 Found 216 hits with Last Name = 'hirai' and Initial = 'g'
Found 216 hits with Last Name = 'hirai' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50099066
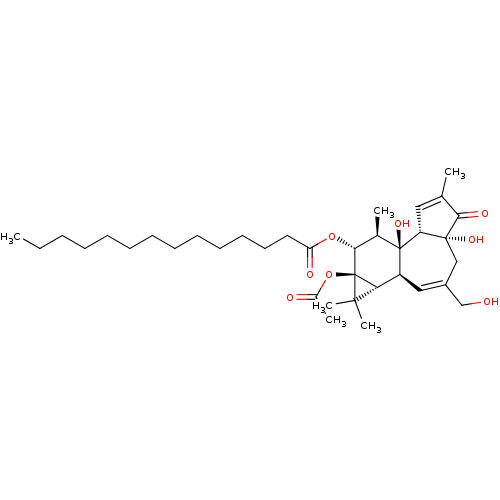
(CHEMBL279115 | phorbol 13-acetate 12-myristate)Show SMILES CCCCCCCCCCCCCC(=O)O[C@@H]1[C@@H](C)[C@]2(O)[C@@H]3C=C(C)C(=O)[C@@]3(O)CC(CO)=C[C@H]2[C@@H]2C(C)(C)[C@]12OC(C)=O |r,c:33,t:22| Show InChI InChI=1S/C36H56O8/c1-7-8-9-10-11-12-13-14-15-16-17-18-29(39)43-32-24(3)35(42)27(30-33(5,6)36(30,32)44-25(4)38)20-26(22-37)21-34(41)28(35)19-23(2)31(34)40/h19-20,24,27-28,30,32,37,41-42H,7-18,21-22H2,1-6H3/t24-,27+,28-,30?,32-,34-,35-,36-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345024

((R)-(4-(dodecyloxy)-1-(hydroxymethyl)-3-oxo-1,3-di...)Show SMILES CCCCCCCCCCCCOc1cccc2c1C(=O)O[C@]2(CO)COC(=O)C(C(C)(C)C)C(C)(C)C |r| Show InChI InChI=1S/C32H52O6/c1-8-9-10-11-12-13-14-15-16-17-21-36-25-20-18-19-24-26(25)28(34)38-32(24,22-33)23-37-29(35)27(30(2,3)4)31(5,6)7/h18-20,27,33H,8-17,21-23H2,1-7H3/t32-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146556

(Acetic acid (R)-4-decyloxy-1-hydroxymethyl-3-oxo-1...)Show SMILES CCCCCCCCCCOc1cccc2c1C(=O)O[C@]2(CO)COC(C)=O Show InChI InChI=1S/C22H32O6/c1-3-4-5-6-7-8-9-10-14-26-19-13-11-12-18-20(19)21(25)28-22(18,15-23)16-27-17(2)24/h11-13,23H,3-10,14-16H2,1-2H3/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146559

(But-3-enoic acid (R)-1-acetoxymethyl-1-hydroxymeth...)Show SMILES CC(=O)OC[C@@]1(CO)OC(=O)c2c1cccc2OC(=O)CC=C Show InChI InChI=1S/C16H16O7/c1-3-5-13(19)22-12-7-4-6-11-14(12)15(20)23-16(11,8-17)9-21-10(2)18/h3-4,6-7,17H,1,5,8-9H2,2H3/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146558
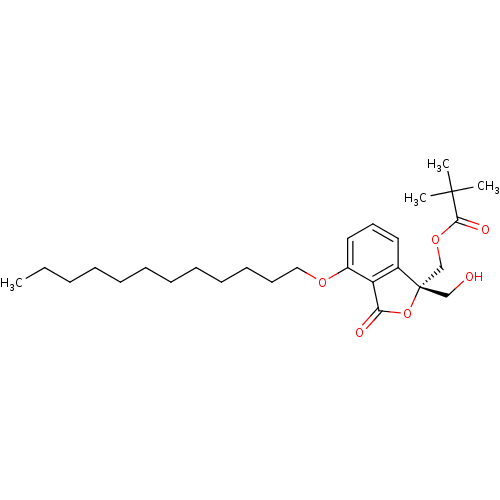
(2,2-Dimethyl-propionic acid (S)-4-dodecyloxy-1-hyd...)Show SMILES CCCCCCCCCCCCOc1cccc2c1C(=O)O[C@@]2(CO)COC(=O)C(C)(C)C Show InChI InChI=1S/C27H42O6/c1-5-6-7-8-9-10-11-12-13-14-18-31-22-17-15-16-21-23(22)24(29)33-27(21,19-28)20-32-25(30)26(2,3)4/h15-17,28H,5-14,18-20H2,1-4H3/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146555

