

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10597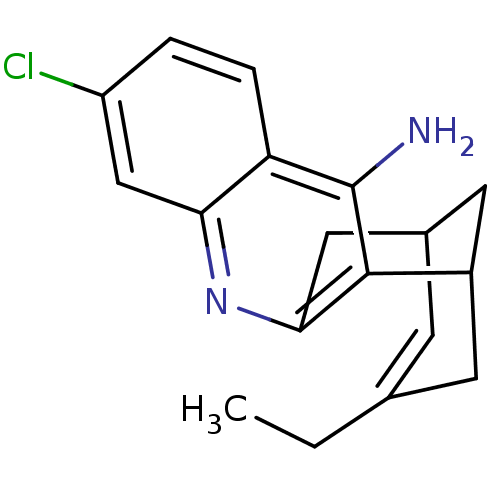 ((1S)-7-chloro-15-ethyl-10-azatetracyclo[11.3.1.0^{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0260 | -60.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139926 (US8901087, 2) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139933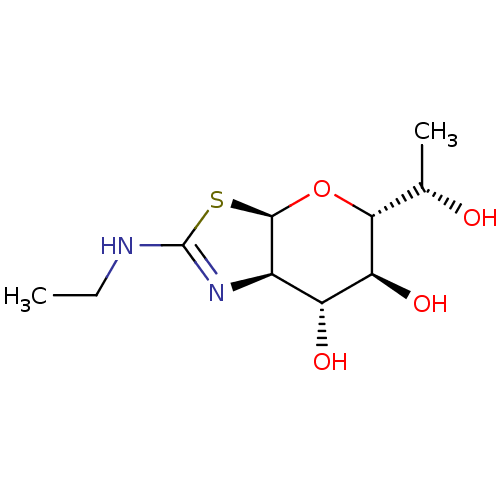 (US8901087, 27) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50262988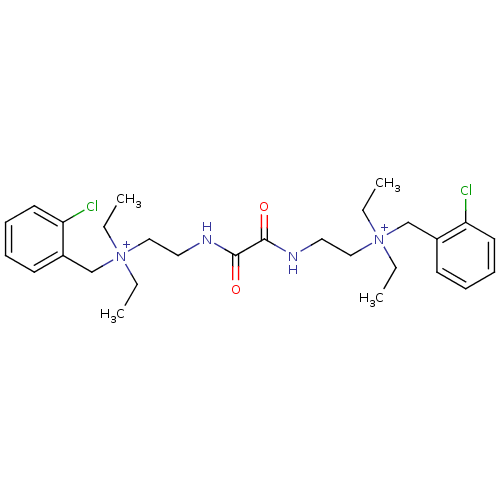 (CHEMBL1200541 | N-(2-chlorobenzyl)-2-(2-(2-((2-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139959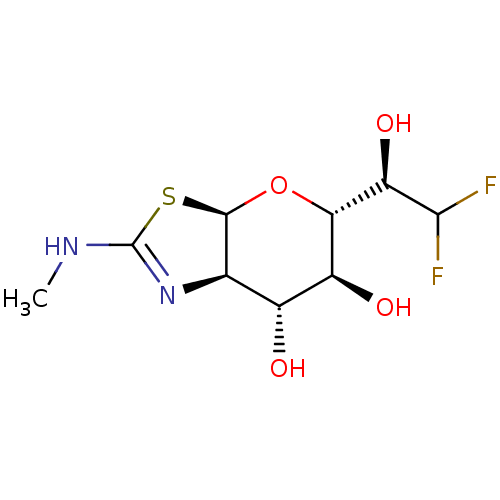 (US8901087, 191) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.140 | -56.2 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139951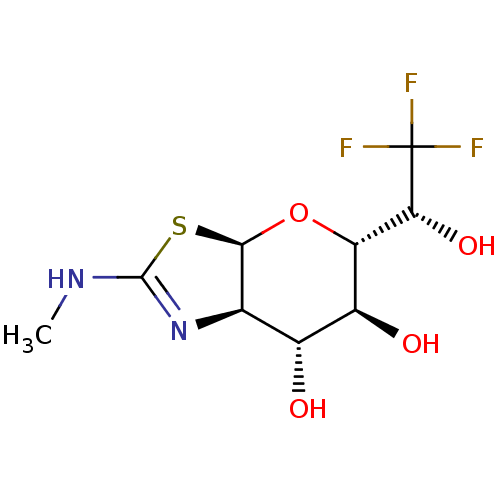 (US8901087, 95) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.160 | -55.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139925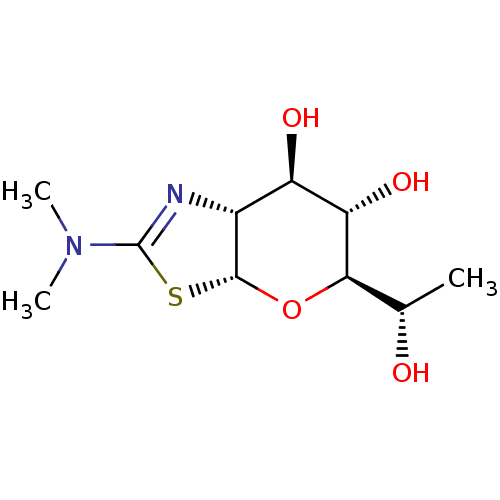 (US8901087, 1) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139952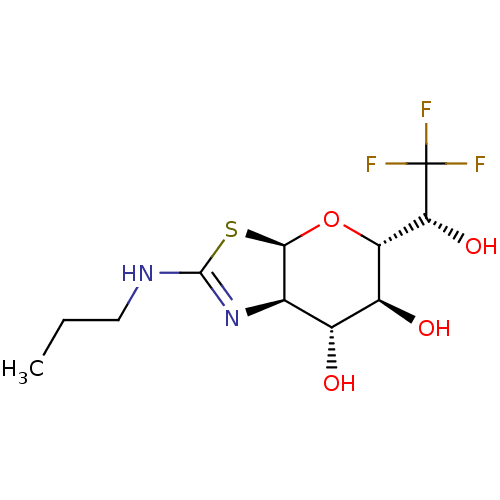 (US8901087, 97) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.170 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139946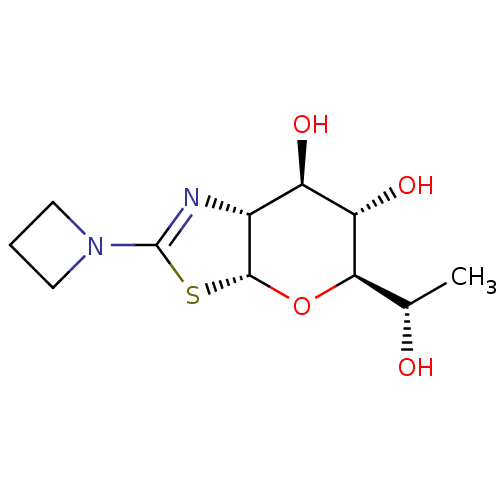 (US8901087, 76) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.180 | -55.