

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359569 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359544 (4-{(6R or S)-1-({2- chloro-6-[1- (difluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359568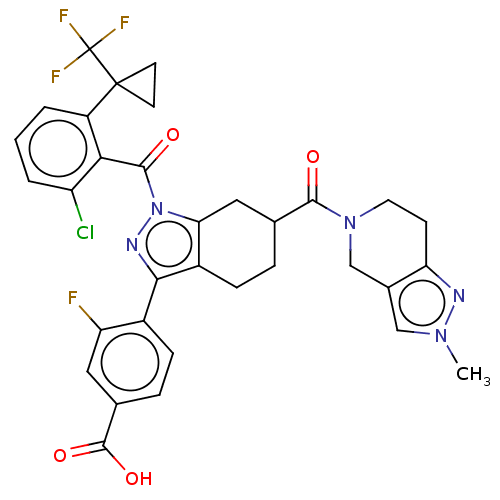 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359566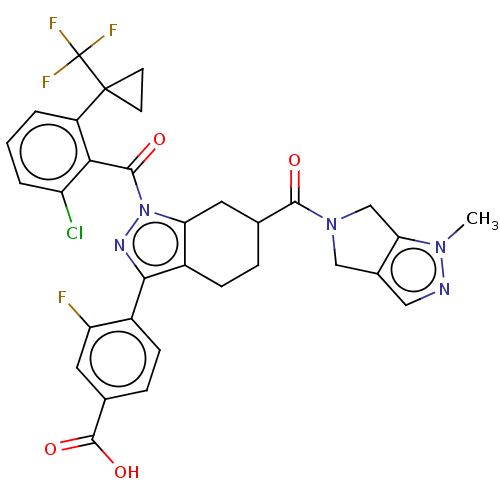 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359571 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359545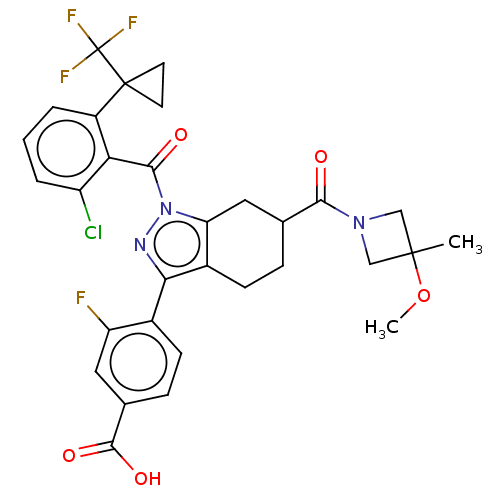 (4-{(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359567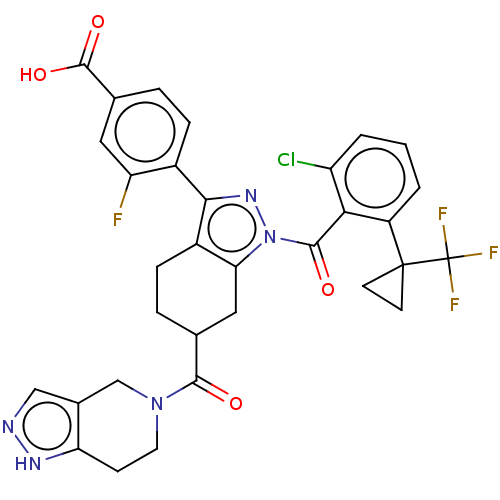 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359543 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359548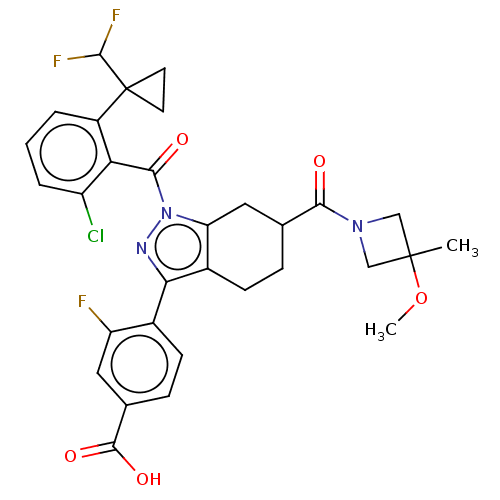 (4-{(6R or S)-1-({2- chloro-6-[1- (difluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359570 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359582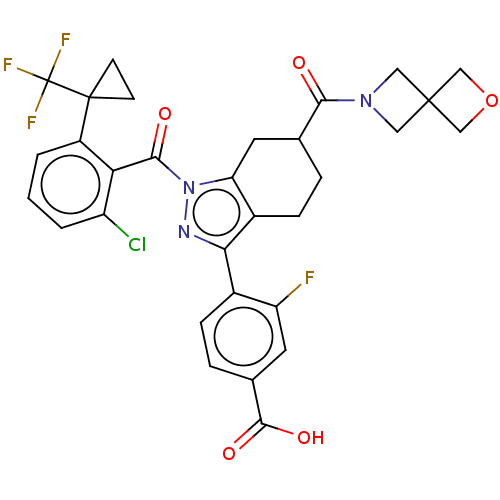 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359535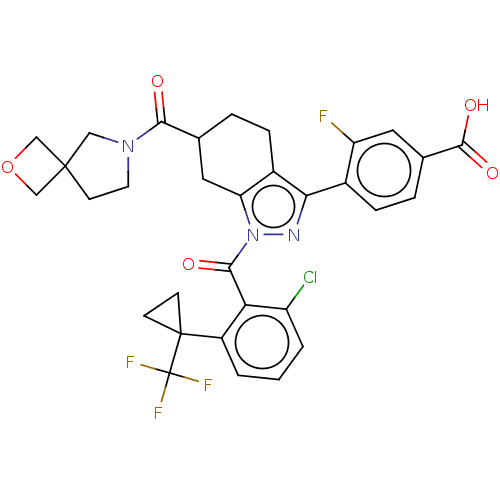 (US10221142, Example 21A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359540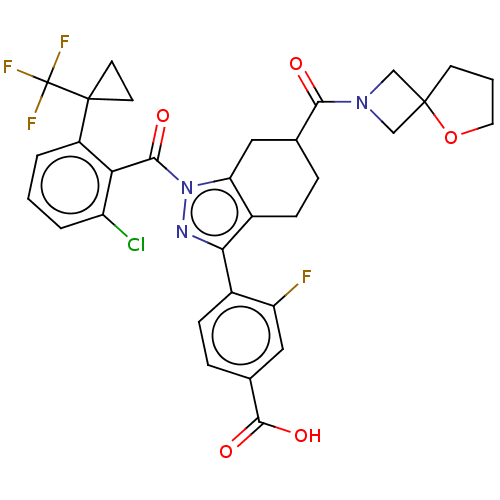 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359577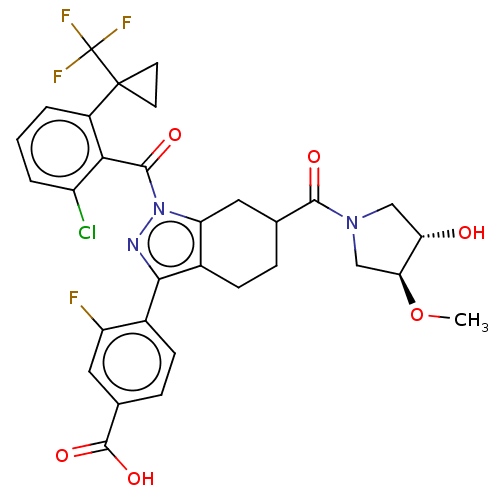 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359547 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359550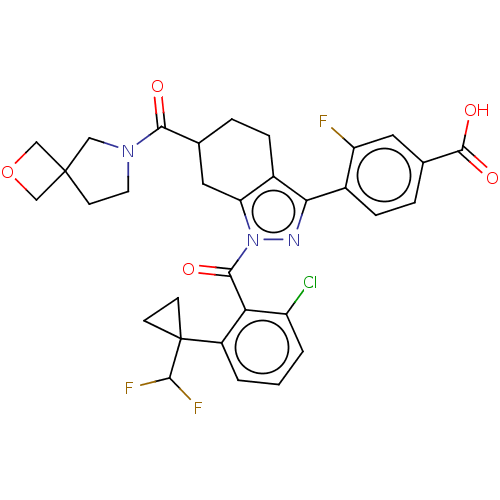 (4-[(6R or S)-1-({2- chloro-6-[1- (difluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359551 (4-[(6R or S)-1-({2- chloro-6-[1- (difluoromethyl)c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359539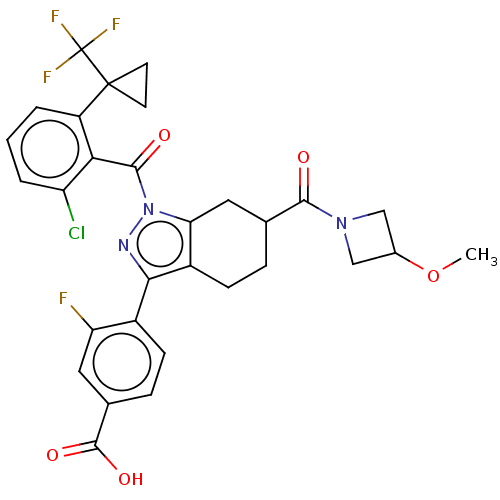 (4-{(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359586 (4-{(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359565 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359578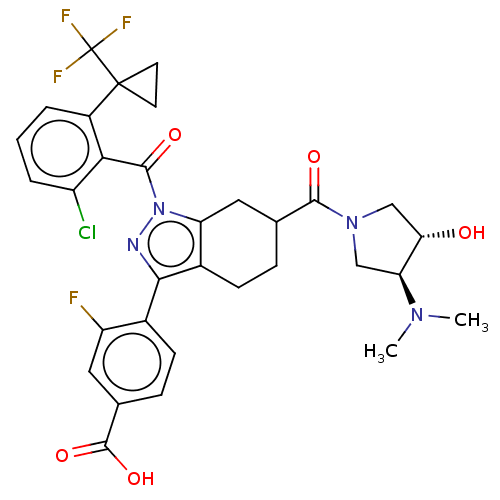 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359584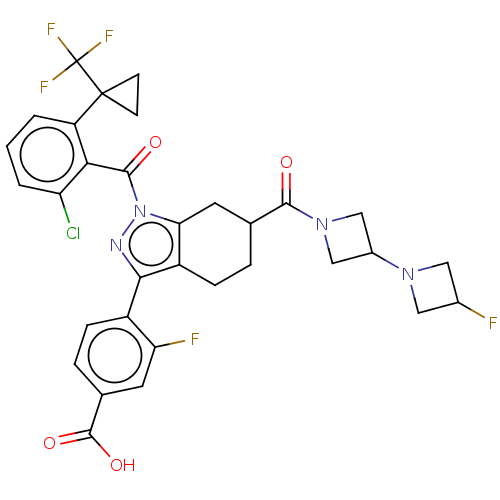 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359541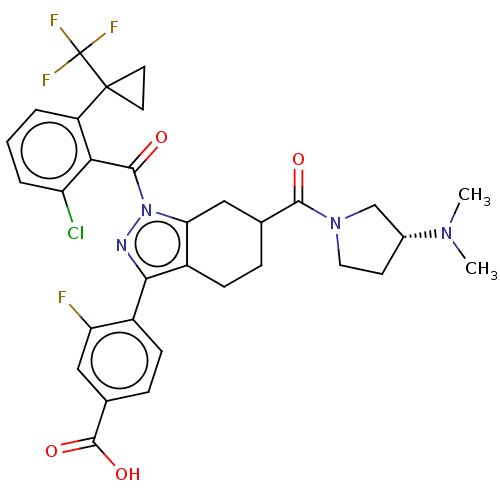 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359574 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359564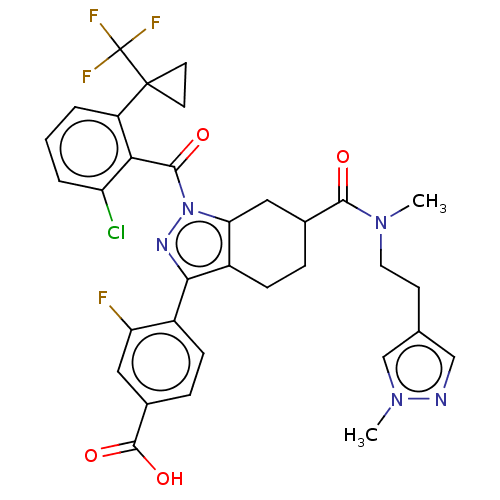 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359541 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359552 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359555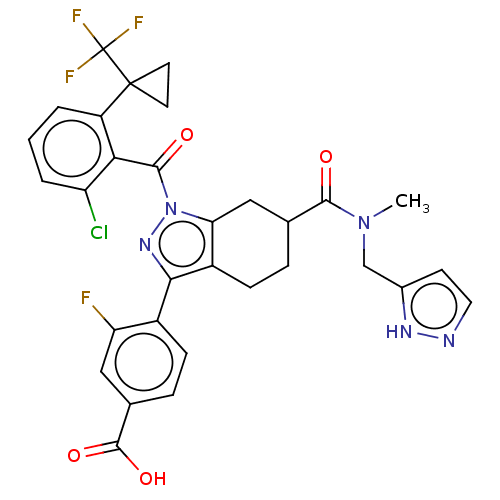 ((R or S)-4-(6-(((1H- pyrazol-5- yl)methyl)(methyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359510 (4-(1-(2-chloro-6- cyclopropylbenzoyl)- 6-(3- metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359556 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359559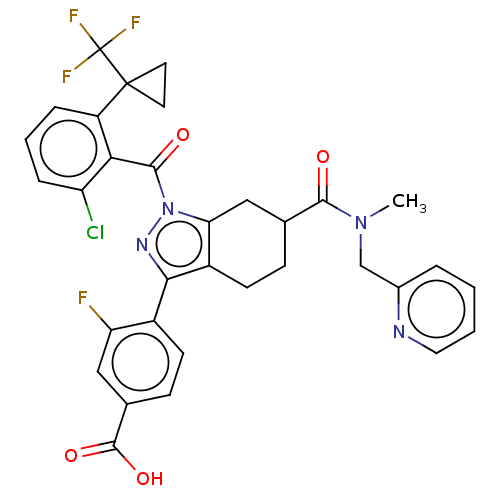 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359579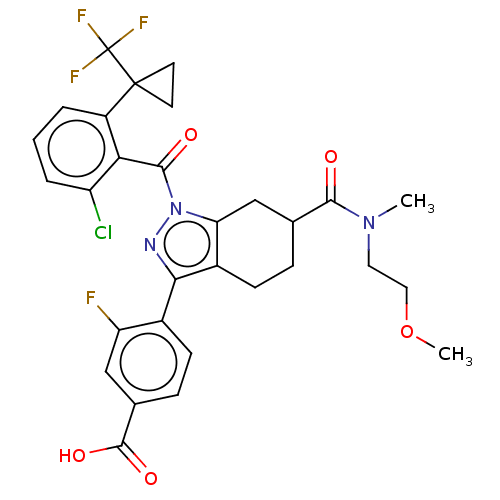 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359557 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359585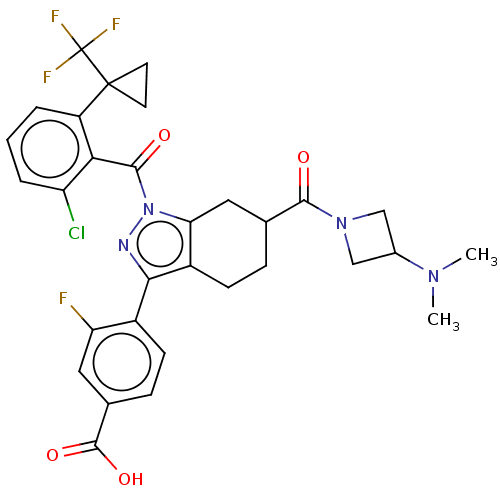 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359558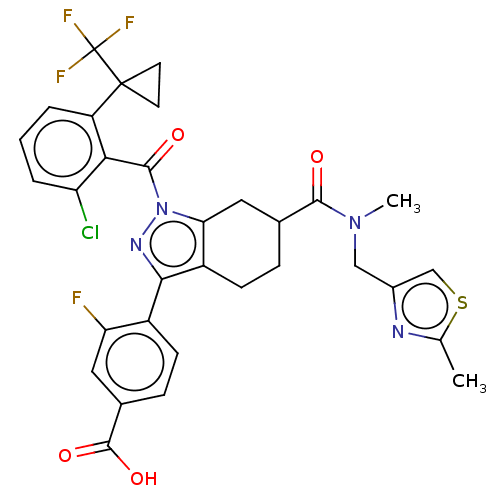 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359572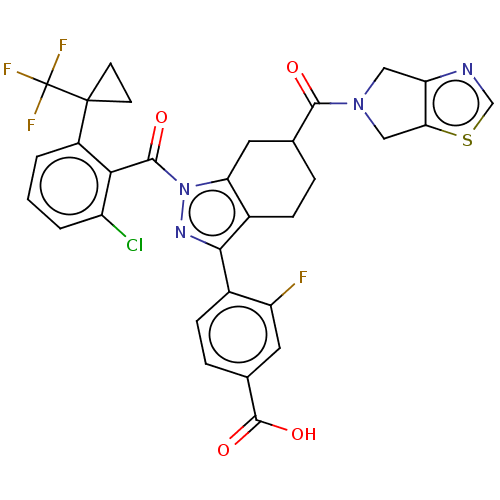 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359573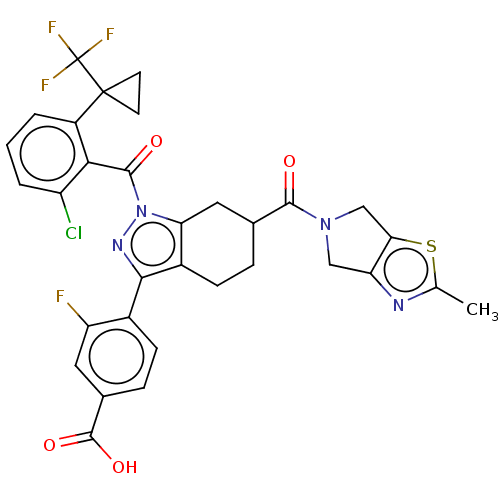 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359587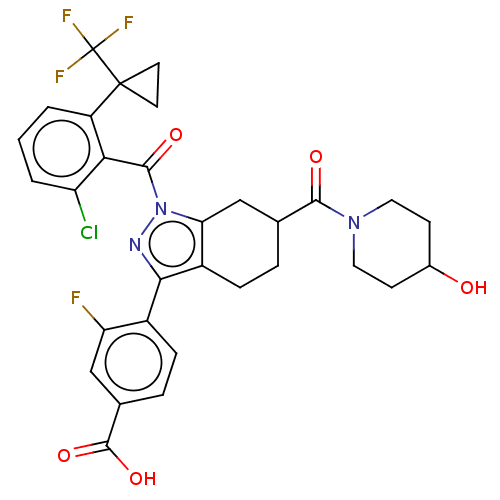 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359576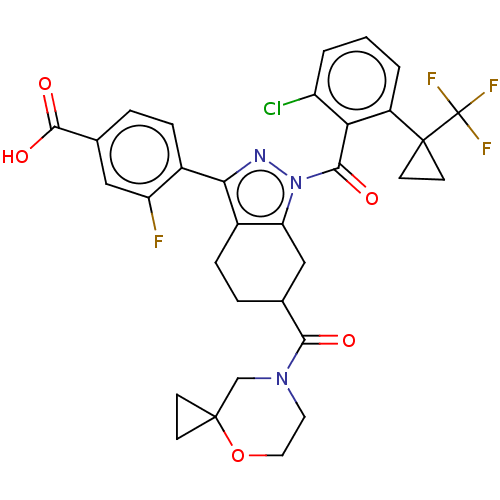 