 Found 115 hits with Last Name = 'stec' and Initial = 'j'
Found 115 hits with Last Name = 'stec' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM50350513
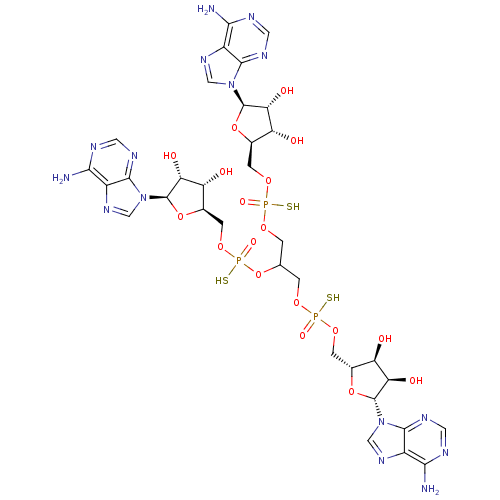
(CHEMBL1812069)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OCC(COP(S)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)OP(S)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C33H44N15O18P3S3/c34-25-16-28(40-6-37-25)46(9-43-16)31-22(52)19(49)13(63-31)3-60-67(55,70)58-1-12(66-69(57,72)62-5-15-21(51)24(54)33(65-15)48-11-45-18-27(36)39-8-42-30(18)48)2-59-68(56,71)61-4-14-20(50)23(53)32(64-14)47-10-44-17-26(35)38-7-41-29(17)47/h6-15,19-24,31-33,49-54H,1-5H2,(H,55,70)(H,56,71)(H,57,72)(H2,34,37,40)(H2,35,38,41)(H2,36,39,42)/t12?,13-,14-,15-,19-,20-,21-,22-,23-,24-,31-,32-,33-,67?,68?,69?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of Fragile histidine triad prorein hydrolytic activity |
Bioorg Med Chem 19: 5053-60 (2011)
Article DOI: 10.1016/j.bmc.2011.06.028
BindingDB Entry DOI: 10.7270/Q28K79GM |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM50350514

(CHEMBL1812068)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OCC(COP(S)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)(COP(S)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)COP(S)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C45H60N20O24P4S4/c46-33-21-37(54-9-50-33)62(13-58-21)41-29(70)25(66)17(86-41)1-78-90(74,94)82-5-45(6-83-91(75,95)79-2-18-26(67)30(71)42(87-18)63-14-59-22-34(47)51-10-55-38(22)63,7-84-92(76,96)80-3-19-27(68)31(72)43(88-19)64-15-60-23-35(48)52-11-56-39(23)64)8-85-93(77,97)81-4-20-28(69)32(73)44(89-20)65-16-61-24-36(49)53-12-57-40(24)65/h9-20,25-32,41-44,66-73H,1-8H2,(H,74,94)(H,75,95)(H,76,96)(H,77,97)(H2,46,50,54)(H2,47,51,55)(H2,48,52,56)(H2,49,53,57)/t17-,18-,19-,20-,25-,26-,27-,28-,29-,30-,31-,32-,41-,42-,43-,44-,45?,90?,91?,92?,93?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of Fragile histidine triad prorein hydrolytic activity |
Bioorg Med Chem 19: 5053-60 (2011)
Article DOI: 10.1016/j.bmc.2011.06.028
BindingDB Entry DOI: 10.7270/Q28K79GM |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM50350515