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM46008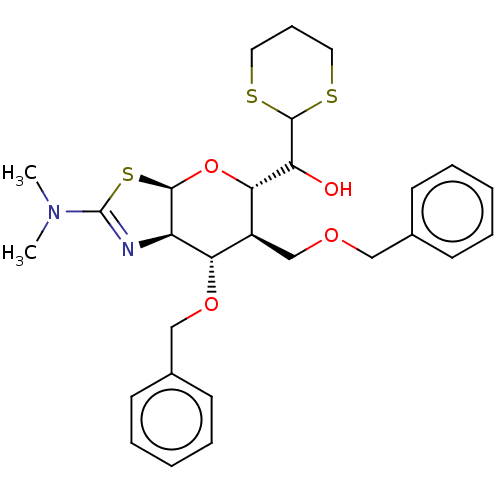 (US8901087, 96) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.190 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139941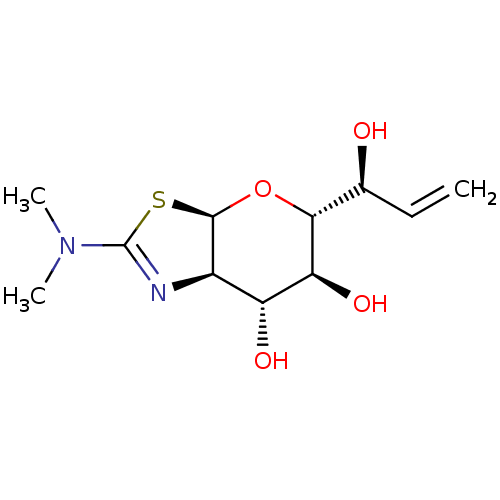 (US8901087, 63) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.220 | -55.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139930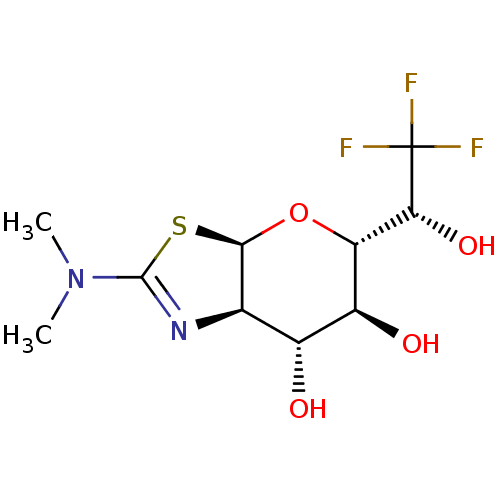 (US8901087, 11) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139939 (US8901087, 57) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.330 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139931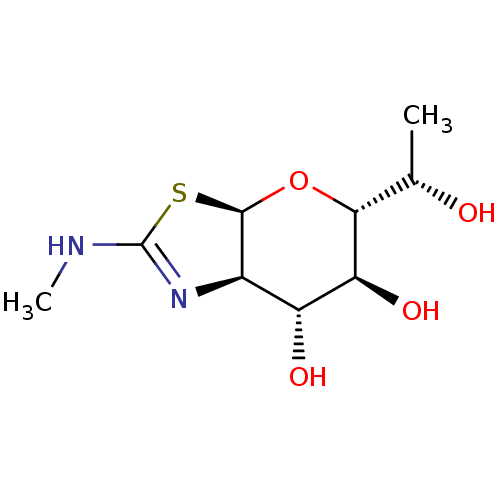 (US8901087, 13) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139961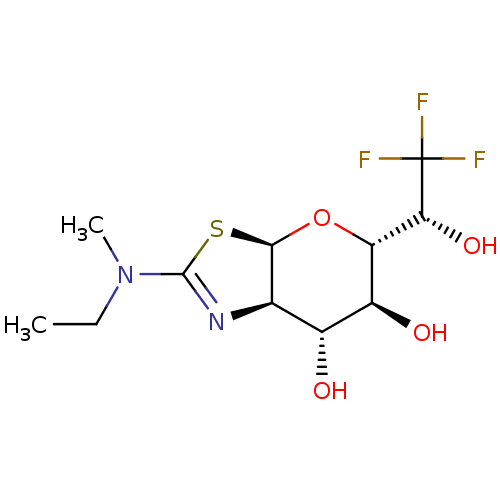 (US8901087, 211) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.440 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139942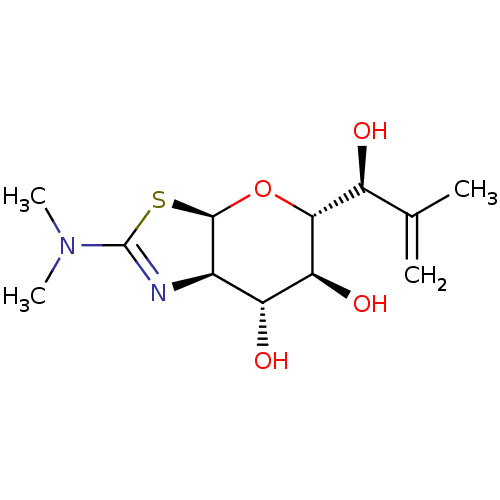 (US8901087, 65) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.450 | -53.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139948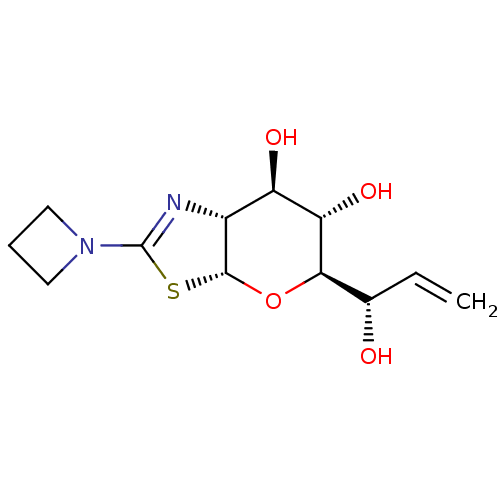 (US8901087, 80) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139950 (US8901087, 91) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.530 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (MOUSE) | BDBM50008780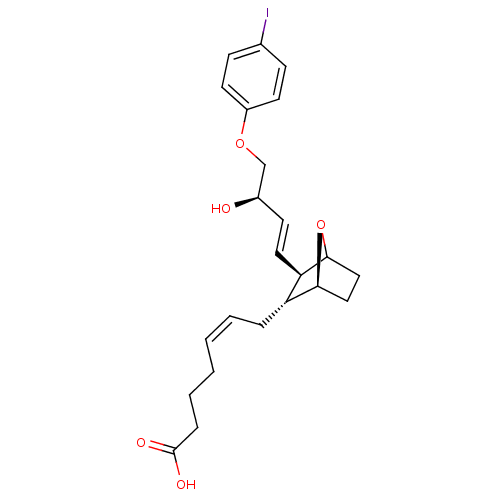 (7-{3-[3-Hydroxy-4-(4-iodo-phenoxy)-but-1-enyl]-7-o...