21,33H,12-15,18H2,1-7H3,(H,34,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Tec |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186485
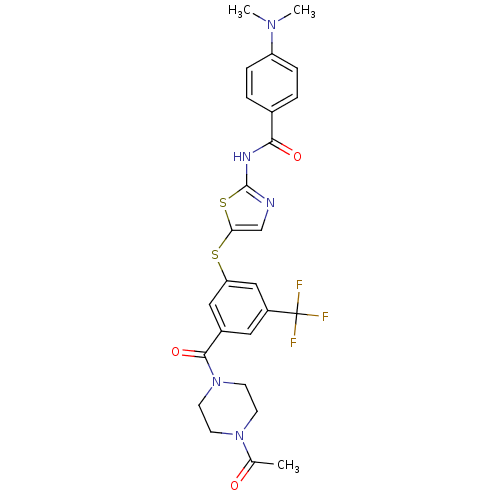
(CHEMBL210599 | N-(5-(3-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2cc(cc(c2)C(F)(F)F)C(=O)N2CCN(CC2)C(C)=O)s1 Show InChI InChI=1S/C26H26F3N5O3S2/c1-16(35)33-8-10-34(11-9-33)24(37)18-12-19(26(27,28)29)14-21(13-18)38-22-15-30-25(39-22)31-23(36)17-4-6-20(7-5-17)32(2)3/h4-7,12-15H,8-11H2,1-3H3,(H,30,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186465

(CHEMBL377355 | N-(5-((3-(1-acetylpiperazine-4-carb...)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cccc(CNCc2cnc(NC(=O)c3ccc(cc3)C(C)(C)C)s2)c1 Show InChI InChI=1S/C29H35N5O3S/c1-20(35)33-12-14-34(15-13-33)27(37)23-7-5-6-21(16-23)17-30-18-25-19-31-28(38-25)32-26(36)22-8-10-24(11-9-22)29(2,3)4/h5-11,16,19,30H,12-15,17-18H2,1-4H3,(H,31,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50186460

(CHEMBL209148 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)c3ccc(CNC(C)C(C)(C)C)cc3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C32H41N5O4S2/c1-20-16-26(41-7)25(30(40)37-14-12-36(13-15-37)22(3)38)17-27(20)42-28-19-34-31(43-28)35-29(39)24-10-8-23(9-11-24)18-33-21(2)32(4,5)6/h8-11,16-17,19,21,33H,12-15,18H2,1-7H3,(H,34,35,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of PKC |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186462
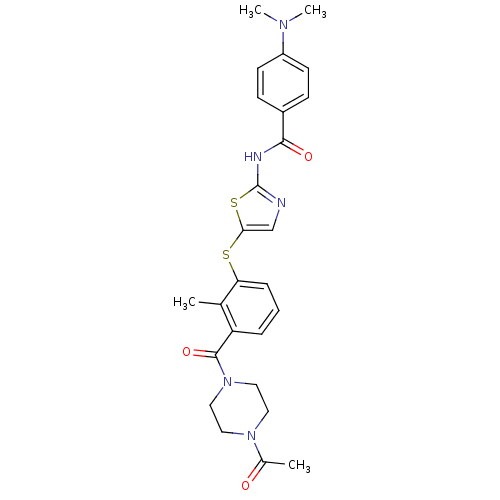
(CHEMBL208867 | N-(5-(3-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2cccc(C(=O)N3CCN(CC3)C(C)=O)c2C)s1 Show InChI InChI=1S/C26H29N5O3S2/c1-17-21(25(34)31-14-12-30(13-15-31)18(2)32)6-5-7-22(17)35-23-16-27-26(36-23)28-24(33)19-8-10-20(11-9-19)29(3)4/h5-11,16H,12-15H2,1-4H3,(H,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data