(Acetic acid (S)-4-decyloxy-1-hydroxymethyl-3-oxo-1...)Show SMILES CCCCCCCCCCOc1cccc2c1C(=O)O[C@@]2(CO)COC(C)=O Show InChI InChI=1S/C22H32O6/c1-3-4-5-6-7-8-9-10-14-26-19-13-11-12-18-20(19)21(25)28-22(18,15-23)16-27-17(2)24/h11-13,23H,3-10,14-16H2,1-2H3/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146561
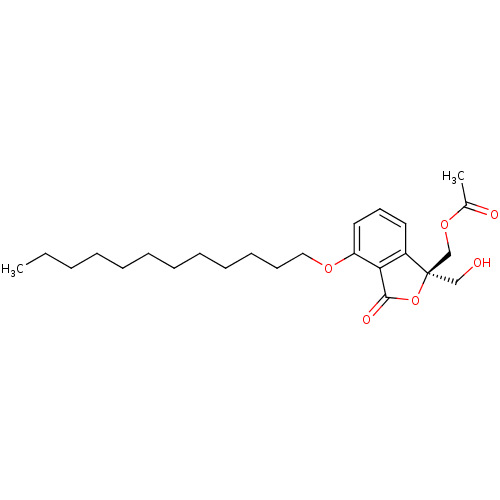
(Acetic acid (R)-4-dodecyloxy-1-hydroxymethyl-3-oxo...)Show SMILES CCCCCCCCCCCCOc1cccc2c1C(=O)O[C@]2(CO)COC(C)=O Show InChI InChI=1S/C24H36O6/c1-3-4-5-6-7-8-9-10-11-12-16-28-21-15-13-14-20-22(21)23(27)30-24(20,17-25)18-29-19(2)26/h13-15,25H,3-12,16-18H2,1-2H3/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146557
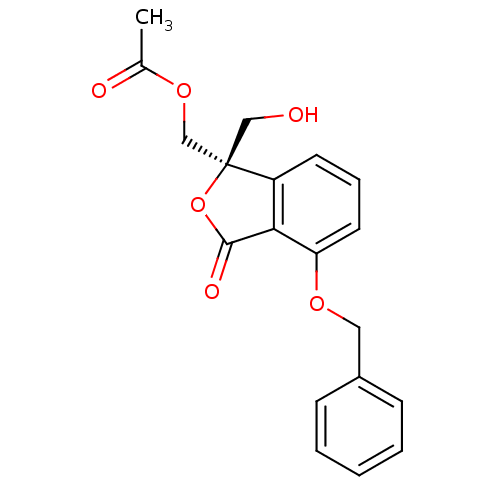
(Acetic acid (R)-4-benzyloxy-1-hydroxymethyl-3-oxo-...)Show SMILES CC(=O)OC[C@@]1(CO)OC(=O)c2c1cccc2OCc1ccccc1 Show InChI InChI=1S/C19H18O6/c1-13(21)24-12-19(11-20)15-8-5-9-16(17(15)18(22)25-19)23-10-14-6-3-2-4-7-14/h2-9,20H,10-12H2,1H3/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146554
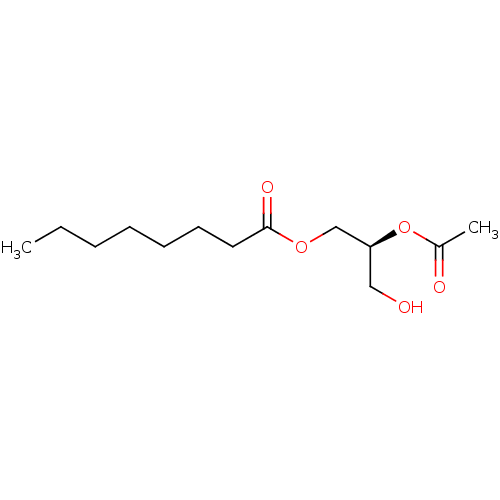
(CHEMBL102042 | Octanoic acid (S)-2-acetoxy-3-hydro...)Show InChI InChI=1S/C13H24O5/c1-3-4-5-6-7-8-13(16)17-10-12(9-14)18-11(2)15/h12,14H,3-10H2,1-2H3/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50146560

