

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291847 (7-[3-(Quinolin-2-ylmethoxy)-benzyloxy]-2-(1H-tetra...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17284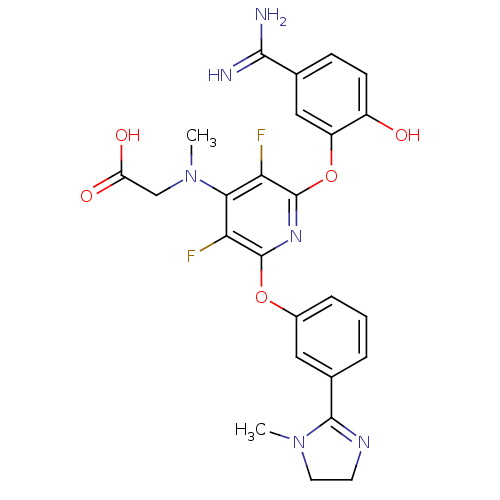 (2-{[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-diflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Biochemistry 39: 12534-42 (2000) Article DOI: 10.1021/bi001477q BindingDB Entry DOI: 10.7270/Q2C827JM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17280 (1-[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-difluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999) Article DOI: 10.1107/s0907444999007350 BindingDB Entry DOI: 10.7270/Q2H1308B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17284 (2-{[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-diflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17280 (1-[2-(5-carbamimidoyl-2-hydroxyphenoxy)-3,5-difluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.110 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Biochemistry 39: 12534-42 (2000) Article DOI: 10.1021/bi001477q BindingDB Entry DOI: 10.7270/Q2C827JM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291855 (7-[3-(Quinolin-2-ylmethoxy)-phenoxymethyl]-2-(1H-t...