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by PDSP Ki Database | Br J Pharmacol 122: 217-24 (1997) Article DOI: 10.1038/sj.bjp.0701367 BindingDB Entry DOI: 10.7270/Q26M35CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM85183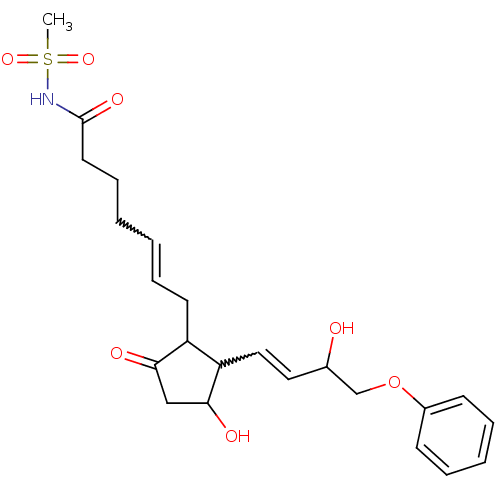 (CAS_60325-46-4 | NSC_43251 | SULPROSTONE) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by PDSP Ki Database | Br J Pharmacol 122: 217-24 (1997) Article DOI: 10.1038/sj.bjp.0701367 BindingDB Entry DOI: 10.7270/Q26M35CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM85177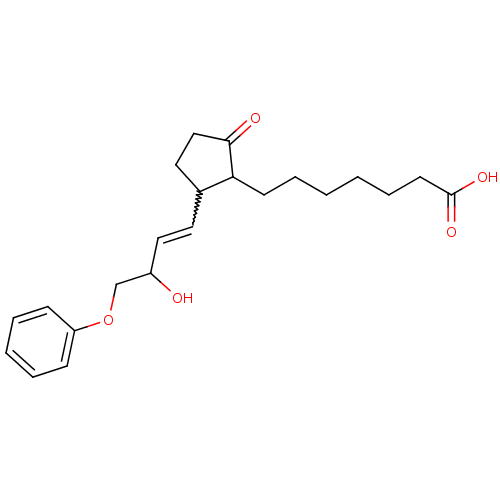 (CAS_80558-61-8 | M&B-28767 | NSC_119139) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by PDSP Ki Database | Br J Pharmacol 122: 217-24 (1997) Article DOI: 10.1038/sj.bjp.0701367 BindingDB Entry DOI: 10.7270/Q26M35CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139929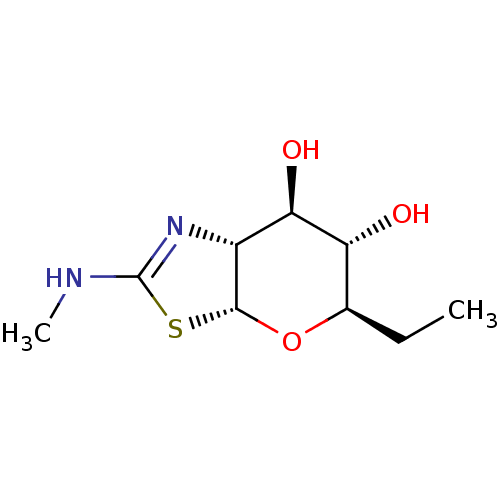 (US8901087, 8) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.680 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (MOUSE) | BDBM50008805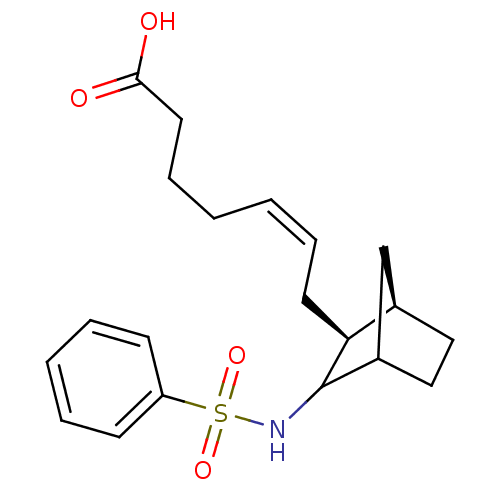 (7-(3-Benzenesulfonylamino-bicyclo[2.2.1]hept-2-yl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by PDSP Ki Database | Br J