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359537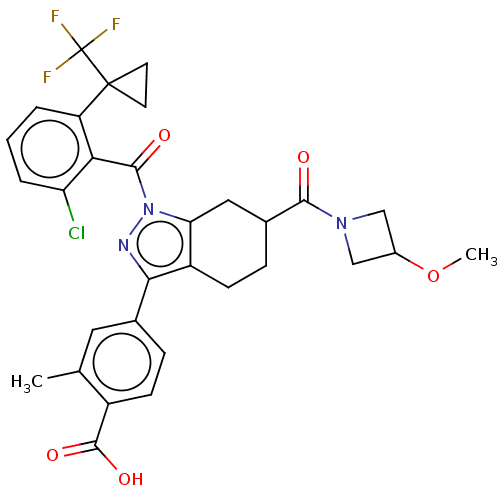 (4-{(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359538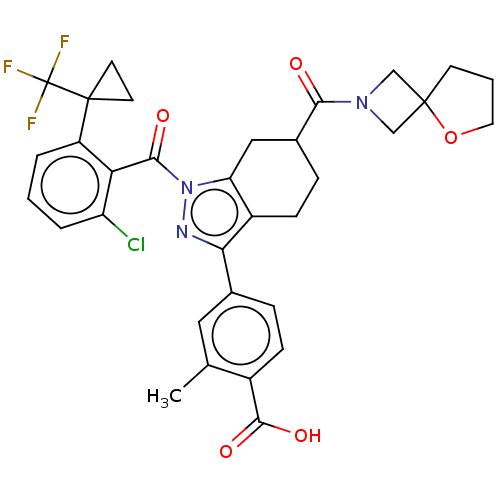 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359601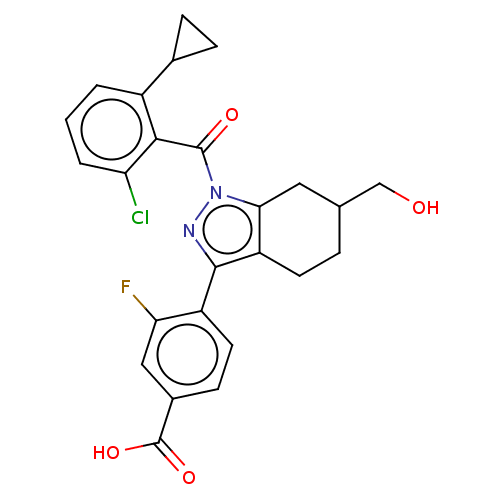 (US10221142, Example 32A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359546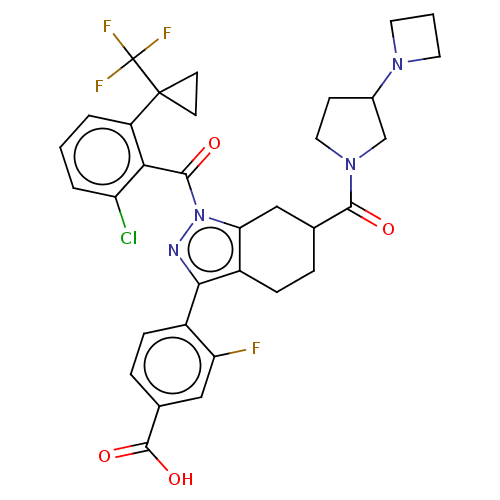 (4-[(6R or S)-6-[(3- azetidin-1- yl)pyrrolidin-1- y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359588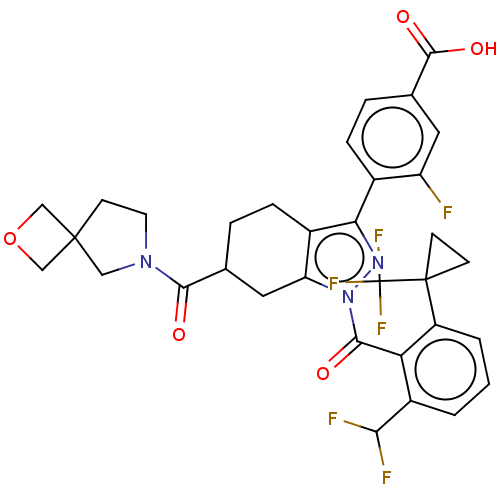 ((R or S)-4-(1-(2- (difluoromethyl)-6- (1- (trifluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359575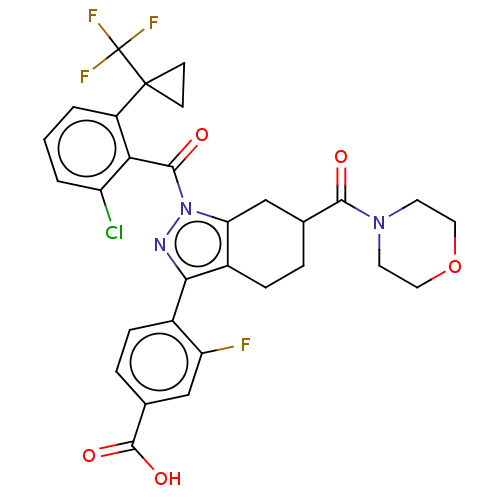 (4-[(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359563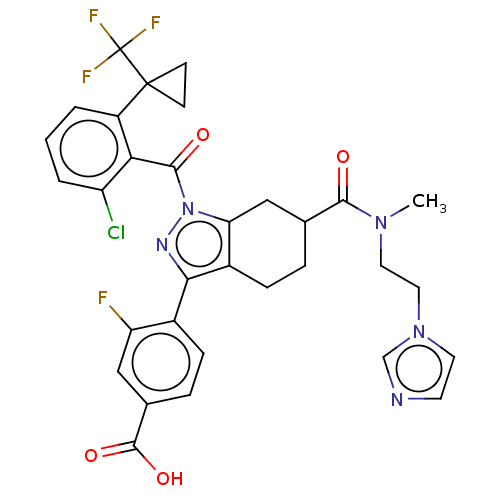 ((R or S)-4-(6-((2- (1H-imidazol-1- yl)ethyl)(methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359560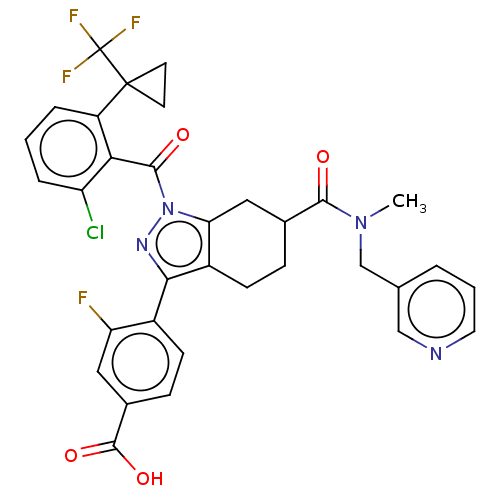 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359536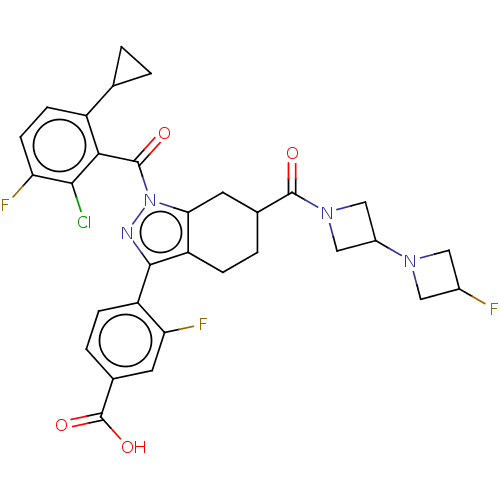 ((R or S)-4-(1-(2- chloro-6-cyclopropyl- 3-fluorobe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359506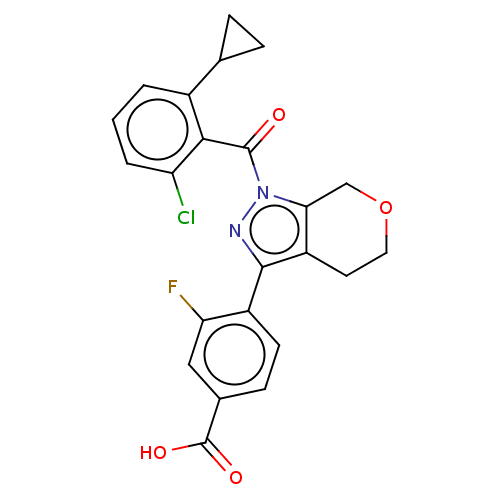 (US10221142, Example 9A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359562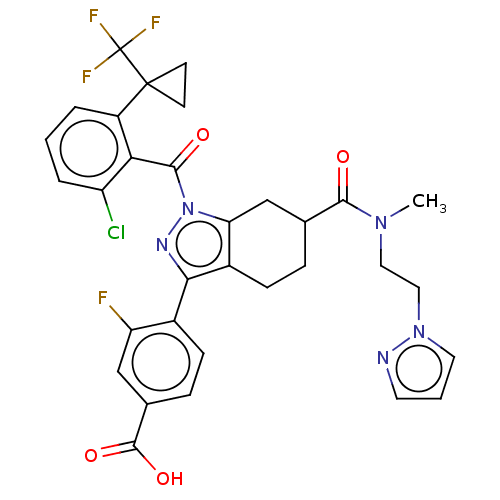 ((R or S)-4-(6-((2- (1H-pyrazol-1- yl)ethyl)(methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359501 (US10221142, Example 6A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359554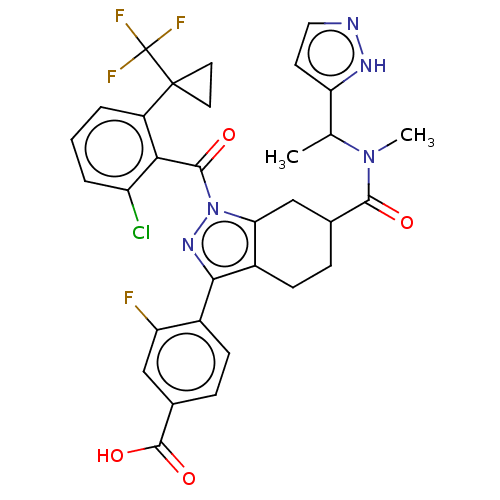 (4-((6R or S)-6-((1- (1H-pyrazol-5- yl)ethyl)(methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359487 (4-(1-(2-chloro-6- cyclopropylbenzoyl)-4,5,6,7- tet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359561 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359607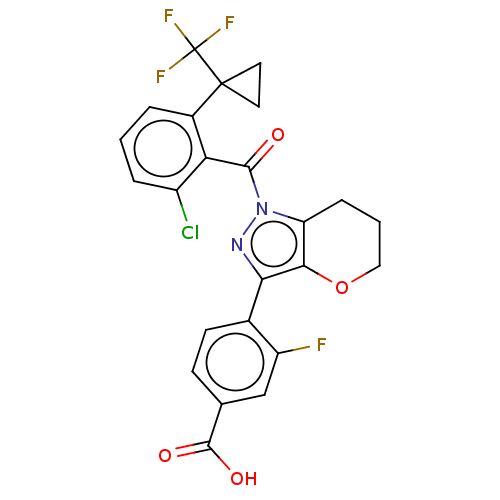 (4-[1-({2-chloro-6-[1- (trifluoromethyl)cyclo propy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359497 (US10221142, Example 2A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359602 (US10221142, Example 33A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359488 (4-(1-(2-chloro-6- cyclopropylbenzoyl)-4,5,6,7- tet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359600 (US10221142, Example 31A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359502 (4-(6-(azetidine-1-carbonyl)-1- (2-chloro-6- cyclop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359495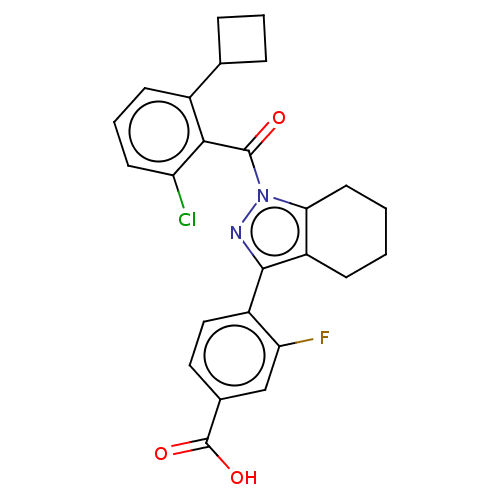 (4-(1-(2-chloro-6- cyclobutylbenzoyl)-4,5,6,7- tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359549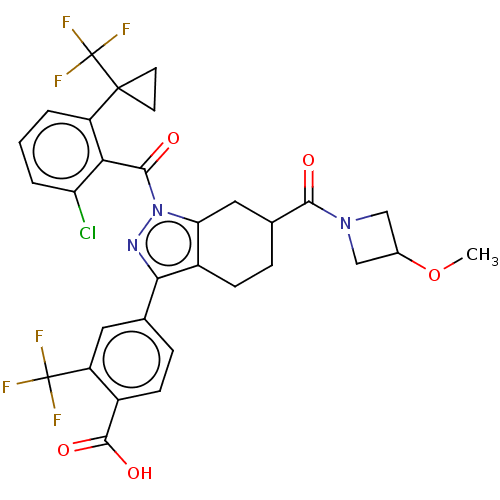 (4-{(6R or S)-1-({2- chloro-6-[1- (trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359606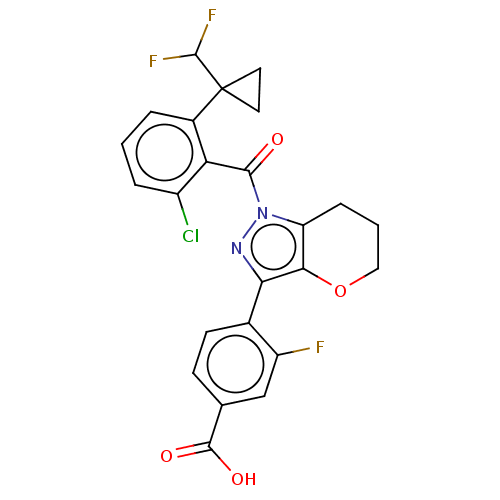 (US10221142, Example 37A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359581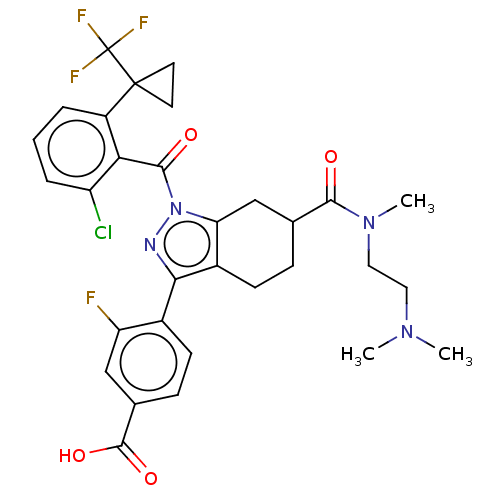 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359507 (4-[1-({2- chloro-6-[1- (trifluoro- methyl) cyclopr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359489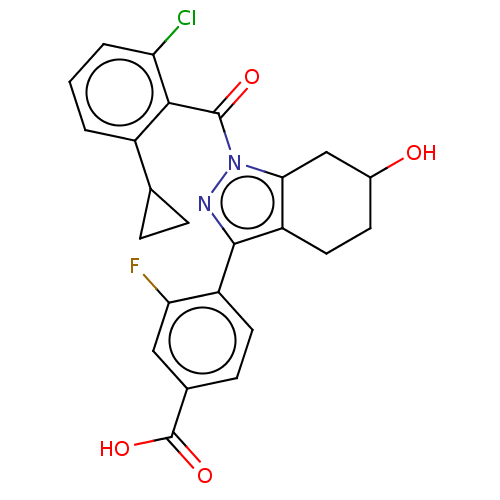 (4-(1-(2-chloro-6- cyclopropylbenzoyl)-6- hydroxy-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359512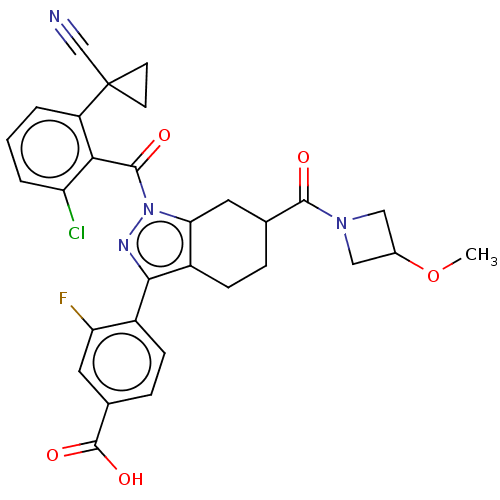 (4-(1-(2-chloro-6-(1- cyanocyclopropyl) benzoyl)-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359484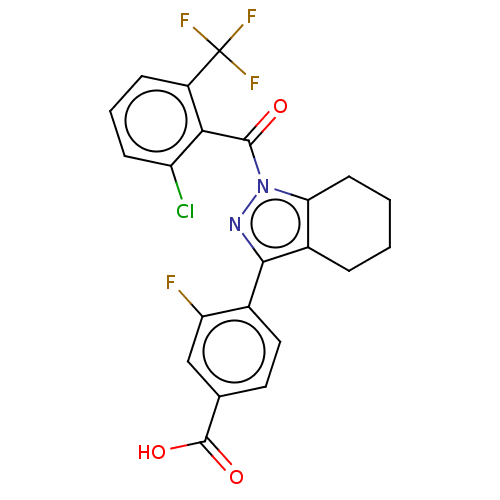 (US10221142, Example 1A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359516 (4-(1-(2-chloro-6-(1- cyanocyclobutyl) benzoyl)-6-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359604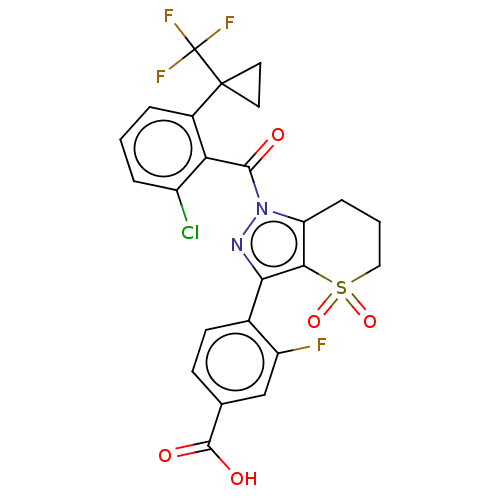 (US10221142, Example 35A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359580 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359598 (US10221142, Example 29A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359518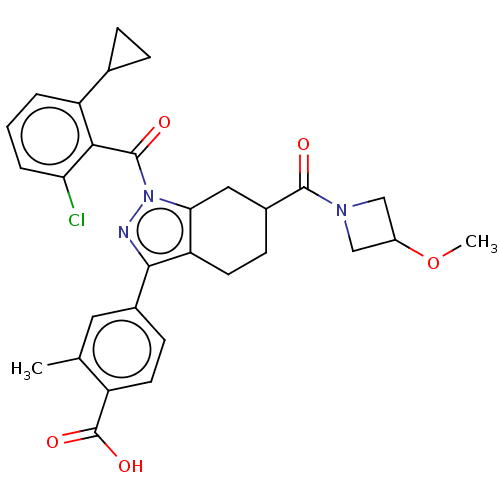 (4-(1-(2-chloro-6- cyclopropylbenzoyl)- 6-(3- metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359503 (4-(1-(2-chloro-6- cyclopropylbenzoyl)-6- (methylca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359595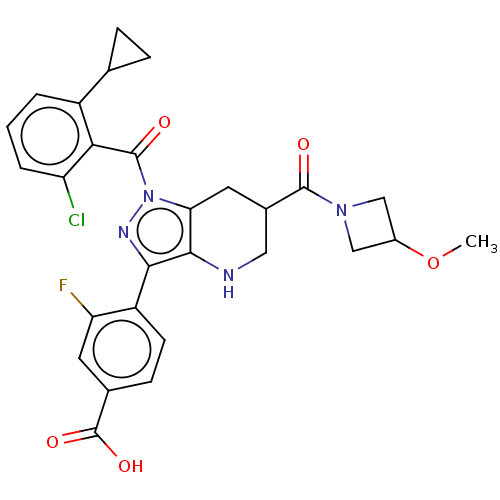 (US10221142, Example 27A | US10221142, Example 27B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359553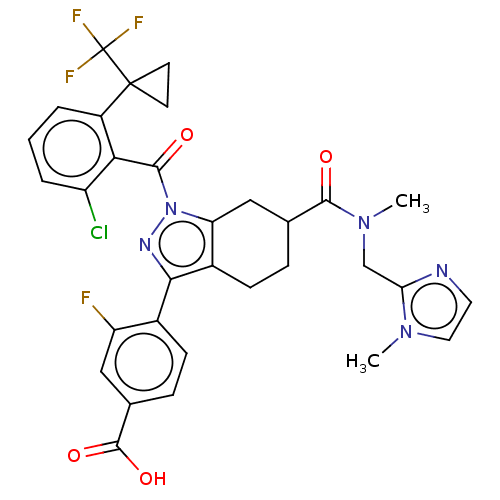 ((R or S)-4-(1-(2- chloro-6-(1- (trifluoromethyl)cy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359514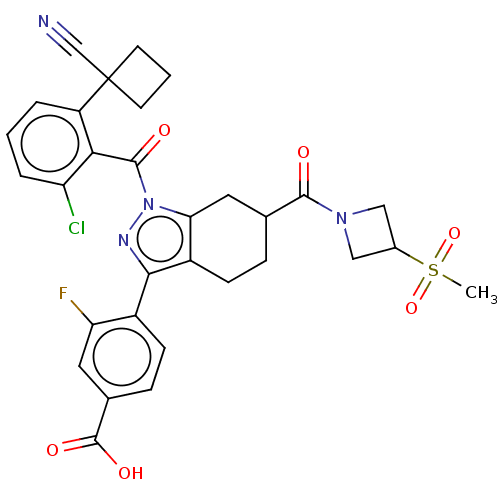 (4-(1-(2-chloro-6-(1- cyanocyclobutyl) benzoyl)-6-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359493 (4-(1-(2-chloro-6- cyclopropylbenzoyl)-6-oxo- 4,5,6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359491 (4-(1-(2-chloro-6- cyclopropylbenzoyl)-4-oxo- 4,5,6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359592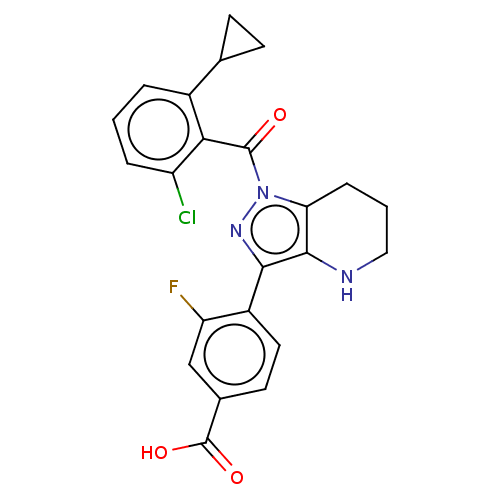 (US10221142, Example 25A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359496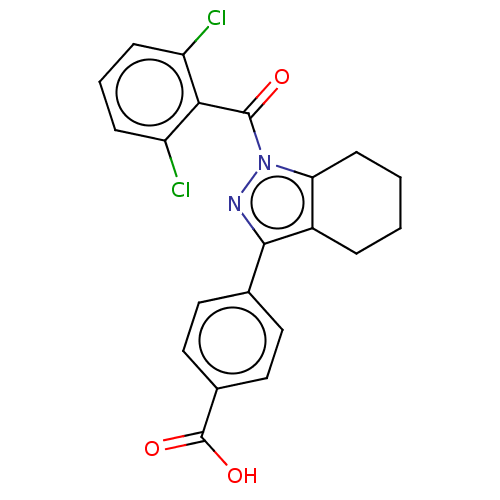 (4-(1-(2,6-dichlorobenzoyl)- 4,5,6,7-tetrahydro-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359523 (US10221142, Example 18A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359492 (4-(1-(2-chloro-6- cyclopropylbenzoyl)-6- methoxy-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359533 (US10221142, Example 20A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359593 (4-[1-({2-chloro-6-[1- (trifluoromethyl)cyclo- prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359485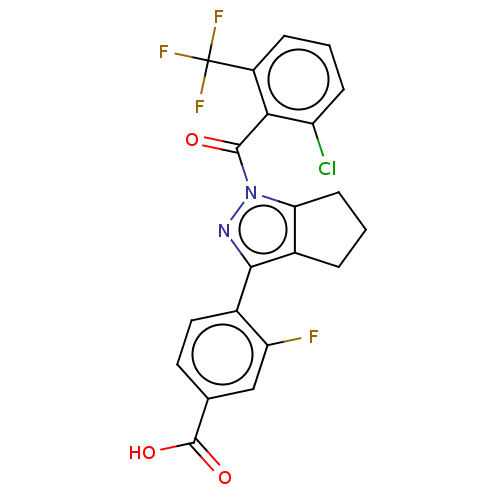 (4-(1-(2-chloro-6- (trifluoromethyl)benzoyl)- 1,4,5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359494 (4-(1-(2-chloro-6- cyclopropylbenzoyl)- 1,4,5,6,7,8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359526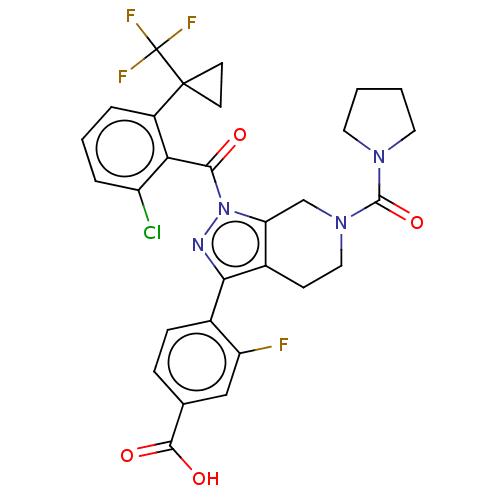 (US10221142, Example 18D) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359534 (4-{1-[(2-chloro-6- cyclopropylphenyl)car bonyl]-4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359583 ((R or S)-4-(1-(2- chloro-6- cyclopropylbenzoyl)- 6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359524 (US10221142, Example 18B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359499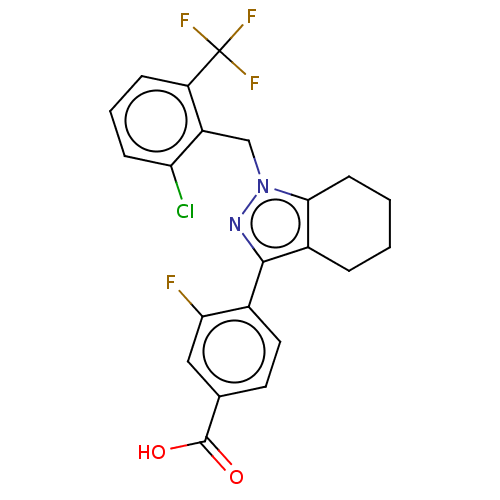 (US10221142, Example 4A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 56.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359594 (US10221142, Example 26A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359590 (US10221142, Example 23A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359603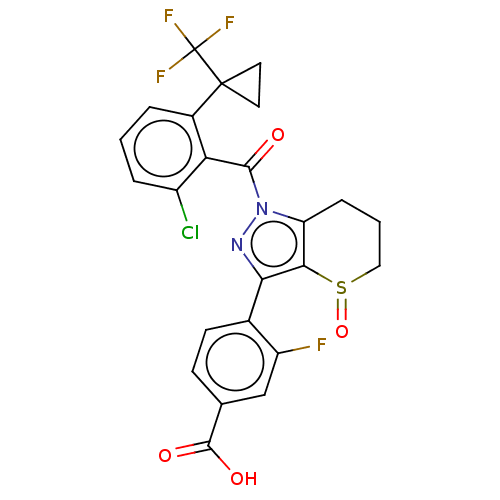 (US10221142, Example 34A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359599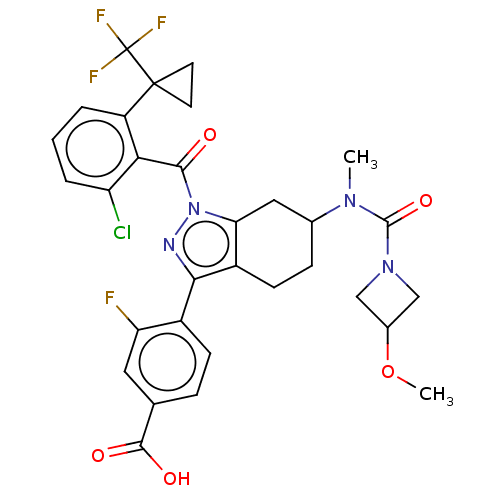 (US10221142, Example 30A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359527 (US10221142, Example 18E) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359505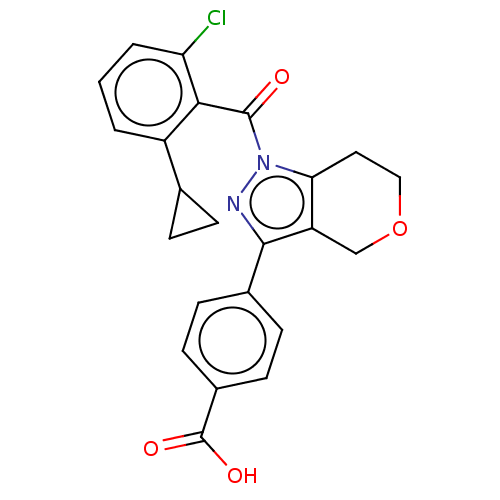 (US10221142, Example 8A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359510 (4-(1-(2-chloro-6- cyclopropylbenzoyl)- 6-(3- metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359532 (US10221142, Example 19C) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359595 (US10221142, Example 27A | US10221142, Example 27B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359500 (US10221142, Example 5A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359528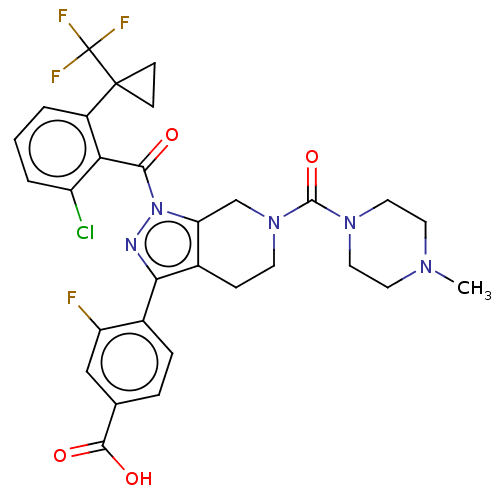 (US10221142, Example 18F) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359591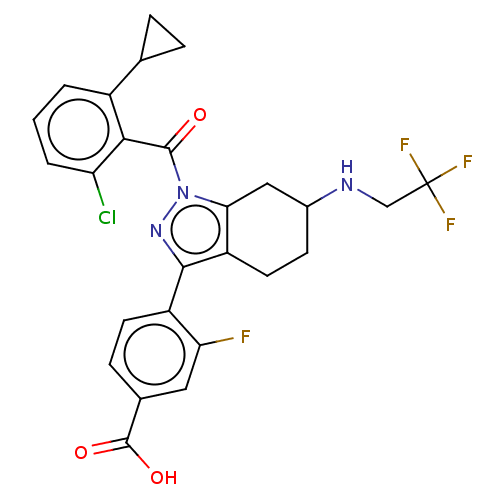 (US10221142, Example 24A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359597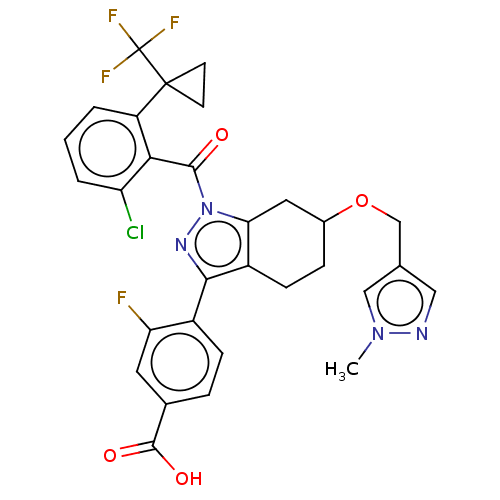 (US10221142, Example 28A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359519 (US10221142, Example 17A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359509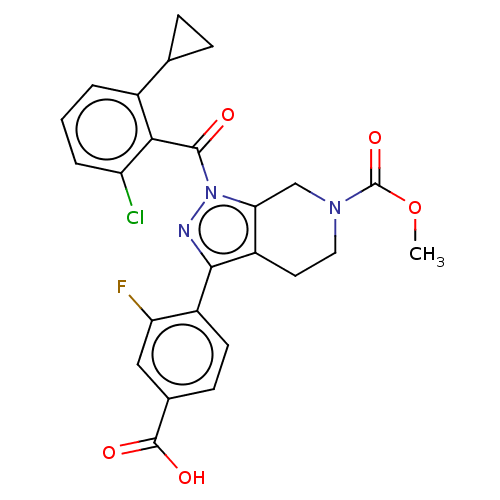 (US10221142, Example 11A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359530 (US10221142, Example 19A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359522 (US10221142, Example 17D) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 235 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359514 (4-(1-(2-chloro-6-(1- cyanocyclobutyl) benzoyl)-6-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 294 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359589 (US10221142, Example 22A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 332 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359525 (US10221142, Example 18C) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 606 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359508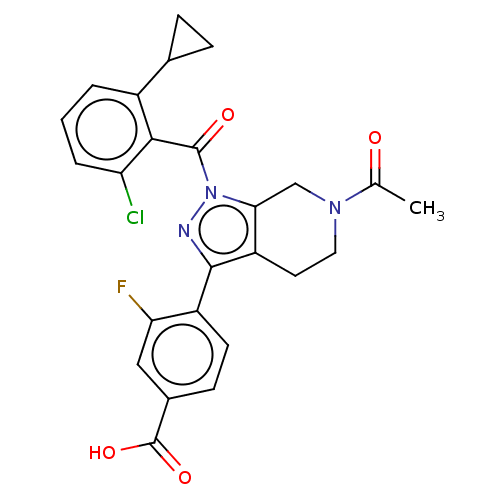 (US10221142, Example 10A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 622 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359516 (4-(1-(2-chloro-6-(1- cyanocyclobutyl) benzoyl)-6-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 