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066635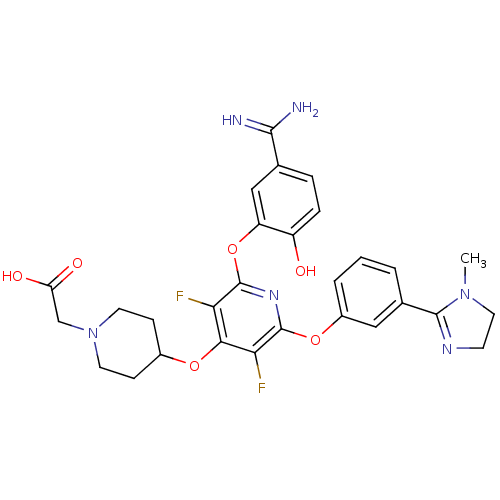 ((4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-difl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066619 (({2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-difluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17282 (7-({6-[(1-ethanimidoylpiperidin-4-yl)oxy]-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.270 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999) Article DOI: 10.1107/s0907444999007350 BindingDB Entry DOI: 10.7270/Q2H1308B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50009075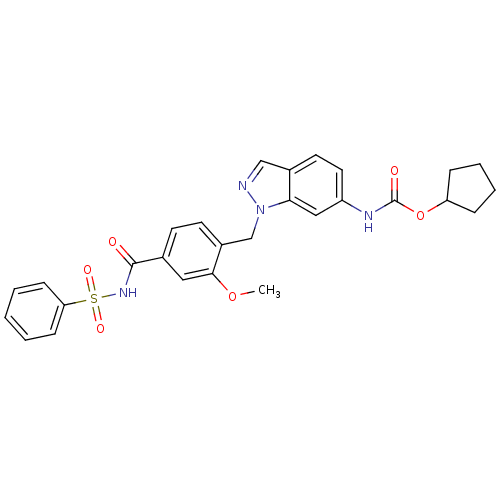 (CHEMBL22033 | ICI 198615 | ICI-198615 | [1-(4-Benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17259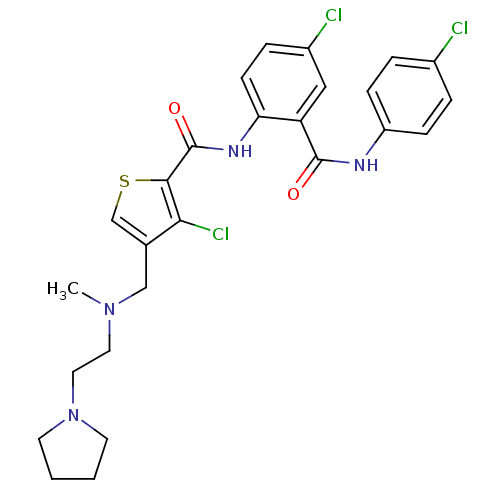 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291848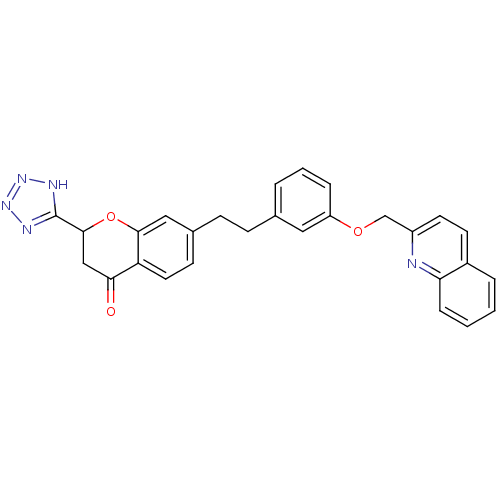 (7-{2-[3-(Quinolin-2-ylmethoxy)-phenyl]-ethyl}-2-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066634 ((4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-difl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17226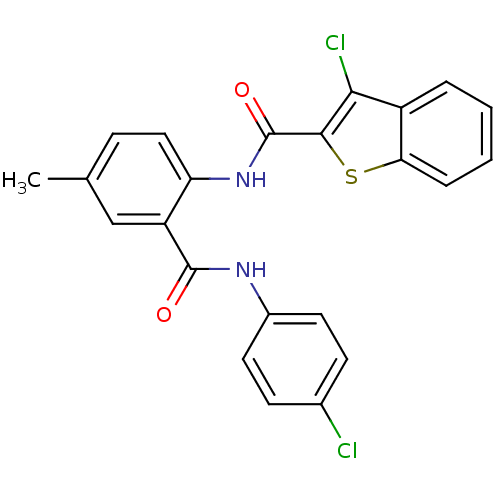 (3-chloro-N-{2-[(4-chlorophenyl)carbamoyl]-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition constant against human factor Xa | Bioorg Med Chem Lett 13: 507-11 (2003) BindingDB Entry DOI: 10.7270/Q21N829C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066641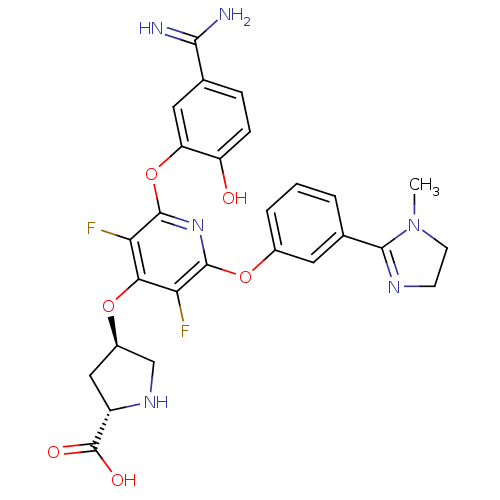 ((2S,4R)-4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17226 (3-chloro-N-{2-[(4-chlorophenyl)carbamoyl]-4-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17261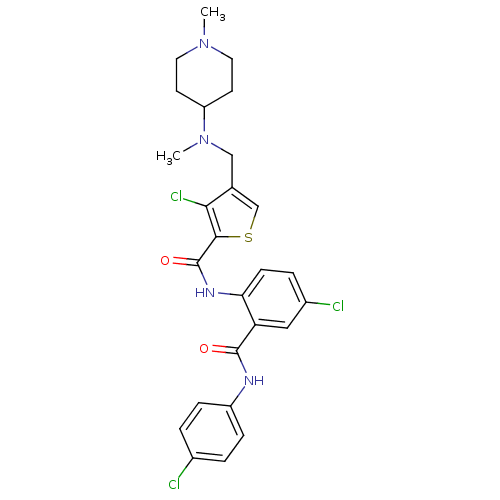 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17269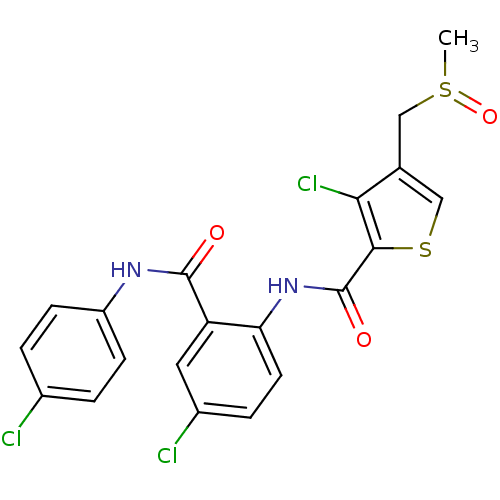 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066623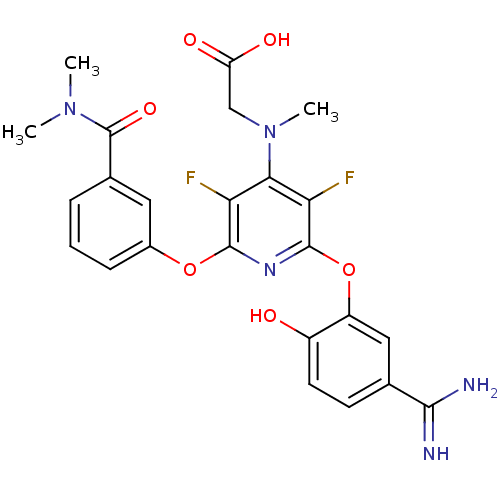 (CHEMBL72318 | {[2-(5-Carbamimidoyl-2-hydroxy-pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066639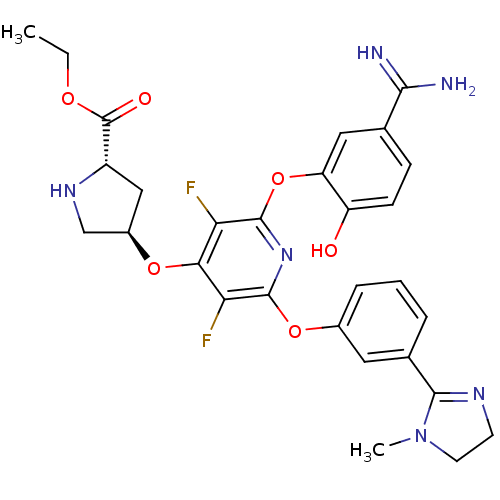 ((2S,4R)-4-{2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17256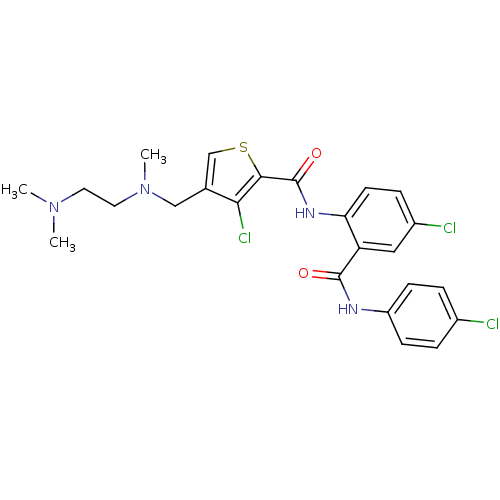 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50404108 (CHEMBL147645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition constant against human factor Xa | Bioorg Med Chem Lett 13: 507-11 (2003) BindingDB Entry DOI: 10.7270/Q21N829C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17257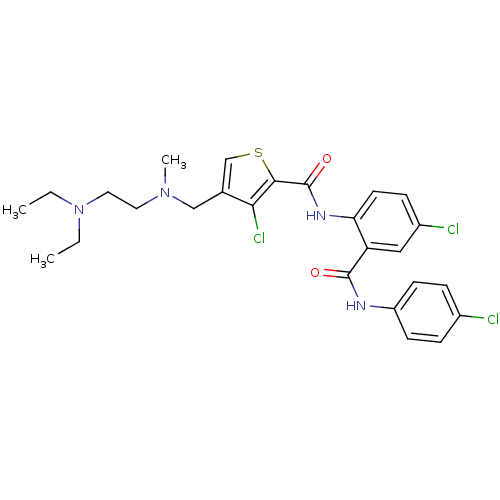 