(Acetic acid (S)-4-dodecyloxy-1-hydroxymethyl-3-oxo...)Show SMILES CCCCCCCCCCCCOc1cccc2c1C(=O)O[C@@]2(CO)COC(C)=O Show InChI InChI=1S/C24H36O6/c1-3-4-5-6-7-8-9-10-11-12-16-28-21-15-13-14-20-22(21)23(27)30-24(20,17-25)18-29-19(2)26/h13-15,25H,3-12,16-18H2,1-2H3/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku University
Curated by ChEMBL
| Assay Description
Binding affinity for protein kinase C alpha |
Bioorg Med Chem Lett 14: 2963-7 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.097
BindingDB Entry DOI: 10.7270/Q2GB23GP |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345027
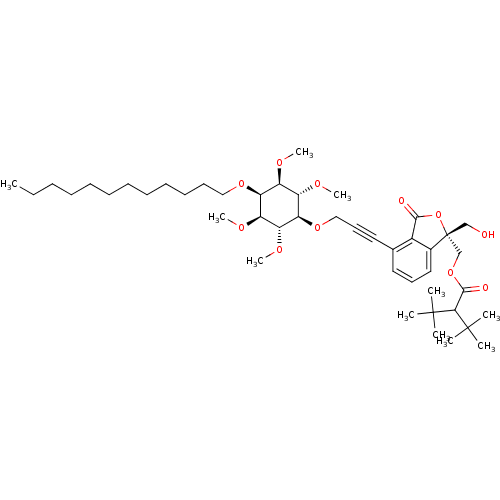
(((R)-4-(3-((2R,3R,5S,6S)-4-(dodecyloxy)-2,3,5,6-te...)Show SMILES CCCCCCCCCCCCO[C@H]1[C@@H](OC)[C@H](OC)[C@@H](OCC#Cc2cccc3c2C(=O)O[C@]3(CO)COC(=O)C(C(C)(C)C)C(C)(C)C)[C@H](OC)[C@H]1OC |r| Show InChI InChI=1S/C45H72O11/c1-12-13-14-15-16-17-18-19-20-21-27-53-38-34(49-8)36(51-10)39(37(52-11)35(38)50-9)54-28-23-25-31-24-22-26-32-33(31)41(47)56-45(32,29-46)30-55-42(48)40(43(2,3)4)44(5,6)7/h22,24,26,34-40,46H,12-21,27-30H2,1-11H3/t34-,35+,36-,37+,38-,39+,45-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345026
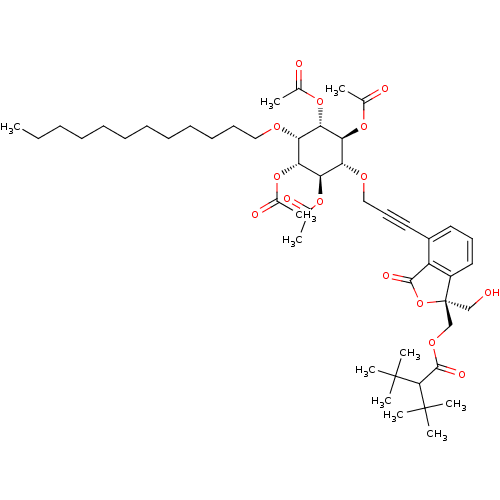
((1R,2R,4S,5S)-3-(3-((R)-1-((2-tert-butyl-3,3-dimet...)Show SMILES CCCCCCCCCCCCO[C@@H]1[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H](OCC#Cc2cccc3c2C(=O)O[C@]3(CO)COC(=O)C(C(C)(C)C)C(C)(C)C)[C@@H](OC(C)=O)[C@@H]1OC(C)=O |r| Show InChI InChI=1S/C49H72O15/c1-12-13-14-15-16-17-18-19-20-21-27-57-38-40(60-31(2)51)42(62-33(4)53)39(43(63-34(5)54)41(38)61-32(3)52)58-28-23-25-35-24-22-26-36-37(35)45(55)64-49(36,29-50)30-59-46(56)44(47(6,7)8)48(9,10)11/h22,24,26,38-44,50H,12-21,27-30H2,1-11H3/t38-,39+,40+,41-,42+,43-,49-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50571956

(CHEMBL4874608)Show SMILES OC(=O)C[C@H](Nc1nc(NCCCCCNC(=O)CCCNc2nc(N[C@@H](CC(O)=O)C(O)=O)nc(N[C@@H](Cc3ccc(O)cc3)C(O)=O)n2)nc(N[C@@H](Cc2c[nH]c3ccccc23)C(O)=O)n1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Competitive inhibition of recombinant human Cdc25B (351 to 380 residues) assessed as hydrolysis of substrate O-methylfluorescein phosphate by Linewea... |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.128265
BindingDB Entry DOI: 10.7270/Q26M3BNG |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345025
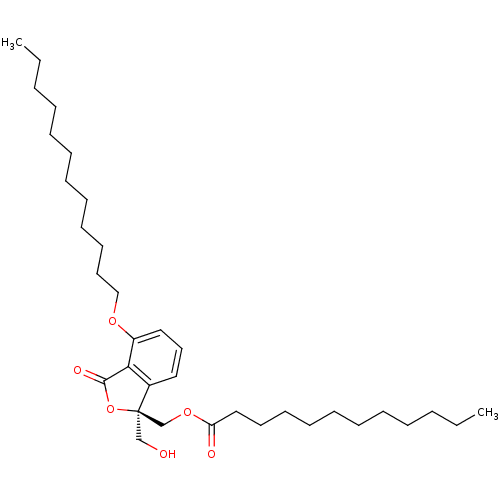
((R)-(4-(dodecyloxy)-1-(hydroxymethyl)-3-oxo-1,3-di...)Show SMILES CCCCCCCCCCCCOc1cccc2c1C(=O)O[C@]2(CO)COC(=O)CCCCCCCCCCC |r| Show InChI InChI=1S/C34H56O6/c1-3-5-7-9-11-13-15-17-19-21-26-38-30-24-22-23-29-32(30)33(37)40-34(29,27-35)28-39-31(36)25-20-18-16-14-12-10-8-6-4-2/h22-24,35H,3-21,25-28H2,1-2H3/t34-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345031
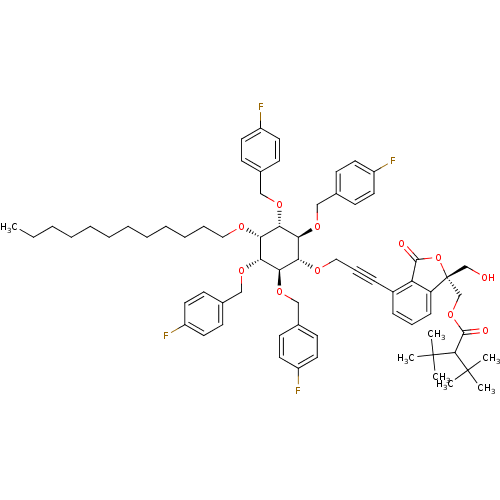
(((R)-4-(3-((2R,3S,5R,6S)-4-(dodecyloxy)-2,3,5,6-te...)Show SMILES CCCCCCCCCCCCO[C@@H]1[C@H](OCc2ccc(F)cc2)[C@@H](OCc2ccc(F)cc2)[C@H](OCC#Cc2cccc3c2C(=O)O[C@]3(CO)COC(=O)C(C(C)(C)C)C(C)(C)C)[C@@H](OCc2ccc(F)cc2)[C@@H]1OCc1ccc(F)cc1 |r| Show InChI InChI=1S/C69H84F4O11/c1-8-9-10-11-12-13-14-15-16-17-39-77-58-60(79-41-47-23-31-52(70)32-24-47)62(81-43-49-27-35-54(72)36-28-49)59(63(82-44-50-29-37-55(73)38-30-50)61(58)80-42-48-25-33-53(71)34-26-48)78-40-19-21-51-20-18-22-56-57(51)65(75)84-69(56,45-74)46-83-66(76)64(67(2,3)4)68(5,6)7/h18,20,22-38,58-64,74H,8-17,39-46H2,1-7H3/t58-,59+,60+,61-,62+,63-,69-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345030

