 Found 194 hits with Last Name = 'broom' and Initial = 'ad'
Found 194 hits with Last Name = 'broom' and Initial = 'ad' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the alpha-Adrenoceptor pA2 blocking activity in vitro in rabbit thoracic aorta |
J Med Chem 30: 675-8 (1987)
BindingDB Entry DOI: 10.7270/Q2G44QW6 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50022736
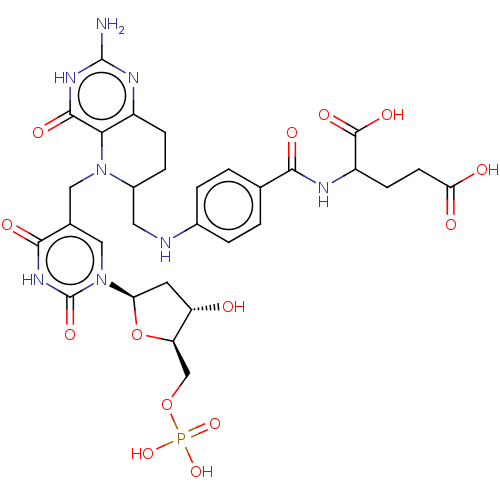
(2-[4-({2-Amino-5-[1-(4-hydroxy-5-phosphonooxymethy...)Show SMILES Nc1nc(O)c2N(Cc3cn([C@H]4C[C@H](O)[C@@H](COP(O)(O)=O)O4)c(=O)[nH]c3=O)C(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)CCc2n1 |r| Show InChI InChI=1S/C30H37N8O14P/c31-29-34-18-6-5-17(10-32-16-3-1-14(2-4-16)25(42)33-19(28(45)46)7-8-23(40)41)37(24(18)27(44)35-29)11-15-12-38(30(47)36-26(15)43)22-9-20(39)21(52-22)13-51-53(48,49)50/h1-4,12,17,19-22,32,39H,5-11,13H2,(H,33,42)(H,40,41)(H,45,46)(H,36,43,47)(H2,48,49,50)(H3,31,34,35,44)/t17?,19?,20-,21+,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase at 600 uM concentration of 5,10-CH2-H4PteGlu |
J Med Chem 31: 2126-32 (1988)
BindingDB Entry DOI: 10.7270/Q26H4J0S |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50227822
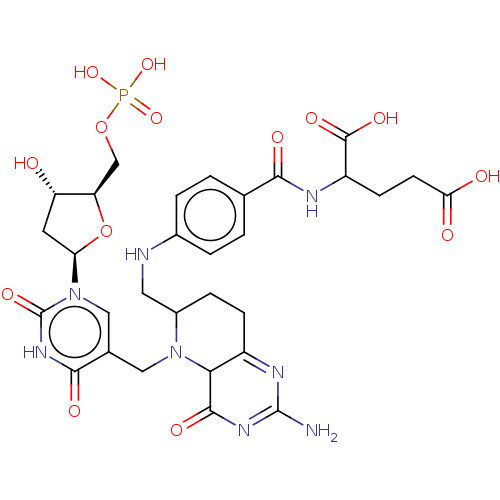
(CHEMBL3392216)Show SMILES NC1=NC(=O)C2N(Cc3cn([C@H]4C[C@H](O)[C@@H](COP(O)(O)=O)O4)c(=O)[nH]c3=O)C(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)CCC2=N1 |r,c:55,t:1| Show InChI InChI=1S/C30H37N8O14P/c31-29-34-18-6-5-17(10-32-16-3-1-14(2-4-16)25(42)33-19(28(45)46)7-8-23(40)41)37(24(18)27(44)35-29)11-15-12-38(30(47)36-26(15)43)22-9-20(39)21(52-22)13-51-53(48,49)50/h1-4,12,17,19-22,24,32,39H,5-11,13H2,(H,33,42)(H,40,41)(H,45,46)(H2,31,35,44)(H,36,43,47)(H2,48,49,50)/t17?,19?,20-,21+,22+,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of compound against thymidylate synthetase |
J Med Chem 32: 2-7 (1989)
BindingDB Entry DOI: 10.7270/Q2BV7H6W |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50022737
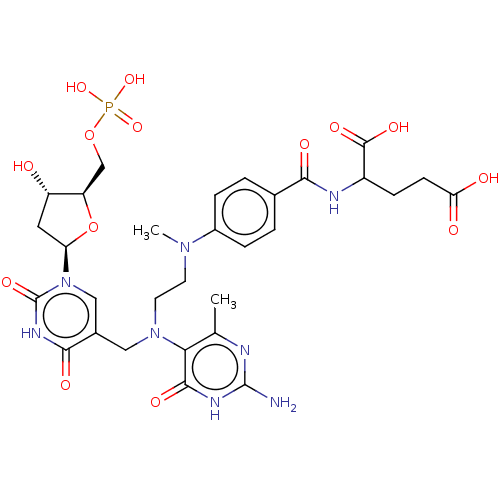
(2-{4-[(2-{(2-Amino-4-methyl-6-oxo-1,6-dihydro-pyri...)Show SMILES CN(CCN(Cc1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O)c1c(C)nc(N)nc1O)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C30H39N8O14P/c1-15-24(27(44)34-29(31)32-15)37(10-9-36(2)18-5-3-16(4-6-18)25(42)33-19(28(45)46)7-8-23(40)41)12-17-13-38(30(47)35-26(17)43)22-11-20(39)21(52-22)14-51-53(48,49)50/h3-6,13,19-22,39H,7-12,14H2,1-2H3,(H,33,42)(H,40,41)(H,45,46)(H,35,43,47)(H2,48,49,50)(H3,31,32,34,44)/t19?,20-,21+,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase at 600 uM concentration of 5,10-CH2-H4PteGlu |
J Med Chem 31: 2126-32 (1988)
BindingDB Entry DOI: 10.7270/Q26H4J0S |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50028107
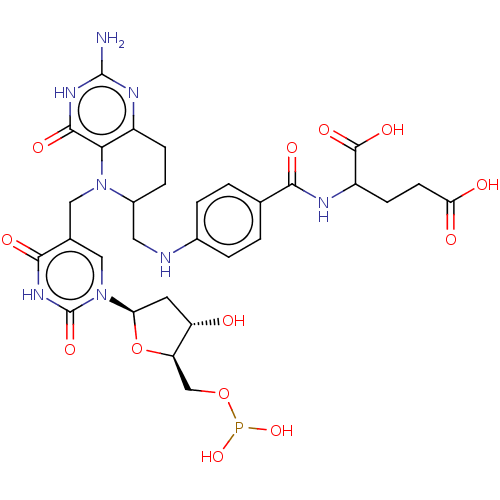
(2-{4-[(2-Amino-5-{1-[5-(dihydroxy-phosphanyloxymet...)Show SMILES Nc1nc2CCC(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)N(Cc3cn([C@H]4C[C@H](O)[C@@H](COP(O)O)O4)c(=O)[nH]c3=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C30H35N8O13P/c31-29-34-18-6-5-17(10-32-16-3-1-14(2-4-16)25(42)33-19(28(45)46)7-8-23(40)41)37(24(18)27(44)35-29)11-15-12-38(30(47)36-26(15)43)22-9-20(39)21(51-22)13-50-52(48)49/h1-4,12,17,19-22,32,39H,5-11,13H2,(H,33,42)(H,40,41)(H,45,46)(H,36,43,47)(H3,31,34,35,44)/q-2/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) |
J Med Chem 27: 1710-7 (1985)
BindingDB Entry DOI: 10.7270/Q2N87BBJ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50017764