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17227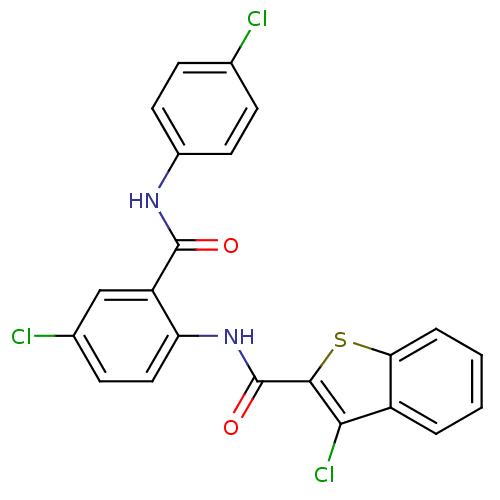 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -52.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17258 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17227 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition constant against human factor Xa | Bioorg Med Chem Lett 13: 507-11 (2003) BindingDB Entry DOI: 10.7270/Q21N829C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066625 (CHEMBL120438 | {4-[2-(5-Carbamimidoyl-2-hydroxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17277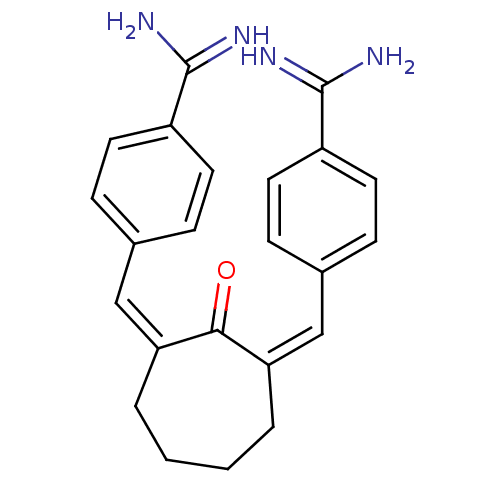 ((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.660 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999) Article DOI: 10.1107/s0907444999007350 BindingDB Entry DOI: 10.7270/Q2H1308B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17277 ((Z,Z)BABCH | 4-{[(1Z,3Z)-3-[(4-carbamimidoylphenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066628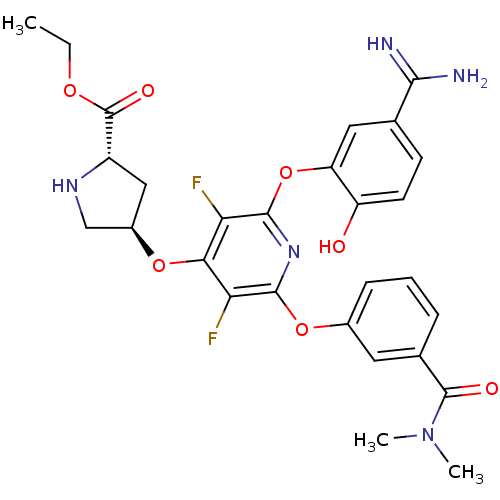 ((2S,4R)-4-[2-(5-Carbamimidoyl-2-hydroxy-phenoxy)-6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17268 