(CHEMBL1812067)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OCC(COP(O)(S)=O)(COP(S)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)COP(S)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C35H49N15O21P4S4/c36-26-17-29(42-8-39-26)48(11-45-17)32-23(54)20(51)14(69-32)1-62-73(59,77)66-5-35(4-65-72(57,58)76,6-67-74(60,78)63-2-15-21(52)24(55)33(70-15)49-12-46-18-27(37)40-9-43-30(18)49)7-68-75(61,79)64-3-16-22(53)25(56)34(71-16)50-13-47-19-28(38)41-10-44-31(19)50/h8-16,20-25,32-34,51-56H,1-7H2,(H,59,77)(H,60,78)(H,61,79)(H2,36,39,42)(H2,37,40,43)(H2,38,41,44)(H2,57,58,76)/t14-,15-,16-,20-,21-,22-,23-,24-,25-,32-,33-,34-,35?,73?,74?,75?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of Fragile histidine triad prorein hydrolytic activity |
Bioorg Med Chem 19: 5053-60 (2011)
Article DOI: 10.1016/j.bmc.2011.06.028
BindingDB Entry DOI: 10.7270/Q28K79GM |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM50350516
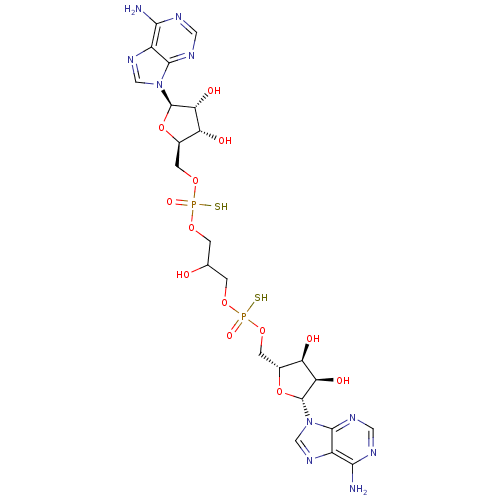
(CHEMBL1812066)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OCC(O)COP(S)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C23H32N10O13P2S2/c24-18-12-20(28-5-26-18)32(7-30-12)22-16(37)14(35)10(45-22)3-43-47(39,49)41-1-9(34)2-42-48(40,50)44-4-11-15(36)17(38)23(46-11)33-8-31-13-19(25)27-6-29-21(13)33/h5-11,14-17,22-23,34-38H,1-4H2,(H,39,49)(H,40,50)(H2,24,26,28)(H2,25,27,29)/t9?,10-,11-,14-,15-,16-,17-,22-,23-,47?,48?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of Fragile histidine triad prorein hydrolytic activity |
Bioorg Med Chem 19: 5053-60 (2011)
Article DOI: 10.1016/j.bmc.2011.06.028
BindingDB Entry DOI: 10.7270/Q28K79GM |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM50350512
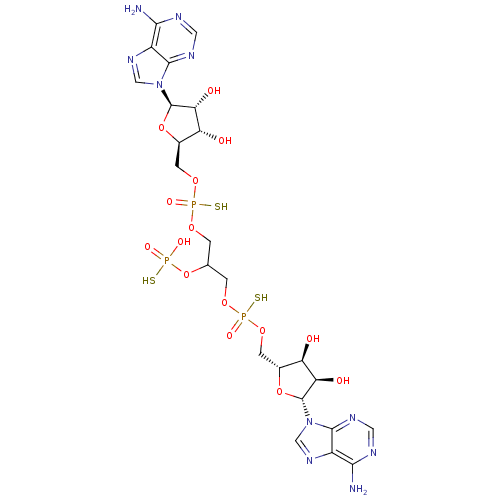
(CHEMBL1812070)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(S)(=O)OCC(COP(S)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)OP(O)(S)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C23H33N10O15P3S3/c24-18-12-20(28-5-26-18)32(7-30-12)22-16(36)14(34)10(46-22)3-44-50(40,53)42-1-9(48-49(38,39)52)2-43-51(41,54)45-4-11-15(35)17(37)23(47-11)33-8-31-13-19(25)27-6-29-21(13)33/h5-11,14-17,22-23,34-37H,1-4H2,(H,40,53)(H,41,54)(H2,24,26,28)(H2,25,27,29)(H2,38,39,52)/t9?,10-,11-,14-,15-,16-,17-,22-,23-,50?,51?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Polish Academy of Sciences
Curated by ChEMBL
| Assay Description
Inhibition of Fragile histidine triad prorein hydrolytic activity |
Bioorg Med Chem 19: 5053-60 (2011)
Article DOI: 10.1016/j.bmc.2011.06.028
BindingDB Entry DOI: 10.7270/Q28K79GM |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81576
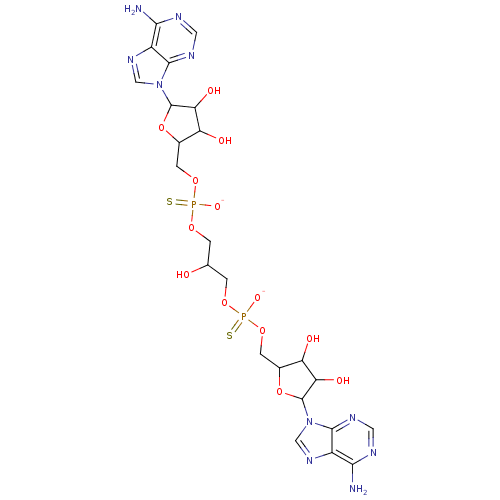
(AppppA analog, 6 (X=S))Show SMILES Nc1ncnc2n(cnc12)C1OC(COP([O-])(=S)OCC(O)COP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)C(O)C1O Show InChI InChI=1S/C23H32N10O13P2S2/c24-18-12-20(28-5-26-18)32(7-30-12)22-16(37)14(35)10(45-22)3-43-47(39,49)41-1-9(34)2-42-48(40,50)44-4-11-15(36)17(38)23(46-11)33-8-31-13-19(25)27-6-29-21(13)33/h5-11,14-17,22-23,34-38H,1-4H2,(H,39,49)(H,40,50)(H2,24,26,28)(H2,25,27,29)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81584
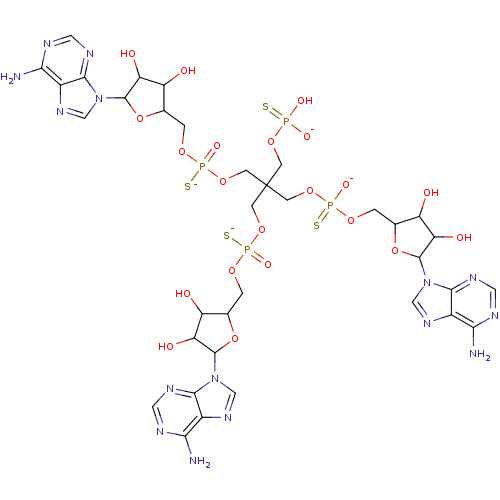
(AppppA analog, 12 (X=S))Show SMILES Nc1ncnc2n(cnc12)C1OC(COP([S-])(=O)OCC(COP(O)([O-])=S)(COP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)COP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)C(O)C1O Show InChI InChI=1S/C35H49N15O21P4S4/c36-26-17-29(42-8-39-26)48(11-45-17)32-23(54)20(51)14(69-32)1-62-73(59,77)66-5-35(4-65-72(57,58)76,6-67-74(60,78)63-2-15-21(52)24(55)33(70-15)49-12-46-18-27(37)40-9-43-30(18)49)7-68-75(61,79)64-3-16-22(53)25(56)34(71-16)50-13-47-19-28(38)41-10-44-31(19)50/h8-16,20-25,32-34,51-56H,1-7H2,(H,59,77)(H,60,78)(H,61,79)(H2,36,39,42)(H2,37,40,43)(H2,38,41,44)(H2,57,58,76)/p-4 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81586