(CHEMBL3392217 | Phosphoric acid mono-{3-hydroxy-5-...)Show SMILES CN1CCN(Cc2cn([C@H]3C[C@H](O)[C@@H](COP(O)(O)=O)O3)c(=O)[nH]c2=O)c2ccccc12 |r| Show InChI InChI=1S/C19H25N4O8P/c1-21-6-7-22(14-5-3-2-4-13(14)21)9-12-10-23(19(26)20-18(12)25)17-8-15(24)16(31-17)11-30-32(27,28)29/h2-5,10,15-17,24H,6-9,11H2,1H3,(H,20,25,26)(H2,27,28,29)/p-2/t15-,16-,17?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of compound against thymidylate synthetase |
J Med Chem 32: 2-7 (1989)
BindingDB Entry DOI: 10.7270/Q2BV7H6W |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50022736

(2-[4-({2-Amino-5-[1-(4-hydroxy-5-phosphonooxymethy...)Show SMILES Nc1nc(O)c2N(Cc3cn([C@H]4C[C@H](O)[C@@H](COP(O)(O)=O)O4)c(=O)[nH]c3=O)C(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)CCc2n1 |r| Show InChI InChI=1S/C30H37N8O14P/c31-29-34-18-6-5-17(10-32-16-3-1-14(2-4-16)25(42)33-19(28(45)46)7-8-23(40)41)37(24(18)27(44)35-29)11-15-12-38(30(47)36-26(15)43)22-9-20(39)21(52-22)13-51-53(48,49)50/h1-4,12,17,19-22,32,39H,5-11,13H2,(H,33,42)(H,40,41)(H,45,46)(H,36,43,47)(H2,48,49,50)(H3,31,34,35,44)/t17?,19?,20-,21+,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Competitive inhibition of the human thymidylate synthase at 600 uM of [dUMP] |
J Med Chem 31: 2126-32 (1988)
BindingDB Entry DOI: 10.7270/Q26H4J0S |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50022737

(2-{4-[(2-{(2-Amino-4-methyl-6-oxo-1,6-dihydro-pyri...)Show SMILES CN(CCN(Cc1cn([C@H]2C[C@H](O)[C@@H](COP(O)(O)=O)O2)c(=O)[nH]c1=O)c1c(C)nc(N)nc1O)c1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C30H39N8O14P/c1-15-24(27(44)34-29(31)32-15)37(10-9-36(2)18-5-3-16(4-6-18)25(42)33-19(28(45)46)7-8-23(40)41)12-17-13-38(30(47)35-26(17)43)22-11-20(39)21(52-22)14-51-53(48,49)50/h3-6,13,19-22,39H,7-12,14H2,1-2H3,(H,33,42)(H,40,41)(H,45,46)(H,35,43,47)(H2,48,49,50)(H3,31,32,34,44)/t19?,20-,21+,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Competitive inhibition of the human thymidylate synthase at 28 uM as Ki(slope) of 5,10-CH2-H4PteGlu |
J Med Chem 31: 2126-32 (1988)
BindingDB Entry DOI: 10.7270/Q26H4J0S |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50028107