(((R)-4-(3-((2R,3S,5R,6S)-4-(dodecyloxy)-2,3,5,6-te...)Show SMILES CCCCCCCCCCCCO[C@@H]1[C@H](OCc2ccc(cc2)C(C)C)[C@@H](OCc2ccc(cc2)C(C)C)[C@H](OCC#Cc2cccc3c2C(=O)O[C@]3(CO)COC(=O)C(C(C)(C)C)C(C)(C)C)[C@@H](OCc2ccc(cc2)C(C)C)[C@@H]1OCc1ccc(cc1)C(C)C |r| Show InChI InChI=1S/C81H112O11/c1-16-17-18-19-20-21-22-23-24-25-47-85-70-72(87-49-59-31-39-63(40-32-59)55(2)3)74(89-51-61-35-43-65(44-36-61)57(6)7)71(75(90-52-62-37-45-66(46-38-62)58(8)9)73(70)88-50-60-33-41-64(42-34-60)56(4)5)86-48-27-29-67-28-26-30-68-69(67)77(83)92-81(68,53-82)54-91-78(84)76(79(10,11)12)80(13,14)15/h26,28,30-46,55-58,70-76,82H,16-25,47-54H2,1-15H3/t70-,71+,72+,73-,74+,75-,81-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345028

(((R)-4-(3-((1S,2S,3R,4R,5S,6R)-4-(dodecyloxy)-2,3,...)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#8]-[#6@@H]-1-[#6@H](-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])-[#6@@H](-[#8]-[#6]\[#6]=[#6]\[#6](-[#6])-[#6])-[#6@H](-[#8]-[#6]C#Cc2cccc3c2-[#6](=O)-[#8][C@]3([#6]-[#8])[#6]-[#8]-[#6](=O)-[#6](C([#6])([#6])[#6])C([#6])([#6])[#6])-[#6@@H](-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])-[#6@@H]-1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C62H98O11/c1-16-17-18-19-20-21-22-23-24-25-36-66-53-54(69-39-33-45(4)5)51(67-37-27-29-44(2)3)52(55(70-40-34-46(6)7)56(53)71-41-35-47(8)9)68-38-28-31-48-30-26-32-49-50(48)58(64)73-62(49,42-63)43-72-59(65)57(60(10,11)12)61(13,14)15/h26-27,29-30,32-35,44,51-57,63H,16-25,36-43H2,1-15H3/b29-27+/t51-,52-,53+,54+,55+,56+,62+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50345029