Pharmacol 122: 217-24 (1997) Article DOI: 10.1038/sj.bjp.0701367 BindingDB Entry DOI: 10.7270/Q26M35CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139932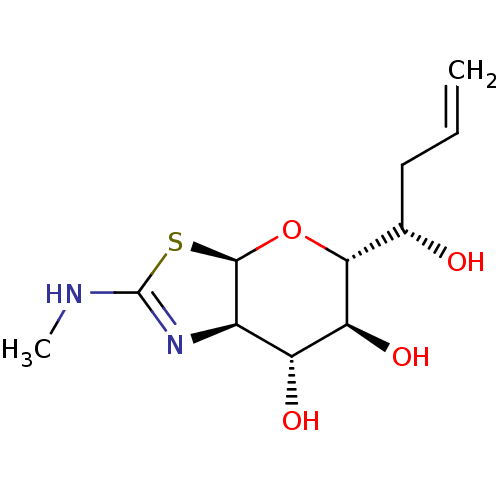 (US8901087, 25) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139938 (US8901087, 53) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139958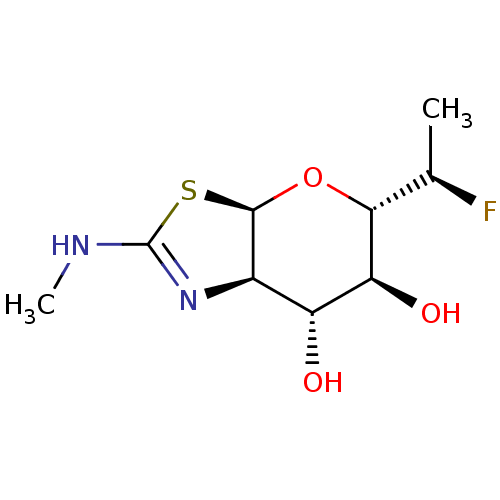 (US8901087, 181) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139956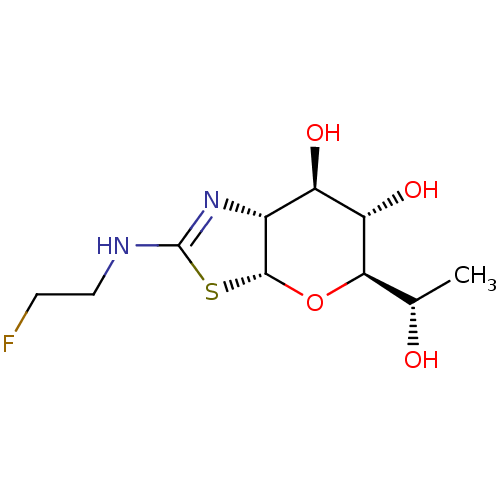 (US8901087, 141) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.820 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by PDSP Ki Database | Br J Pharmacol 122: 217-24 (1997) Article DOI: 10.1038/sj.bjp.0701367 BindingDB Entry DOI: 10.7270/Q26M35CT | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139927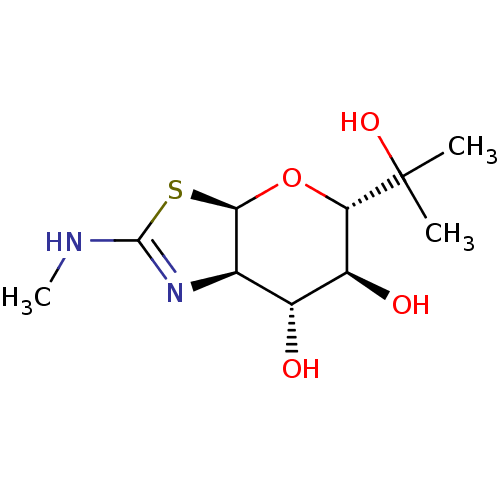 (US8901087, 3) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139935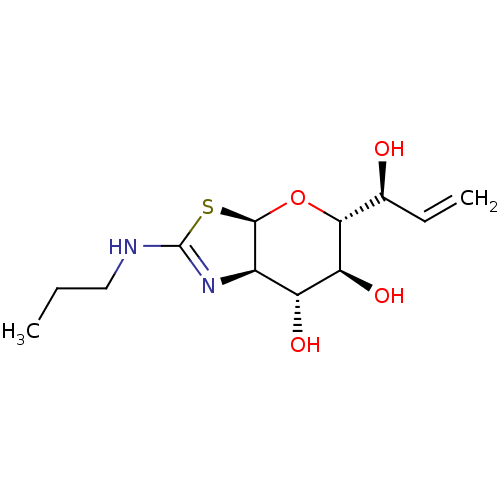 (US8901087, 42) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.03 | -51.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101828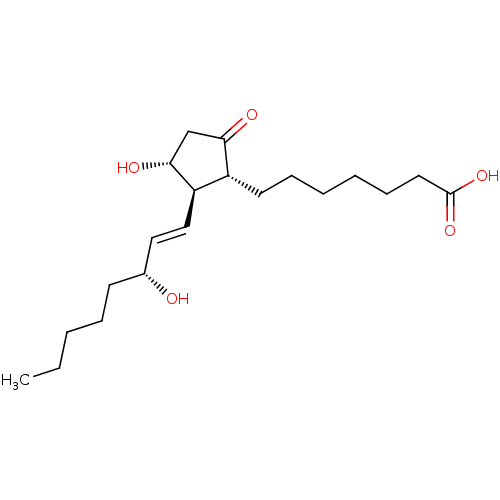 (7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-oct-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by PDSP Ki Database | Br J Pharmacol 122: 217-24 (1997) Article DOI: 10.1038/sj.bjp.0701367 BindingDB Entry