(2-{4-[(2-Amino-5-{1-[5-(dihydroxy-phosphanyloxymet...)Show SMILES Nc1nc2CCC(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)N(Cc3cn([C@H]4C[C@H](O)[C@@H](COP(O)O)O4)c(=O)[nH]c3=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C30H35N8O13P/c31-29-34-18-6-5-17(10-32-16-3-1-14(2-4-16)25(42)33-19(28(45)46)7-8-23(40)41)37(24(18)27(44)35-29)11-15-12-38(30(47)36-26(15)43)22-9-20(39)21(51-22)13-50-52(48)49/h1-4,12,17,19-22,32,39H,5-11,13H2,(H,33,42)(H,40,41)(H,45,46)(H,36,43,47)(H3,31,34,35,44)/q-2/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) |
J Med Chem 27: 1710-7 (1985)
BindingDB Entry DOI: 10.7270/Q2N87BBJ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50017762
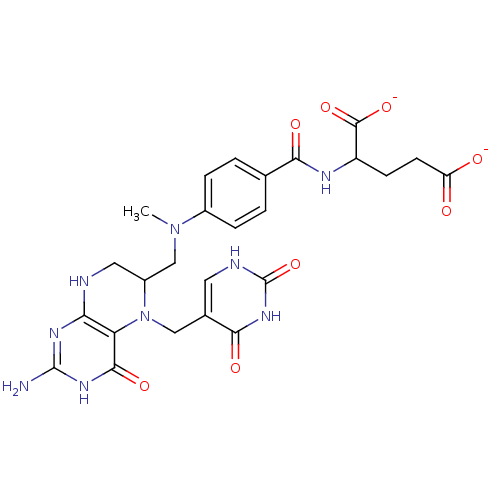
(2-(4-{[2-Amino-5-(2,4-dioxo-1,2,3,4-tetrahydro-pyr...)Show SMILES CN(CC1CNc2nc(N)[nH]c(=O)c2N1Cc1c[nH]c(=O)[nH]c1=O)c1ccc(cc1)C(=O)NC(CCC([O-])=O)C([O-])=O Show InChI InChI=1S/C25H29N9O8/c1-33(14-4-2-12(3-5-14)20(37)29-16(23(40)41)6-7-17(35)36)11-15-9-27-19-18(22(39)31-24(26)30-19)34(15)10-13-8-28-25(42)32-21(13)38/h2-5,8,15-16H,6-7,9-11H2,1H3,(H,29,37)(H,35,36)(H,40,41)(H2,28,32,38,42)(H4,26,27,30,31,39)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of compound against thymidylate synthetase |
J Med Chem 32: 2-7 (1989)
BindingDB Entry DOI: 10.7270/Q2BV7H6W |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against thymidylate synthase from murine leukemia L1210 |
J Med Chem 30: 675-8 (1987)
BindingDB Entry DOI: 10.7270/Q2G44QW6 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50023905
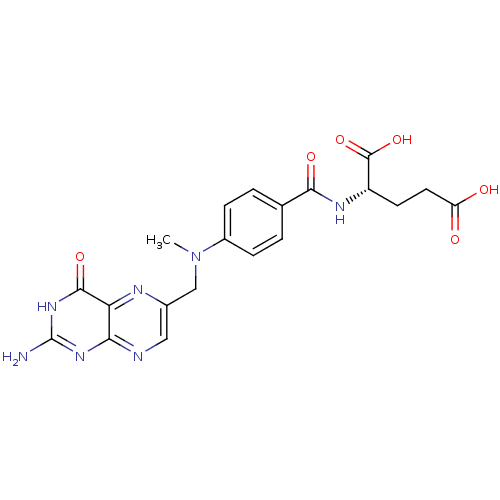
(2-{4-[(2-Amino-4-oxo-1,4-dihydro-pteridin-6-ylmeth...)Show SMILES CN(Cc1cnc2nc(N)[nH]c(=O)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C20H21N7O6/c1-27(9-11-8-22-16-15(23-11)18(31)26-20(21)25-16)12-4-2-10(3-5-12)17(30)24-13(19(32)33)6-7-14(28)29/h2-5,8,13H,6-7,9H2,1H3,(H,24,30)(H,28,29)(H,32,33)(H3,21,22,25,26,31)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant of thymidylate synthase was determined in human AML cells |
J Med Chem 30: 675-8 (1987)
BindingDB Entry DOI: 10.7270/Q2G44QW6 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50028108
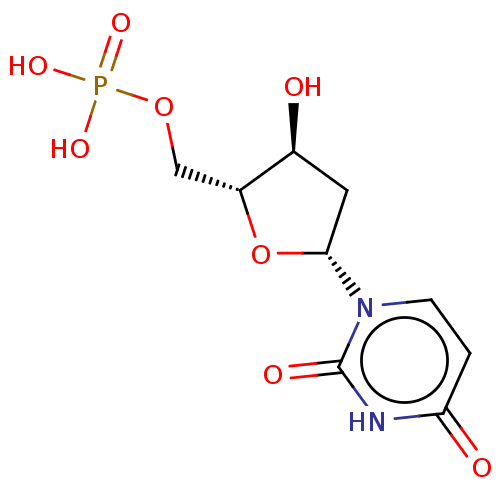
(2'-Deoxyuridinemonophosphate | DEOXYURIDINE MONOPH...)Show SMILES O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C9H13N2O8P/c12-5-3-8(11-2-1-7(13)10-9(11)14)19-6(5)4-18-20(15,16)17/h1-2,5-6,8,12H,3-4H2,(H,10,13,14)(H2,15,16,17)/t5-,6+,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) |
J Med Chem 27: 1710-7 (1985)
BindingDB Entry DOI: 10.7270/Q2N87BBJ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Indolethylamine N-methyltransferase
(Homo sapiens (Human)) | BDBM50367839