(((R)-1-(hydroxymethyl)-3-oxo-4-(3-((2R,3S,5R,6S)-2...)Show SMILES CCCCCCCCCCCCO[C@@H]1[C@H](OCc2ccccc2)[C@@H](OCc2ccccc2)[C@H](OCC#Cc2cccc3c2C(=O)O[C@]3(CO)COC(=O)C(C(C)(C)C)C(C)(C)C)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C69H88O11/c1-8-9-10-11-12-13-14-15-16-29-43-73-58-60(75-45-51-32-21-17-22-33-51)62(77-47-53-36-25-19-26-37-53)59(63(78-48-54-38-27-20-28-39-54)61(58)76-46-52-34-23-18-24-35-52)74-44-31-41-55-40-30-42-56-57(55)65(71)80-69(56,49-70)50-79-66(72)64(67(2,3)4)68(5,6)7/h17-28,30,32-40,42,58-64,70H,8-16,29,43-50H2,1-7H3/t58-,59+,60+,61-,62+,63-,69-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Advanced Science Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PDBu from human recombinant PKCalpha after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 21: 3587-90 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.108
BindingDB Entry DOI: 10.7270/Q2HM58TF |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Miyagi Cancer Center Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Influenza A virus A/PR/8/34(H1N1) neuraminidase by fluorometric method using 4MU-NeuAc substrate |
Antimicrob Agents Chemother 52: 3484-91 (2008)
Article DOI: 10.1128/AAC.00344-08
BindingDB Entry DOI: 10.7270/Q2BR8T42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Miyagi Cancer Center Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human influenza A virus A/Aichi/2/1968(H3N2) neuraminidase by fluorometric method using 4MU-NeuAc substrate |
Antimicrob Agents Chemother 52: 3484-91 (2008)
Article DOI: 10.1128/AAC.00344-08
BindingDB Entry DOI: 10.7270/Q2BR8T42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM50330326

((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...)Show SMILES [#6]-[#6](=O)-[#7]-[#6@@H]-1-[#6@H](-[#6]=[#6](-[#8]-[#6@H]-1-[#6@H](-[#8])-[#6@H](-[#8])-[#6]-[#8])-[#6](-[#8])=O)\[#7]=[#6](\[#7])-[#7] |r,c:6| Show InChI InChI=1S/C12H20N4O7/c1-4(18)15-8-5(16-12(13)14)2-7(11(21)22)23-10(8)9(20)6(19)3-17/h2,5-6,8-10,17,19-20H,3H2,1H3,(H,15,18)(H,21,22)(H4,13,14,16)/t5-,6+,8+,9+,10+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.66 | n/a | n/a | n/a | n/a | n/a | n/a |
Miyagi Cancer Center Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human influenza A virus A/Aichi/2/1968(H3N2) neuraminidase by fluorometric method using 4MU-NeuAc substrate |
Antimicrob Agents Chemother 52: 3484-91 (2008)
Article DOI: 10.1128/AAC.00344-08
BindingDB Entry DOI: 10.7270/Q2BR8T42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuraminidase
(Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Miyagi Cancer Center Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human Influenza A virus A/PR/8/34(H1N1) neuraminidase by fluorometric method using 4MU-NeuAc substrate |
Antimicrob Agents Chemother 52: 3484-91 (2008)
Article DOI: 10.1128/AAC.00344-08
BindingDB Entry DOI: 10.7270/Q2BR8T42 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50388826

(CHEMBL2062715)Show SMILES Oc1c(Cl)c(N=CCN2CCCCC2)c(O)c2ncccc12 |w:5.4| Show InChI InChI=1S/C16H18ClN3O2/c17-12-14(19-7-10-20-8-2-1-3-9-20)16(22)13-11(15(12)21)5-4-6-18-13/h4-7,21-22H,1-3,8-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25B in presence of 2 mM DTT |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50388826

(CHEMBL2062715)Show SMILES Oc1c(Cl)c(N=CCN2CCCCC2)c(O)c2ncccc12 |w:5.4| Show InChI InChI=1S/C16H18ClN3O2/c17-12-14(19-7-10-20-8-2-1-3-9-20)16(22)13-11(15(12)21)5-4-6-18-13/h4-7,21-22H,1-3,8-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25A in presence of 2 mM DTT |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50388822
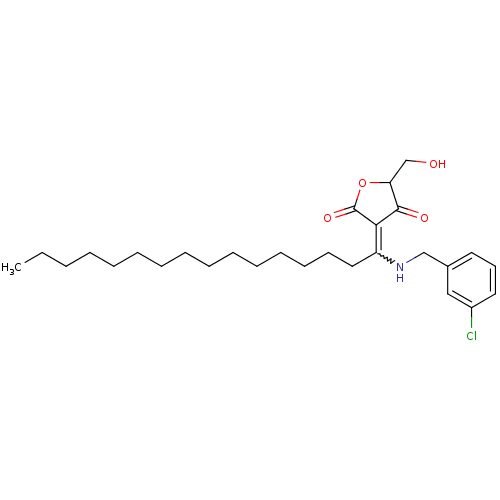
(CHEMBL2062592)Show SMILES CCCCCCCCCCCCCCCC(NCc1cccc(Cl)c1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H42ClNO4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-18-24(26-27(32)25(21-31)34-28(26)33)30-20-22-16-15-17-23(29)19-22/h15-17,19,25,30-31H,2-14,18,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of VHR |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50012280