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17252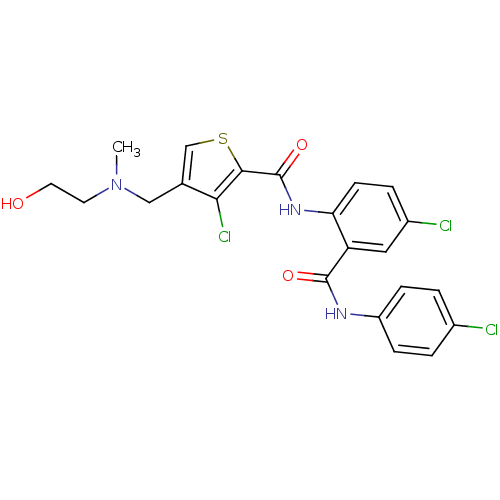 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17228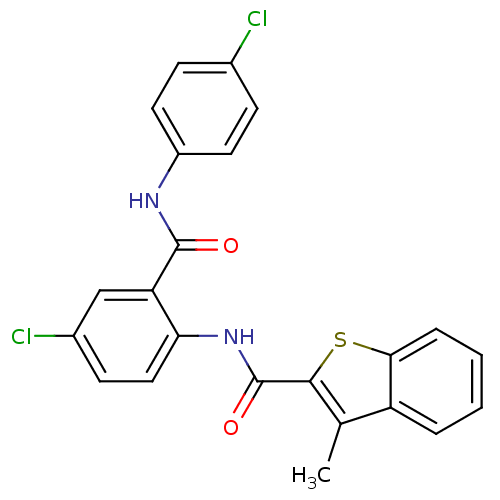 (N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]phenyl}-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.820 | -51.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17231 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.950 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17085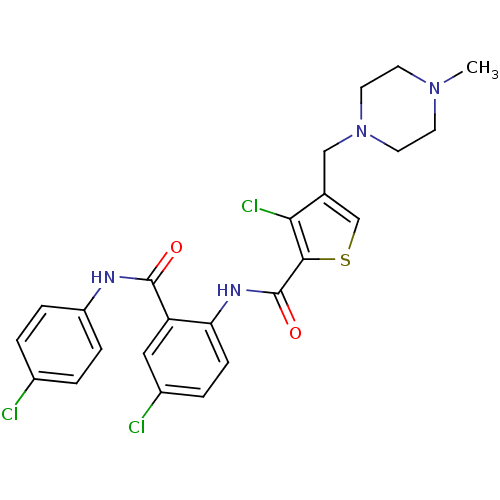 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291849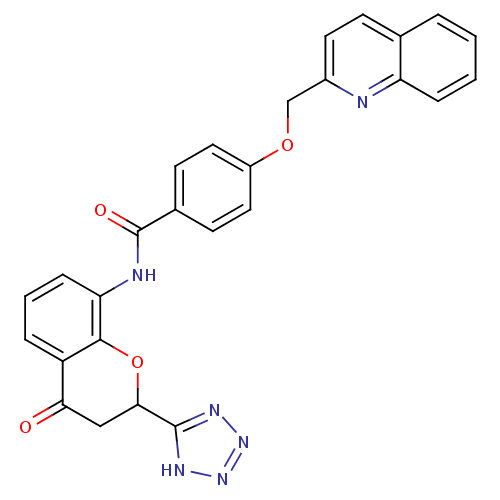 (CHEMBL47662 | N-[4-Oxo-2-(1H-tetrazol-5-yl)-chroma...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291850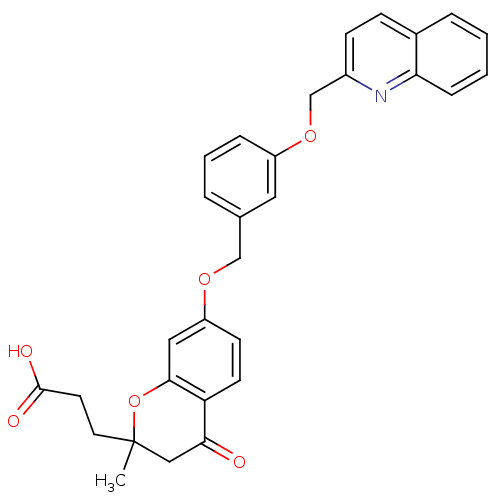 (3-{2-Methyl-4-oxo-7-[3-(quinolin-2-ylmethoxy)-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50066638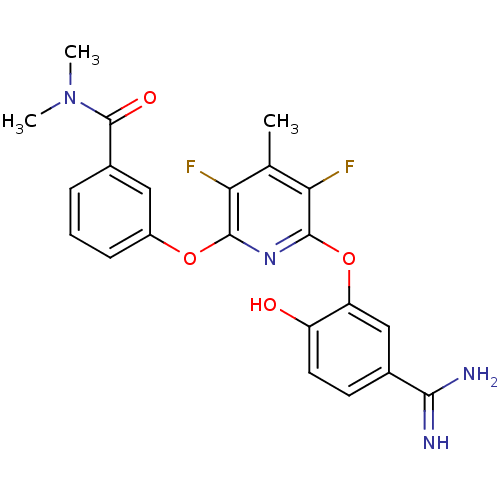 (3-[6-(5-Carbamimidoyl-2-hydroxy-phenoxy)-3,5-diflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibitory potency was measured against human coagulation factor X | J Med Chem 41: 3557-62 (1998) Article DOI: 10.1021/jm980280h BindingDB Entry DOI: 10.7270/Q2KS6QPR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50291854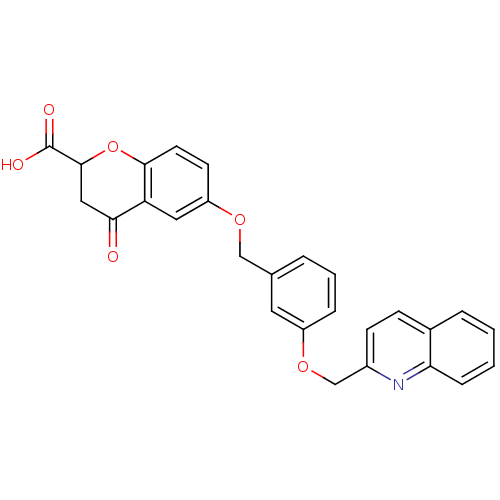 (4-Oxo-6-[3-(quinolin-2-ylmethoxy)-benzyloxy]-chrom...