(CHEMBL2368635)Show SMILES [I-].C[S+](CCCCc1c[nH]c2ccccc12)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C23H29N6O3S.HI/c1-33(9-5-4-6-14-10-25-16-8-3-2-7-15(14)16)11-17-19(30)20(31)23(32-17)29-13-28-18-21(24)26-12-27-22(18)29;/h2-3,7-8,10,12-13,17,19-20,23,25,30-31H,4-6,9,11H2,1H3,(H2,24,26,27);1H/q+1;/p-1/t17-,19-,20-,23-,33?;/m1./s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of compound against indole N-methyl transferase |
J Med Chem 32: 2-7 (1989)
BindingDB Entry DOI: 10.7270/Q2BV7H6W |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human enzyme thymidylate synthase derived from either HeLa or KB cells |
J Med Chem 30: 675-8 (1987)
BindingDB Entry DOI: 10.7270/Q2G44QW6 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50367343
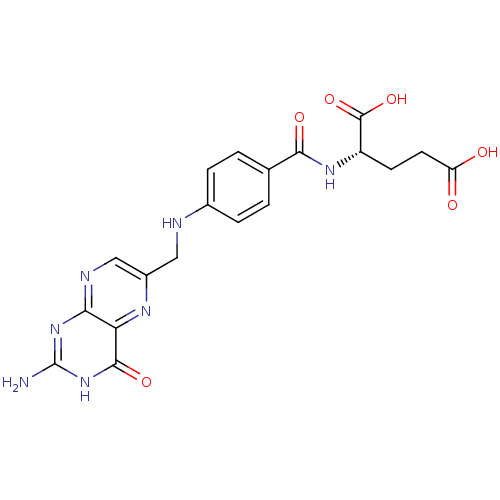
((2S)-2-[[4-[(2-amino-4-oxo-1H-pteridin-6-yl)methyl...)Show SMILES Nc1nc2ncc(CNc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)nc2c(=O)[nH]1 |r| Show InChI InChI=1S/C19H19N7O6/c20-19-25-15-14(17(30)26-19)23-11(8-22-15)7-21-10-3-1-9(2-4-10)16(29)24-12(18(31)32)5-6-13(27)28/h1-4,8,12,21H,5-7H2,(H,24,29)(H,27,28)(H,31,32)(H3,20,22,25,26,30)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory constant of thymidylate synthase was determined in human AML cells |
J Med Chem 30: 675-8 (1987)
BindingDB Entry DOI: 10.7270/Q2G44QW6 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50227823