(CHEMBL3260030)Show SMILES CCCCCCCCCCCCCCC\C(NCc1cccc(C)c1)=C1\C(=O)OC(CO)C1=O Show InChI InChI=1S/C29H45NO4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-19-25(27-28(32)26(22-31)34-29(27)33)30-21-24-18-16-17-23(2)20-24/h16-18,20,26,30-31H,3-15,19,21-22H2,1-2H3/b27-25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Science and Technology Agency
Curated by ChEMBL
| Assay Description
Inhibition of Vaccinia H1-related phosphatase (unknown origin) by fluorescence emission assay |
Bioorg Med Chem 22: 2771-82 (2014)
Article DOI: 10.1016/j.bmc.2014.03.012
BindingDB Entry DOI: 10.7270/Q24T6KXJ |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50388821

(CHEMBL2062591)Show SMILES CCCCCCCCCCCCCCCC(NCc1cccc(C)c1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C29H45NO4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-19-25(27-28(32)26(22-31)34-29(27)33)30-21-24-18-16-17-23(2)20-24/h16-18,20,26,30-31H,3-15,19,21-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of VHR |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50388809

(CHEMBL2062597)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccc(C)cc1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C29H45NO4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-25(27-28(32)26(22-31)34-29(27)33)30-21-24-19-17-23(2)18-20-24/h17-20,26,30-31H,3-16,21-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of VHR |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50388824
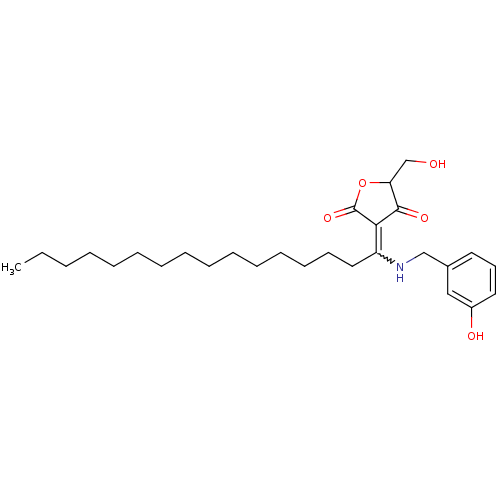
(CHEMBL2062594)Show SMILES CCCCCCCCCCCCCCCC(NCc1cccc(O)c1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H43NO5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-18-24(26-27(32)25(21-30)34-28(26)33)29-20-22-16-15-17-23(31)19-22/h15-17,19,25,29-31H,2-14,18,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25B |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50388809

(CHEMBL2062597)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccc(C)cc1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C29H45NO4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-25(27-28(32)26(22-31)34-29(27)33)30-21-24-19-17-23(2)18-20-24/h17-20,26,30-31H,3-16,21-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25B |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50388810
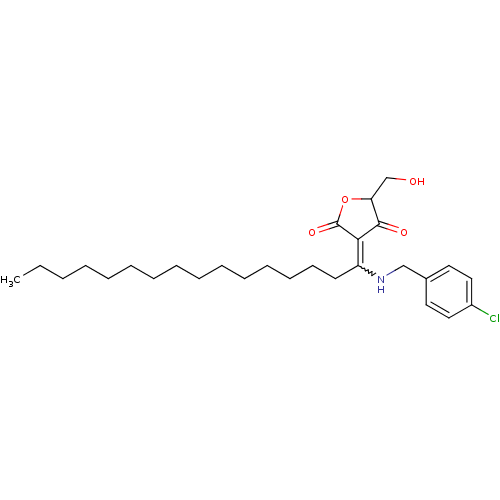
(CHEMBL2062598)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccc(Cl)cc1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H42ClNO4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-24(26-27(32)25(21-31)34-28(26)33)30-20-22-16-18-23(29)19-17-22/h16-19,25,30-31H,2-15,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of VHR |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50388812

(CHEMBL2062707)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccc(O)cc1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H43NO5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-24(26-27(32)25(21-30)34-28(26)33)29-20-22-16-18-23(31)19-17-22/h16-19,25,29-31H,2-15,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25B |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50388818

(CHEMBL2062712)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccccc1O)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H43NO5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-18-23(26-27(32)25(21-30)34-28(26)33)29-20-22-17-15-16-19-24(22)31/h15-17,19,25,29-31H,2-14,18,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25B |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50388818

(CHEMBL2062712)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccccc1O)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H43NO5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-18-23(26-27(32)25(21-30)34-28(26)33)29-20-22-17-15-16-19-24(22)31/h15-17,19,25,29-31H,2-14,18,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25B in presence of 2 mM DTT |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50012323
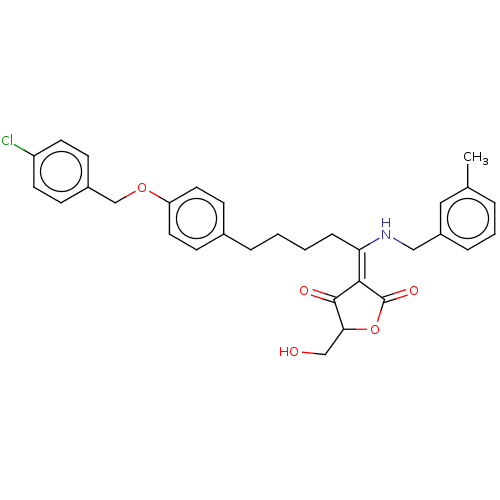
(CHEMBL3260378)Show SMILES Cc1cccc(CN\C(CCCCc2ccc(OCc3ccc(Cl)cc3)cc2)=C2/C(=O)OC(CO)C2=O)c1 Show InChI InChI=1S/C31H32ClNO5/c1-21-5-4-7-24(17-21)18-33-27(29-30(35)28(19-34)38-31(29)36)8-3-2-6-22-11-15-26(16-12-22)37-20-23-9-13-25(32)14-10-23/h4-5,7,9-17,28,33-34H,2-3,6,8,18-20H2,1H3/b29-27- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Japan Science and Technology Agency
Curated by ChEMBL
| Assay Description
Inhibition of Vaccinia H1-related phosphatase (unknown origin) by fluorescence emission assay in presence of 0.001% NP-40 |
Bioorg Med Chem 22: 2771-82 (2014)
Article DOI: 10.1016/j.bmc.2014.03.012
BindingDB Entry DOI: 10.7270/Q24T6KXJ |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50388823