DOI: 10.7270/Q26M35CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098853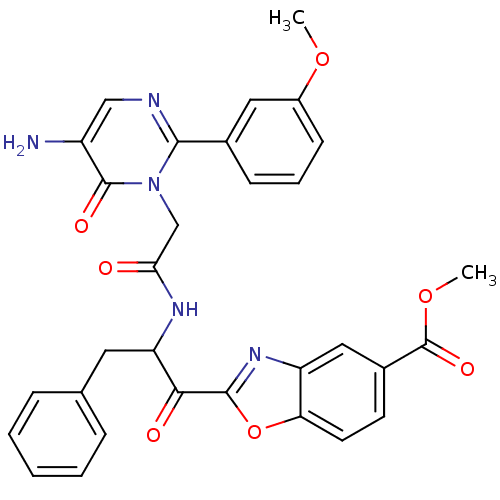 (2-(2-{2-[5-Amino-2-(3-methoxy-phenyl)-6-oxo-6H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description Inhibitory activity against canine skin chymase | J Med Chem 44: 1286-96 (2001) BindingDB Entry DOI: 10.7270/Q26H4J4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139957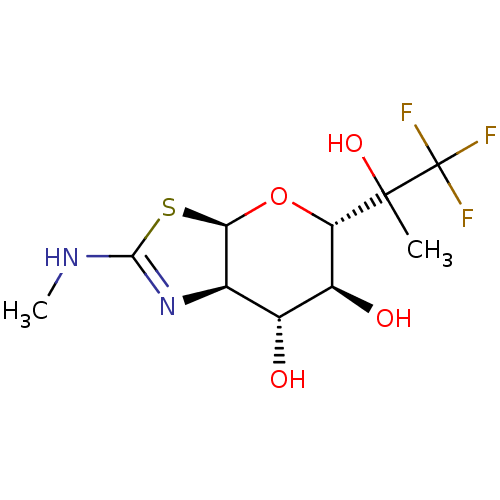 (US8901087, 159) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.25 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139943 (US8901087, 67) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.25 | -50.8 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50200028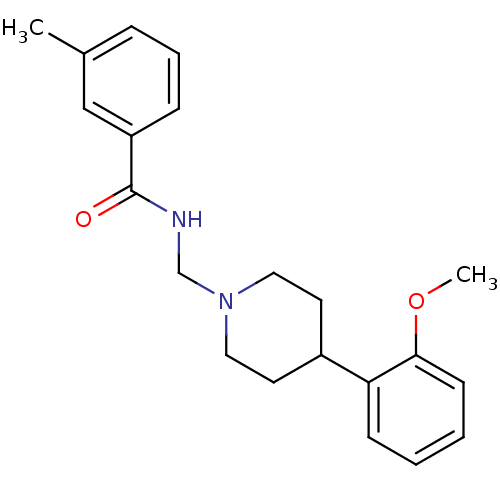 (2-methoxy-N-(3',4',5',6'-tetrahydro-2'H-[2,4'-bipy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]A369508 from human D4 receptor expressed in HEK293 cell membrane | J Med Chem 49: 7450-65 (2006) Article DOI: 10.1021/jm060662k BindingDB Entry DOI: 10.7270/Q25X28MM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068901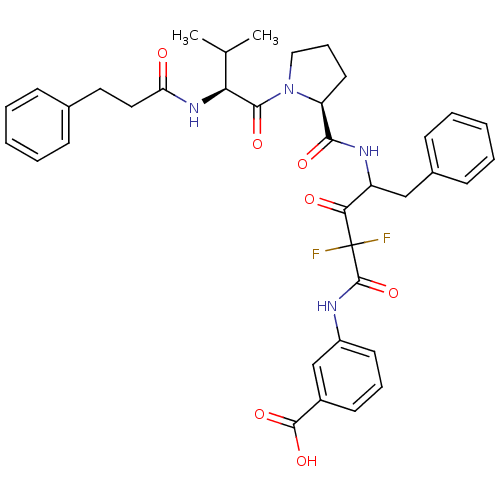 (3-[2,2-Difluoro-4-({(S)-1-[(S)-3-methyl-2-(3-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 913-8 (1999) BindingDB Entry DOI: 10.7270/Q2GQ6WXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139953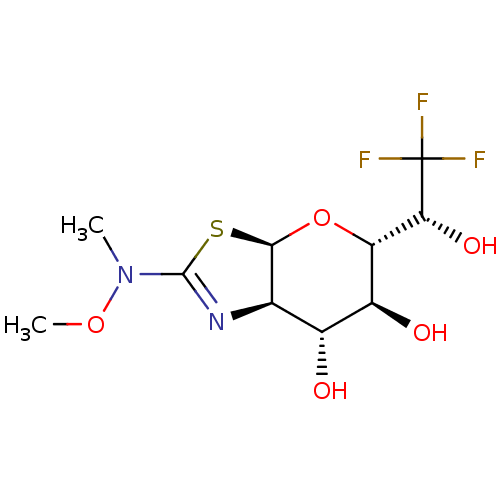 (US8901087, 98) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.43 | -50.5 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM85189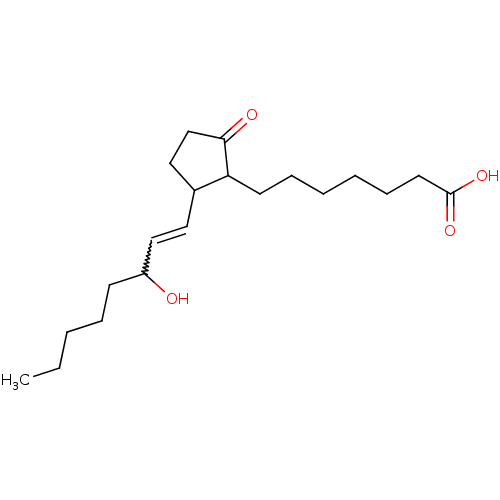 (PGE1,11-DEOXY) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by PDSP Ki Database | Br J Pharmacol 122: 217-24 (1997) Article DOI: 10.1038/sj.bjp.0701367 BindingDB Entry DOI: 10.7270/Q26M35CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50089616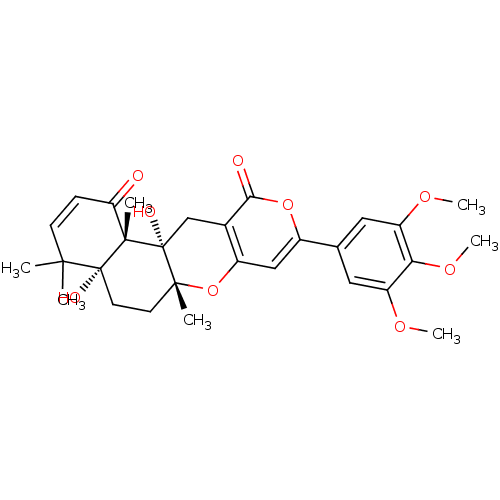 (4a,12a-Dihydroxy-4,4,6a,12b-tetramethyl-9-(3,4,5-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.70 | -50.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Mayo Clinic College of Medicine | Assay Description Assays were conducted at 25 C in 20 mM sodium phosphate buffer (pH 7.0) and 0.01% bovine serum albumin unless otherwise noted. AChE concentrations we... | Biochemistry 52: 7486-99 (2013) Article DOI: 10.1021/bi401043w BindingDB Entry DOI: 10.7270/Q24X56GT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139962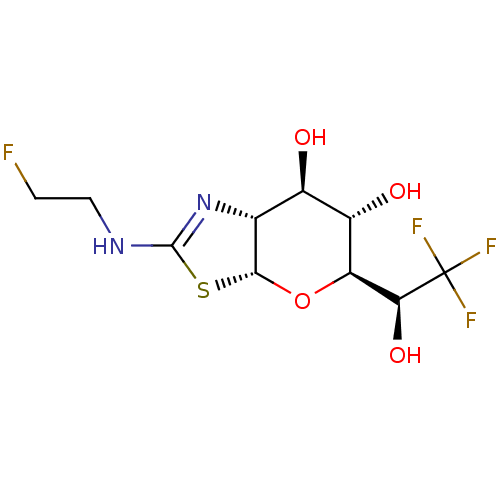 (US8901087, 215) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.80 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM85174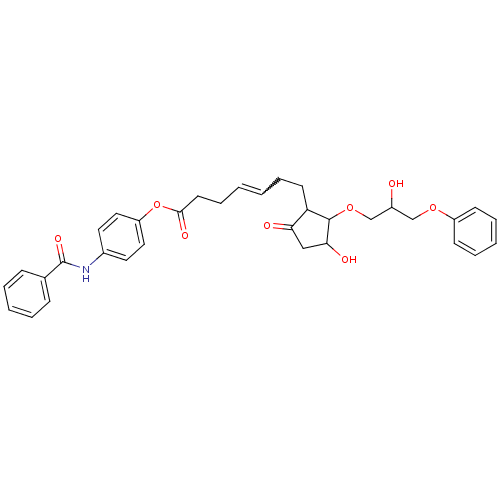 (CAS_5311224 | GR 63799X | NSC_5311224) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by PDSP Ki Database | Br J Pharmacol 122: 