(CHEMBL3392214)Show SMILES Nc1nc(O)c2cc(CSC[C@H]3O[C@H]([C@H](O)[C@@H]3O)n3cnc4c(N)ncnc34)[nH]c2n1 |r| Show InChI InChI=1S/C17H19N9O4S/c18-12-9-14(21-4-20-12)26(5-22-9)16-11(28)10(27)8(30-16)3-31-2-6-1-7-13(23-6)24-17(19)25-15(7)29/h1,4-5,8,10-11,16,27-28H,2-3H2,(H2,18,20,21)(H4,19,23,24,25,29)/t8-,10-,11-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of compound against Guanine-7-methyl transferase |
J Med Chem 32: 2-7 (1989)
BindingDB Entry DOI: 10.7270/Q2BV7H6W |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50028109
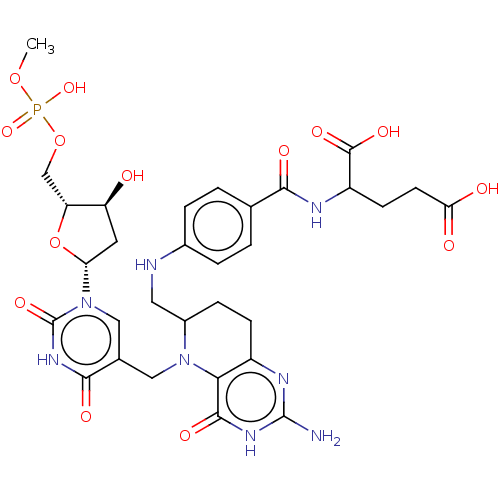
(2-{4-[(2-Amino-5-{1-[4-hydroxy-5-(hydroxy-methoxy-...)Show SMILES COP(O)(=O)OC[C@H]1O[C@H](C[C@@H]1O)n1cc(CN2C(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)CCc3nc(N)[nH]c(=O)c23)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C31H39N8O14P/c1-51-54(49,50)52-14-22-21(40)10-23(53-22)39-13-16(27(44)37-31(39)48)12-38-18(6-7-19-25(38)28(45)36-30(32)35-19)11-33-17-4-2-15(3-5-17)26(43)34-20(29(46)47)8-9-24(41)42/h2-5,13,18,20-23,33,40H,6-12,14H2,1H3,(H,34,43)(H,41,42)(H,46,47)(H,49,50)(H,37,44,48)(H3,32,35,36,45)/p-3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) |
J Med Chem 27: 1710-7 (1985)
BindingDB Entry DOI: 10.7270/Q2N87BBJ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
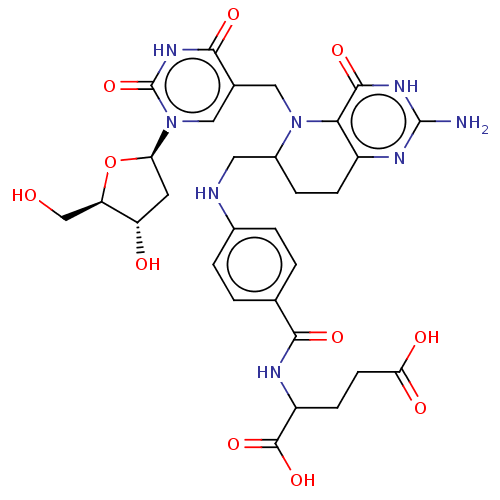
(Homo sapiens (Human)) | BDBM50028110
(2-[4-({2-Amino-5-[1-(4-hydroxy-5-hydroxymethyl-tet...)Show SMILES Nc1nc2CCC(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)N(Cc3cn([C@H]4C[C@H](O)[C@@H](CO)O4)c(=O)[nH]c3=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C30H36N8O11/c31-29-34-18-6-5-17(10-32-16-3-1-14(2-4-16)25(43)33-19(28(46)47)7-8-23(41)42)37(24(18)27(45)35-29)11-15-12-38(30(48)36-26(15)44)22-9-20(40)21(13-39)49-22/h1-4,12,17,19-22,32,39-40H,5-11,13H2,(H,33,43)(H,41,42)(H,46,47)(H,36,44,48)(H3,31,34,35,45)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) |
J Med Chem 27: 1710-7 (1985)
BindingDB Entry DOI: 10.7270/Q2N87BBJ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
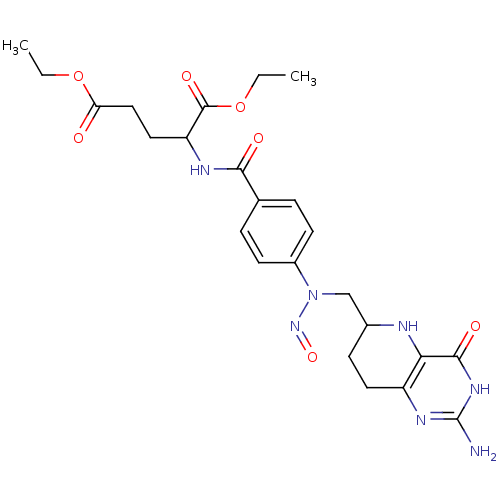
(Homo sapiens (Human)) | BDBM50028106
(CHEMBL46945 | diethyl 2-{4-[2-amino-4-oxo-3,4,5,6,...)Show SMILES CCOC(=O)CCC(NC(=O)c1ccc(cc1)N(CC1CCc2nc(N)[nH]c(=O)c2N1)N=O)C(=O)OCC Show InChI InChI=1S/C24H31N7O7/c1-3-37-19(32)12-11-18(23(35)38-4-2)27-21(33)14-5-8-16(9-6-14)31(30-36)13-15-7-10-17-20(26-15)22(34)29-24(25)28-17/h5-6,8-9,15,18,26H,3-4,7,10-13H2,1-2H3,(H,27,33)(H3,25,28,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidylate synthase with variable concentration of N5, 10-CH2-H4PteGlu and fixed dUMP (100 uM) |
J Med Chem 27: 1710-7 (1985)
BindingDB Entry DOI: 10.7270/Q2N87BBJ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50028106

(CHEMBL46945 | diethyl 2-{4-[2-amino-4-oxo-3,4,5,6,...)Show SMILES CCOC(=O)CCC(NC(=O)c1ccc(cc1)N(CC1CCc2nc(N)[nH]c(=O)c2N1)N=O)C(=O)OCC Show InChI InChI=1S/C24H31N7O7/c1-3-37-19(32)12-11-18(23(35)38-4-2)27-21(33)14-5-8-16(9-6-14)31(30-36)13-15-7-10-17-20(26-15)22(34)29-24(25)28-17/h5-6,8-9,15,18,26H,3-4,7,10-13H2,1-2H3,(H,27,33)(H3,25,28,29,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidylate synthase with variable concentration of N5, 10-CH2-H4PteGlu and fixed dUMP (100 uM) |
J Med Chem 27: 1710-7 (1985)
BindingDB Entry DOI: 10.7270/Q2N87BBJ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50028110

(2-[4-({2-Amino-5-[1-(4-hydroxy-5-hydroxymethyl-tet...)Show SMILES Nc1nc2CCC(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)N(Cc3cn([C@H]4C[C@H](O)[C@@H](CO)O4)c(=O)[nH]c3=O)c2c(=O)[nH]1 |r| Show InChI InChI=1S/C30H36N8O11/c31-29-34-18-6-5-17(10-32-16-3-1-14(2-4-16)25(43)33-19(28(46)47)7-8-23(41)42)37(24(18)27(45)35-29)11-15-12-38(30(48)36-26(15)44)22-9-20(40)21(13-39)49-22/h1-4,12,17,19-22,32,39-40H,5-11,13H2,(H,33,43)(H,41,42)(H,46,47)(H,36,44,48)(H3,31,34,35,45)/p-2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) |
J Med Chem 27: 1710-7 (1985)
BindingDB Entry DOI: 10.7270/Q2N87BBJ |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50028109