(CHEMBL2062593)Show SMILES CCCCCCCCCCCCCCCC(NCc1cccc(F)c1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H42FNO4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-18-24(26-27(32)25(21-31)34-28(26)33)30-20-22-16-15-17-23(29)19-22/h15-17,19,25,30-31H,2-14,18,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of VHR |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50388807
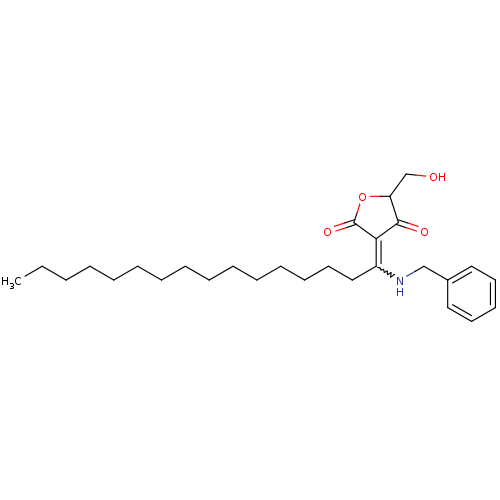
(CHEMBL2062590)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccccc1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H43NO4/c1-2-3-4-5-6-7-8-9-10-11-12-13-17-20-24(29-21-23-18-15-14-16-19-23)26-27(31)25(22-30)33-28(26)32/h14-16,18-19,25,29-30H,2-13,17,20-22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25A in presence of 100 mM DTT |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50388823

(CHEMBL2062593)Show SMILES CCCCCCCCCCCCCCCC(NCc1cccc(F)c1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H42FNO4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-18-24(26-27(32)25(21-31)34-28(26)33)30-20-22-16-15-17-23(29)19-22/h15-17,19,25,30-31H,2-14,18,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25B |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50388808

(CHEMBL2062596)Show SMILES CCCCCCCCCCCCCCCC(NCc1cccc(c1)N(C)C)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C30H48N2O4/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-20-26(28-29(34)27(23-33)36-30(28)35)31-22-24-18-17-19-25(21-24)32(2)3/h17-19,21,27,31,33H,4-16,20,22-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of VHR |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50388820
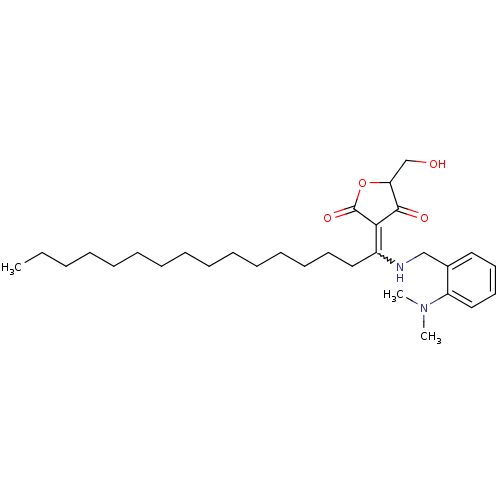
(CHEMBL2062714)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccccc1N(C)C)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C30H48N2O4/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-20-25(28-29(34)27(23-33)36-30(28)35)31-22-24-19-17-18-21-26(24)32(2)3/h17-19,21,27,31,33H,4-16,20,22-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of VHR |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50388810

(CHEMBL2062598)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccc(Cl)cc1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H42ClNO4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-24(26-27(32)25(21-31)34-28(26)33)30-20-22-16-18-23(29)19-17-22/h16-19,25,30-31H,2-15,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25B |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50388825
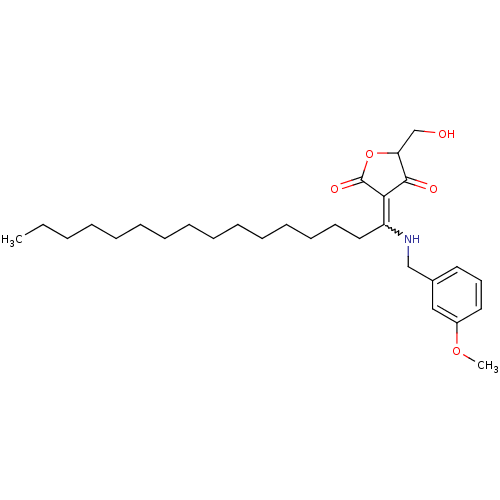
(CHEMBL2062595)Show SMILES CCCCCCCCCCCCCCCC(NCc1cccc(OC)c1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C29H45NO5/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-19-25(27-28(32)26(22-31)35-29(27)33)30-21-23-17-16-18-24(20-23)34-2/h16-18,20,26,30-31H,3-15,19,21-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of VHR |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50388818