625 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359521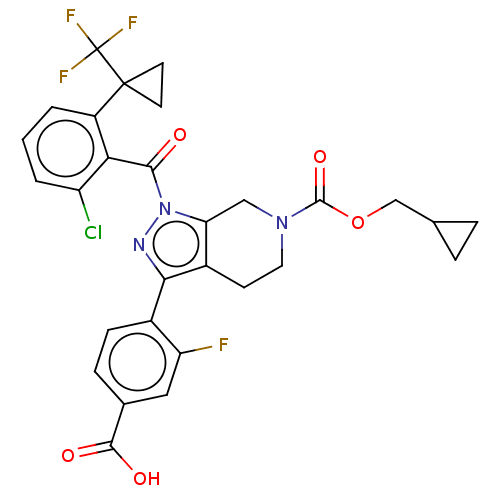 (US10221142, Example 17C) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 642 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359512 (4-(1-(2-chloro-6-(1- cyanocyclopropyl) benzoyl)-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 642 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359498 (US10221142, Example 3A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 728 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359531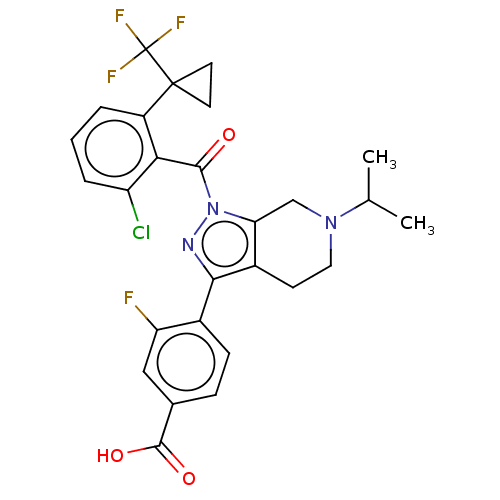 (US10221142, Example 19B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 773 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359529 (US10221142, Example 18G) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 814 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359520 (US10221142, Example 17B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359504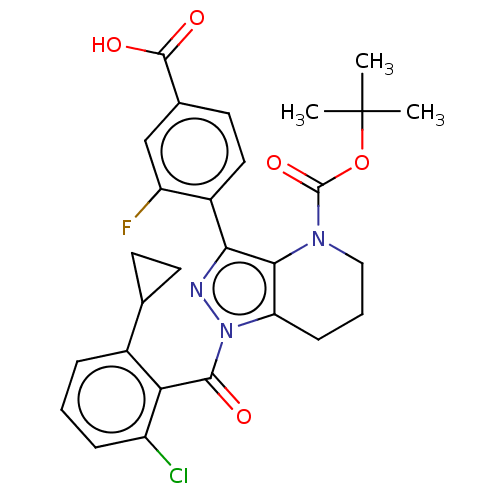 (US10221142, Example 7A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359605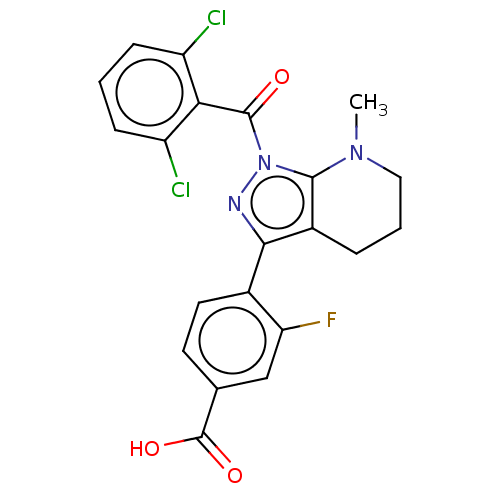 (US10221142, Example 36A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359490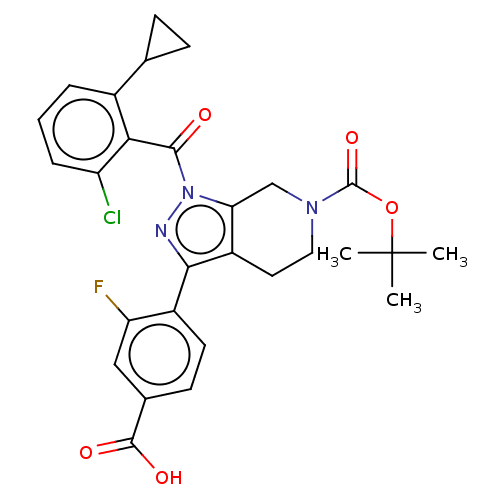 (4-(6-(tert-butoxycarbonyl)-1-(2- chloro-6-cyclopro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM359486 (4-(6-(tert-butoxycarbonyl)-1-(2- chloro-6- (triflu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The compounds of the invention inhibit RORgammaT activity. Activation of RORgammaT activity can be measured using, e.g., biochemical TR-FRET assay. I... | US Patent US10221142 (2019) BindingDB Entry DOI: 10.7270/Q2M90BZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||