(2-{4-[(2-Amino-5-{1-[4-hydroxy-5-(hydroxy-methoxy-...)Show SMILES COP(O)(=O)OC[C@H]1O[C@H](C[C@@H]1O)n1cc(CN2C(CNc3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)CCc3nc(N)[nH]c(=O)c23)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C31H39N8O14P/c1-51-54(49,50)52-14-22-21(40)10-23(53-22)39-13-16(27(44)37-31(39)48)12-38-18(6-7-19-25(38)28(45)36-30(32)35-19)11-33-17-4-2-15(3-5-17)26(43)34-20(29(46)47)8-9-24(41)42/h2-5,13,18,20-23,33,40H,6-12,14H2,1H3,(H,34,43)(H,41,42)(H,46,47)(H,49,50)(H,37,44,48)(H3,32,35,36,45)/p-3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidylate synthase with variable concentration of dUMP and fixed N5,10-CH2-H4PteGlu (200 uM) |
J Med Chem 27: 1710-7 (1985)
BindingDB Entry DOI: 10.7270/Q2N87BBJ |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50049575

(1-{4-[(2,4-Diamino-pyrido[3,2-d]pyrimidin-6-ylmeth...)Show InChI InChI=1S/C16H16N6O/c1-9(23)10-2-4-11(5-3-10)19-8-12-6-7-13-14(20-12)15(17)22-16(18)21-13/h2-7,19H,8H2,1H3,(H4,17,18,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
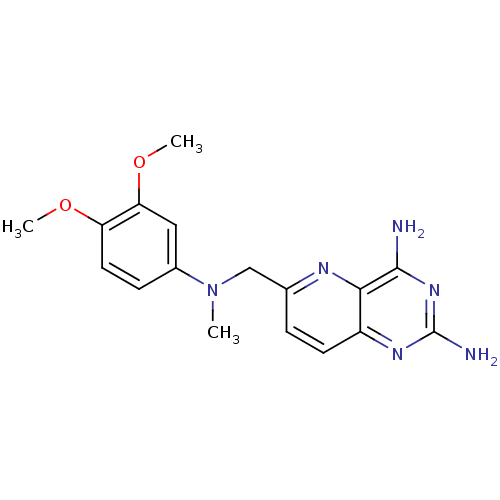
(Rattus norvegicus (rat)) | BDBM50049609
(6-{[(3,4-Dimethoxy-phenyl)-methyl-amino]-methyl}-p...)Show InChI InChI=1S/C17H20N6O2/c1-23(11-5-7-13(24-2)14(8-11)25-3)9-10-4-6-12-15(20-10)16(18)22-17(19)21-12/h4-8H,9H2,1-3H3,(H4,18,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50031867

(6-((methyl(3,4,5-trimethoxyphenyl)amino)methyl)pyr...)Show SMILES COc1cc(cc(OC)c1OC)N(C)Cc1ccc2nc(N)nc(N)c2n1 Show InChI InChI=1S/C18H22N6O3/c1-24(11-7-13(25-2)16(27-4)14(8-11)26-3)9-10-5-6-12-15(21-10)17(19)23-18(20)22-12/h5-8H,9H2,1-4H3,(H4,19,20,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50049587

(1-{4-[(2,4-Diamino-pyrido[3,2-d]pyrimidin-6-ylmeth...)Show InChI InChI=1S/C17H18N6O/c1-10(24)11-3-6-13(7-4-11)23(2)9-12-5-8-14-15(20-12)16(18)22-17(19)21-14/h3-8H,9H2,1-2H3,(H4,18,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50049583

(6-[(Methyl-naphthalen-2-yl-amino)-methyl]-pyrido[3...)Show InChI InChI=1S/C19H18N6/c1-25(15-8-6-12-4-2-3-5-13(12)10-15)11-14-7-9-16-17(22-14)18(20)24-19(21)23-16/h2-10H,11H2,1H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50049604
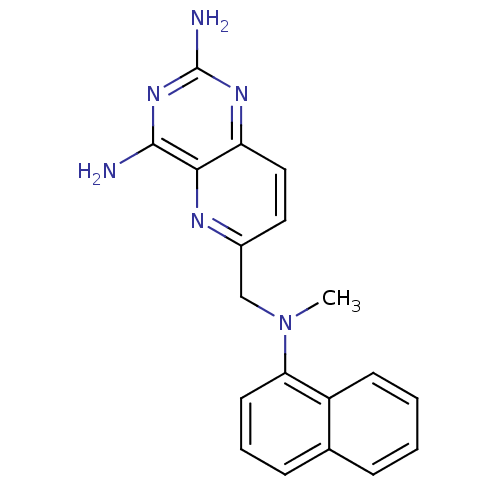
(6-[(Methyl-naphthalen-1-yl-amino)-methyl]-pyrido[3...)Show InChI InChI=1S/C19H18N6/c1-25(16-8-4-6-12-5-2-3-7-14(12)16)11-13-9-10-15-17(22-13)18(20)24-19(21)23-15/h2-10H,11H2,1H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50049585