(CHEMBL2062712)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccccc1O)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H43NO5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-18-23(26-27(32)25(21-30)34-28(26)33)29-20-22-17-15-16-19-24(22)31/h15-17,19,25,29-31H,2-14,18,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25A in presence of 100 mM DTT |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50388820

(CHEMBL2062714)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccccc1N(C)C)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C30H48N2O4/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-20-25(28-29(34)27(23-33)36-30(28)35)31-22-24-19-17-18-21-26(24)32(2)3/h17-19,21,27,31,33H,4-16,20,22-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25B |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50388823

(CHEMBL2062593)Show SMILES CCCCCCCCCCCCCCCC(NCc1cccc(F)c1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H42FNO4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-18-24(26-27(32)25(21-31)34-28(26)33)30-20-22-16-15-17-23(29)19-22/h15-17,19,25,30-31H,2-14,18,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25A |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50388814

(CHEMBL2062709)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccc(cc1)N(C)C)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C30H48N2O4/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26(28-29(34)27(23-33)36-30(28)35)31-22-24-18-20-25(21-19-24)32(2)3/h18-21,27,31,33H,4-17,22-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25B |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50388810

(CHEMBL2062598)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccc(Cl)cc1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C28H42ClNO4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-24(26-27(32)25(21-31)34-28(26)33)30-20-22-16-18-23(29)19-17-22/h16-19,25,30-31H,2-15,20-21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25A |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50388808

(CHEMBL2062596)Show SMILES CCCCCCCCCCCCCCCC(NCc1cccc(c1)N(C)C)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C30H48N2O4/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-20-26(28-29(34)27(23-33)36-30(28)35)31-22-24-18-17-19-25(21-24)32(2)3/h17-19,21,27,31,33H,4-16,20,22-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CDC25B |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
(Homo sapiens (Human)) | BDBM50388819
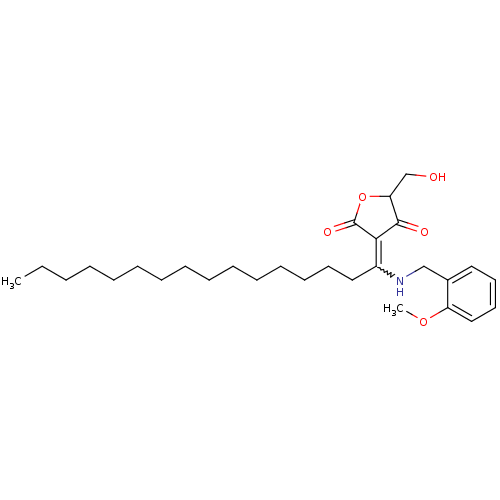
(CHEMBL2062713)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccccc1OC)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C29H45NO5/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-19-24(27-28(32)26(22-31)35-29(27)33)30-21-23-18-16-17-20-25(23)34-2/h16-18,20,26,30-31H,3-15,19,21-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of VHR |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |
Dual specificity protein phosphatase 3
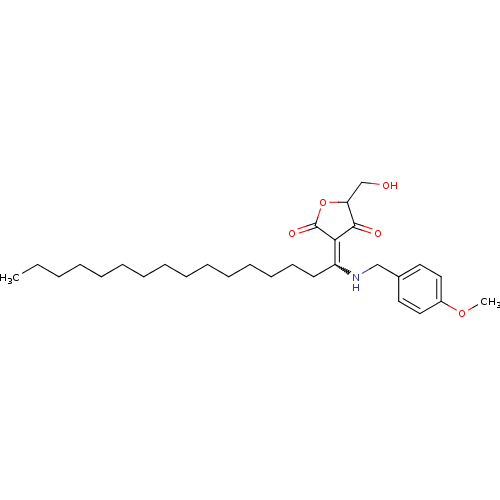
(Homo sapiens (Human)) | BDBM50388813
(CHEMBL2062708)Show SMILES CCCCCCCCCCCCCCCC(NCc1ccc(OC)cc1)=C1C(=O)OC(CO)C1=O |w:15.15| Show InChI InChI=1S/C29H45NO5/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-25(27-28(32)26(22-31)35-29(27)33)30-21-23-17-19-24(34-2)20-18-23/h17-20,26,30-31H,3-16,21-22H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of VHR |
ACS Med Chem Lett 3: 294-298 (2012)
Article DOI: 10.1021/ml2002778
BindingDB Entry DOI: 10.7270/Q2ZW1N0H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data