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against LTD4 receptor in guinea pig lung membranes. | J Med Chem 34: 1704-7 (1991) BindingDB Entry DOI: 10.7270/Q2FJ2FR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17264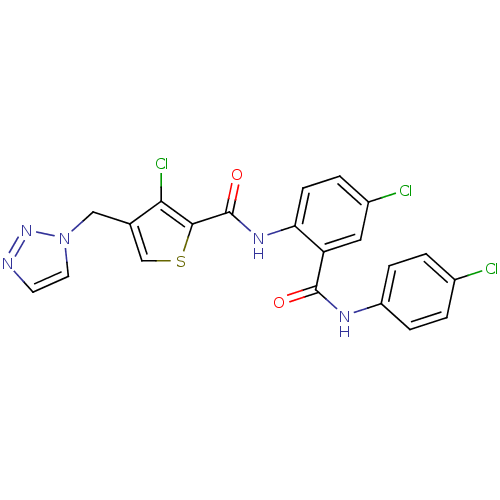 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17253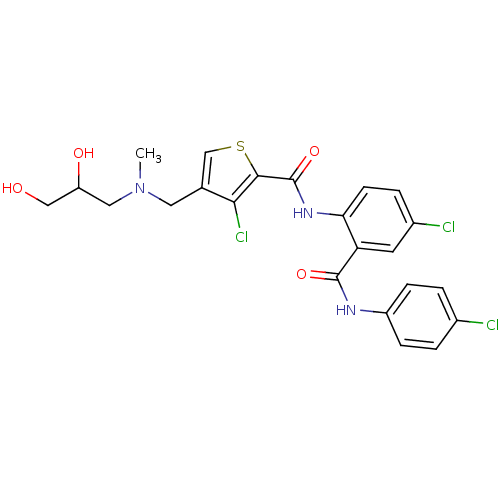 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17255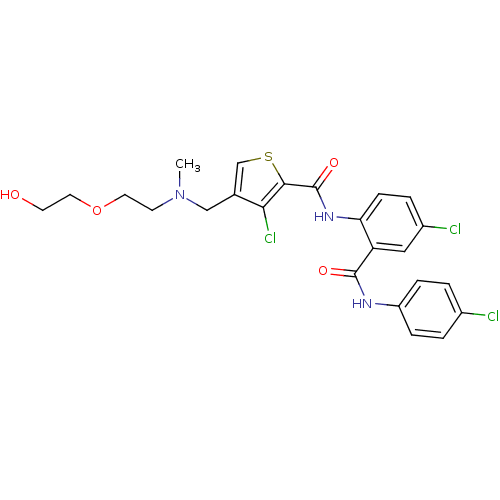 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17281 (7-({2-[(1-ethanimidoylpiperidin-4-yl)oxy]-9H-carba...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.60 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Acta Crystallogr D Biol Crystallogr 55: 1395-404 (1999) Article DOI: 10.1107/s0907444999007350 BindingDB Entry DOI: 10.7270/Q2H1308B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17263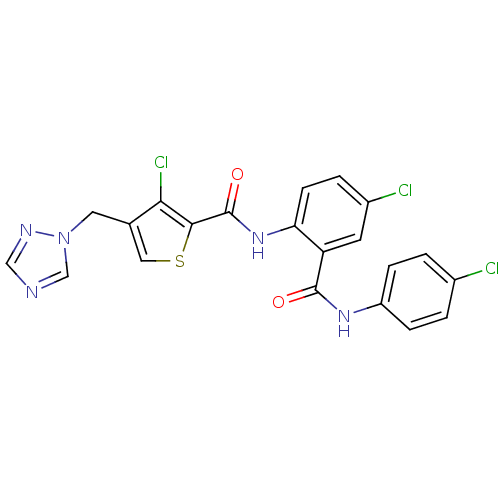 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17248 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17266 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50404133 (CHEMBL356112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex Biosciences Curated by ChEMBL | Assay Description Inhibition constant against human factor Xa | Bioorg Med Chem Lett 13: 507-11 (2003) BindingDB Entry DOI: 10.7270/Q21N829C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17242 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17267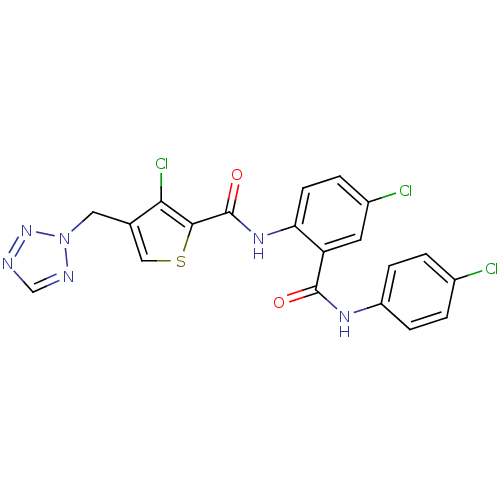 (3-chloro-N-{4-chloro-2-[(4-chlorophenyl)carbamoyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Berlex | Assay Description The enzyme activities were determined kinetically as the initial rate of cleavage of a peptide p-nitroanilide. Km for enzyme and substrate was determ... | Bioorg Med Chem 15: 2127-46 (2007) Article DOI: 10.1016/j.bmc.2006.12.019 BindingDB Entry DOI: 10.7270/Q2MS3R1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50013556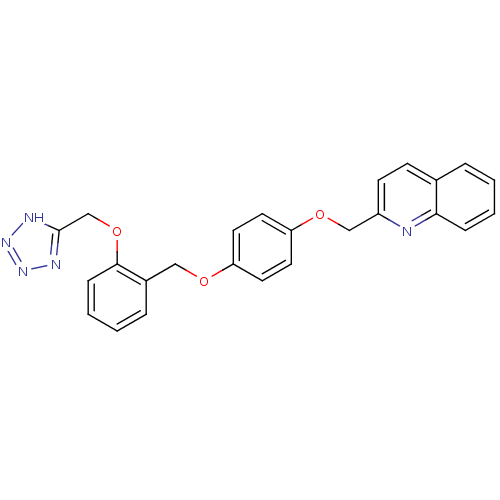 (2-{4-[2-(1H-Tetrazol-5-ylmethoxy)-benzyloxy]-pheno...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description Binding affinity against Cysteinyl leukotriene D4 receptor from guinea pig lung was determined using [3H]-LTD4 (0.2 nM) | J Med Chem 33: 1194-200 (1990) BindingDB Entry DOI: 10.7270/Q2PK0F4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2888 total ) | Next | Last >> |