(1-{4-[(2,4-Diamino-pyrido[3,2-d]pyrimidin-6-ylmeth...)Show SMILES Nc1nc(N)c2nc(CN(CC#C)c3ccc(cc3)C(=O)C(F)(F)F)ccc2n1 Show InChI InChI=1S/C19H15F3N6O/c1-2-9-28(13-6-3-11(4-7-13)16(29)19(20,21)22)10-12-5-8-14-15(25-12)17(23)27-18(24)26-14/h1,3-8H,9-10H2,(H4,23,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50049611
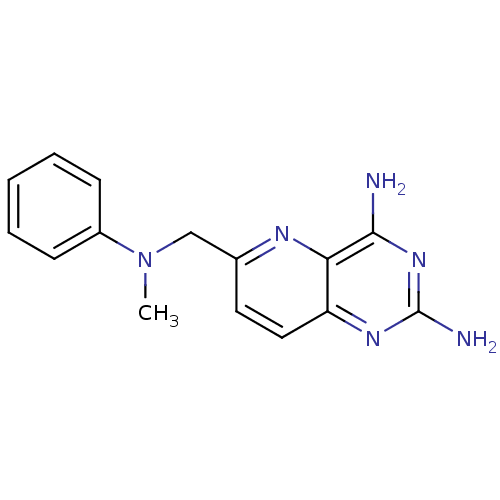
(6-[(Methyl-phenyl-amino)-methyl]-pyrido[3,2-d]pyri...)Show InChI InChI=1S/C15H16N6/c1-21(11-5-3-2-4-6-11)9-10-7-8-12-13(18-10)14(16)20-15(17)19-12/h2-8H,9H2,1H3,(H4,16,17,19,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50049609

(6-{[(3,4-Dimethoxy-phenyl)-methyl-amino]-methyl}-p...)Show InChI InChI=1S/C17H20N6O2/c1-23(11-5-7-13(24-2)14(8-11)25-3)9-10-4-6-12-15(20-10)16(18)22-17(19)21-12/h4-8H,9H2,1-3H3,(H4,18,19,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18268

(5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...)Show InChI InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50049603

(6-{[(2,4-Dimethoxy-phenyl)-methyl-amino]-methyl}-p...)Show InChI InChI=1S/C17H20N6O2/c1-23(13-7-5-11(24-2)8-14(13)25-3)9-10-4-6-12-15(20-10)16(18)22-17(19)21-12/h4-8H,9H2,1-3H3,(H4,18,19,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50049587

(1-{4-[(2,4-Diamino-pyrido[3,2-d]pyrimidin-6-ylmeth...)Show InChI InChI=1S/C17H18N6O/c1-10(24)11-3-6-13(7-4-11)23(2)9-12-5-8-14-15(20-12)16(18)22-17(19)21-14/h3-8H,9H2,1-2H3,(H4,18,19,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50049607

(6-{[(3-Methoxy-phenyl)-methyl-amino]-methyl}-pyrid...)Show InChI InChI=1S/C16H18N6O/c1-22(11-4-3-5-12(8-11)23-2)9-10-6-7-13-14(19-10)15(17)21-16(18)20-13/h3-8H,9H2,1-2H3,(H4,17,18,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50049576
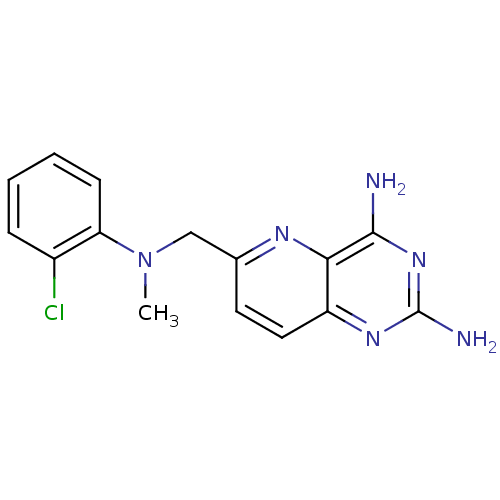
(6-{[(2-Chloro-phenyl)-methyl-amino]-methyl}-pyrido...)Show InChI InChI=1S/C15H15ClN6/c1-22(12-5-3-2-4-10(12)16)8-9-6-7-11-13(19-9)14(17)21-15(18)20-11/h2-7H,8H2,1H3,(H4,17,18,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50049599
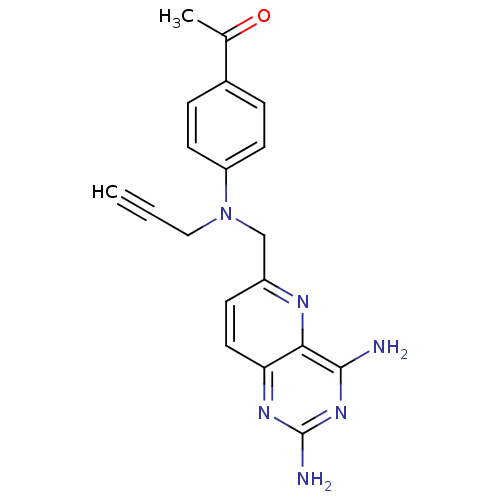
(1-{4-[(2,4-Diamino-pyrido[3,2-d]pyrimidin-6-ylmeth...)Show SMILES CC(=O)c1ccc(cc1)N(CC#C)Cc1ccc2nc(N)nc(N)c2n1 Show InChI InChI=1S/C19H18N6O/c1-3-10-25(15-7-4-13(5-8-15)12(2)26)11-14-6-9-16-17(22-14)18(20)24-19(21)23-16/h1,4-9H,10-11H2,2H3,(H4,20,21,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50049603