217-24 (1997) Article DOI: 10.1038/sj.bjp.0701367 BindingDB Entry DOI: 10.7270/Q26M35CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM85185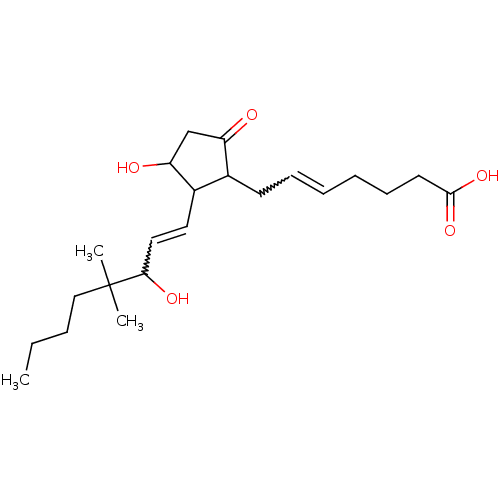 (PGE2,16,16-DIMETHYL) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by PDSP Ki Database | Br J Pharmacol 122: 217-24 (1997) Article DOI: 10.1038/sj.bjp.0701367 BindingDB Entry DOI: 10.7270/Q26M35CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by PDSP Ki Database | Br J Pharmacol 122: 217-24 (1997) Article DOI: 10.1038/sj.bjp.0701367 BindingDB Entry DOI: 10.7270/Q26M35CT | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Mus musculus (Mouse)) | BDBM50101828 (7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-oct-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by PDSP Ki Database | Br J Pharmacol 122: 217-24 (1997) Article DOI: 10.1038/sj.bjp.0701367 BindingDB Entry DOI: 10.7270/Q26M35CT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor | Bioorg Med Chem Lett 17: 4030-4 (2007) Article DOI: 10.1016/j.bmcl.2007.04.093 BindingDB Entry DOI: 10.7270/Q2P55N60 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139940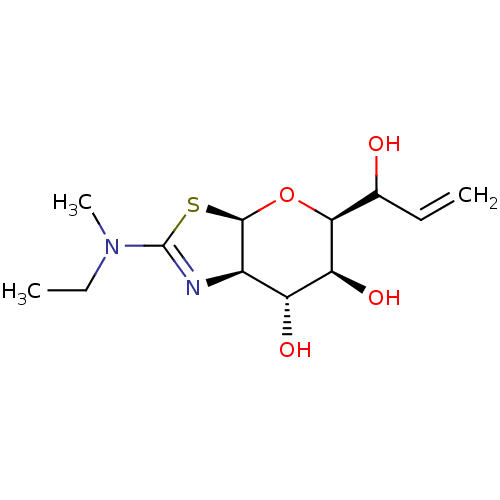 (US8901087, 61) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.46 | -49.