(6-{[(2,4-Dimethoxy-phenyl)-methyl-amino]-methyl}-p...)Show InChI InChI=1S/C17H20N6O2/c1-23(13-7-5-11(24-2)8-14(13)25-3)9-10-4-6-12-15(20-10)16(18)22-17(19)21-12/h4-8H,9H2,1-3H3,(H4,18,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50049601
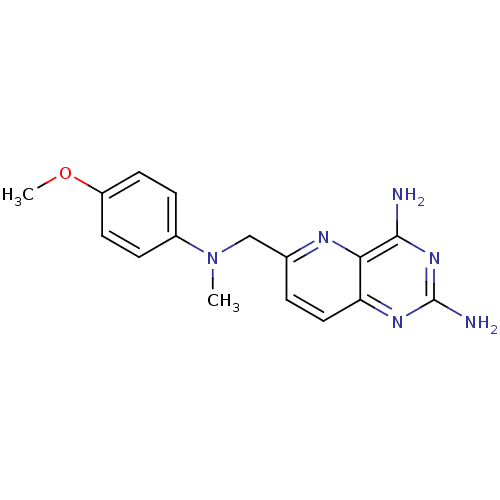
(6-{[(4-Methoxy-phenyl)-methyl-amino]-methyl}-pyrid...)Show InChI InChI=1S/C16H18N6O/c1-22(11-4-6-12(23-2)7-5-11)9-10-3-8-13-14(19-10)15(17)21-16(18)20-13/h3-8H,9H2,1-2H3,(H4,17,18,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50049583

(6-[(Methyl-naphthalen-2-yl-amino)-methyl]-pyrido[3...)Show InChI InChI=1S/C19H18N6/c1-25(15-8-6-12-4-2-3-5-13(12)10-15)11-14-7-9-16-17(22-14)18(20)24-19(21)23-16/h2-10H,11H2,1H3,(H4,20,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50031869
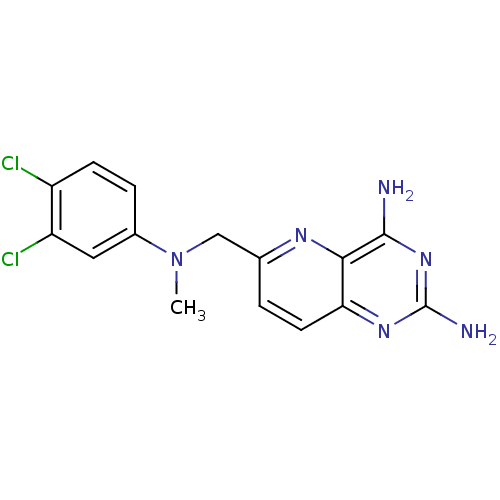
(6-(((3,4-dichlorophenyl)(methyl)amino)methyl)pyrid...)Show InChI InChI=1S/C15H14Cl2N6/c1-23(9-3-4-10(16)11(17)6-9)7-8-2-5-12-13(20-8)14(18)22-15(19)21-12/h2-6H,7H2,1H3,(H4,18,19,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM18224

(6-[(2,5-dimethoxyphenyl)methyl]-5-methylpyrido[2,3...)Show InChI InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Rattus norvegicus (rat)) | BDBM50049601

(6-{[(4-Methoxy-phenyl)-methyl-amino]-methyl}-pyrid...)Show InChI InChI=1S/C16H18N6O/c1-22(11-4-6-12(23-2)7-5-11)9-10-3-8-13-14(19-10)15(17)21-16(18)20-13/h3-8H,9H2,1-2H3,(H4,17,18,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50049604

(6-[(Methyl-naphthalen-1-yl-amino)-methyl]-pyrido[3...)Show InChI InChI=1S/C19H18N6/c1-25(16-8-4-6-12-5-2-3-7-14(12)16)11-13-9-10-15-17(22-13)18(20)24-19(21)23-15/h2-10H,11H2,1H3,(H4,20,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50031866

(6-(((3-chlorophenyl)(methyl)amino)methyl)pyrido[3,...)Show InChI InChI=1S/C15H15ClN6/c1-22(11-4-2-3-9(16)7-11)8-10-5-6-12-13(19-10)14(17)21-15(18)20-12/h2-7H,8H2,1H3,(H4,17,18,20,21) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Bifunctional dihydrofolate reductase-thymidylate synthase
(Toxoplasma gondii) | BDBM50049599

(1-{4-[(2,4-Diamino-pyrido[3,2-d]pyrimidin-6-ylmeth...)Show SMILES CC(=O)c1ccc(cc1)N(CC#C)Cc1ccc2nc(N)nc(N)c2n1 Show InChI InChI=1S/C19H18N6O/c1-3-10-25(15-7-4-13(5-8-15)12(2)26)11-14-6-9-16-17(22-14)18(20)24-19(21)23-16/h1,4-9H,10-11H2,2H3,(H4,20,21,23,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Toxoplasma gondii dihydrofolate reductase. |
J Med Chem 39: 1836-45 (1996)
Article DOI: 10.1021/jm950918e
BindingDB Entry DOI: 10.7270/Q2319TXB |
More data for this
Ligand-Target Pair | |
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM50127511

(4-hydroxy-2-(methoxymethyl)-5-(6-oxo-1-prop-2-ynyl...)Show SMILES COC[C@H]1O[C@H](C(O)[C@H]1OP(C)([O-])=O)n1cnc2c1ncn(CC#C)c2=O Show InChI InChI=1S/C15H19N4O7P/c1-4-5-18-7-17-13-10(14(18)21)16-8-19(13)15-11(20)12(26-27(3,22)23)9(25-15)6-24-2/h1,7-9,11-12,15,20H,5-6H2,2-3H3,(H,22,23)/p-1/t9-,11?,12+,15-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against reverse transcriptase (RT) in cell free RT. |
J Med Chem 46: 1878-85 (2003)
Article DOI: 10.1021/jm0203276
BindingDB Entry DOI: 10.7270/Q25X29NG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data