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50098874 (4-{2-[5-Amino-2-(3-chloro-phenyl)-6-oxo-6H-pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Welfide Corporation Curated by ChEMBL | Assay Description In vitro inhibitory activity was determined against human heart chymase | J Med Chem 44: 1297-304 (2001) BindingDB Entry DOI: 10.7270/Q22R3SCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50234380 (CHEMBL245876 | quinolin-8-yl 4-methyl-3-(piperidin...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK cells | Bioorg Med Chem Lett 18: 1725-9 (2008) Article DOI: 10.1016/j.bmcl.2008.01.042 BindingDB Entry DOI: 10.7270/Q2X92B1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50068918 ((S)-4-((2S,3S)-2-Benzyloxycarbonylamino-3-methyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human heart chymase (HHC) | Bioorg Med Chem Lett 8: 919-24 (1999) BindingDB Entry DOI: 10.7270/Q2BZ656D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein O-GlcNAcase (Homo sapiens (Human)) | BDBM139928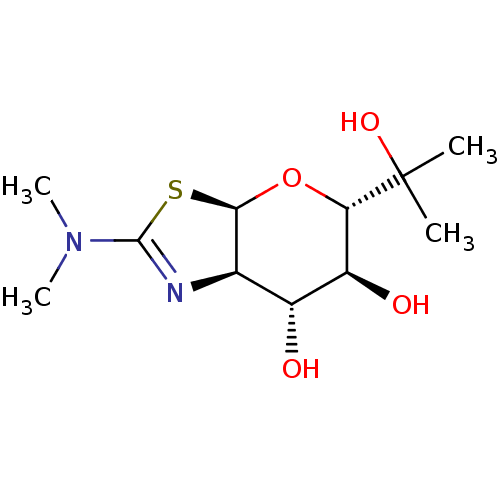 (US8901087, 4) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.10 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzymatic reactions are carried out in a reaction containing 50 mM NaH2PO4, 100 mM NaCl and 0.1% BSA (pH 7.0) using 2 mM 4-Methylumbelliferyl N-acety... | US Patent US8901087 (2014) BindingDB Entry DOI: 10.7270/Q24B301W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5332 total ) | Next | Last >> |