(AppppA analog, 13 (X=S))Show SMILES Nc1ncnc2n(cnc12)C1OC(COP([O-])(=S)OCC(COP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)(COP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)COP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)C(O)C1O Show InChI InChI=1S/C45H60N20O24P4S4/c46-33-21-37(54-9-50-33)62(13-58-21)41-29(70)25(66)17(86-41)1-78-90(74,94)82-5-45(6-83-91(75,95)79-2-18-26(67)30(71)42(87-18)63-14-59-22-34(47)51-10-55-38(22)63,7-84-92(76,96)80-3-19-27(68)31(72)43(88-19)64-15-60-23-35(48)52-11-56-39(23)64)8-85-93(77,97)81-4-20-28(69)32(73)44(89-20)65-16-61-24-36(49)53-12-57-40(24)65/h9-20,25-32,41-44,66-73H,1-8H2,(H,74,94)(H,75,95)(H,76,96)(H,77,97)(H2,46,50,54)(H2,47,51,55)(H2,48,52,56)(H2,49,53,57)/p-4 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81590

(AppppA analog, 15 (X=S))Show SMILES Nc1ncnc2n(cnc12)C1OC(COP([O-])(=S)OCC(COP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)OP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)C(O)C1O Show InChI InChI=1S/C33H44N15O18P3S3/c34-25-16-28(40-6-37-25)46(9-43-16)31-22(52)19(49)13(63-31)3-60-67(55,70)58-1-12(66-69(57,72)62-5-15-21(51)24(54)33(65-15)48-11-45-18-27(36)39-8-42-30(18)48)2-59-68(56,71)61-4-14-20(50)23(53)32(64-14)47-10-44-17-26(35)38-7-41-29(17)47/h6-15,19-24,31-33,49-54H,1-5H2,(H,55,70)(H,56,71)(H,57,72)(H2,34,37,40)(H2,35,38,41)(H2,36,39,42)/p-3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81593

(AppppA analog, 17 (X=S))Show SMILES Nc1ncnc2n(cnc12)C1OC(COP([O-])(=S)OCC(COP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)OP([O-])([S-])=O)C(O)C1O Show InChI InChI=1S/C23H33N10O15P3S3/c24-18-12-20(28-5-26-18)32(7-30-12)22-16(36)14(34)10(46-22)3-44-50(40,53)42-1-9(48-49(38,39)52)2-43-51(41,54)45-4-11-15(35)17(37)23(47-11)33-8-31-13-19(25)27-6-29-21(13)33/h5-11,14-17,22-23,34-37H,1-4H2,(H,40,53)(H,41,54)(H2,24,26,28)(H2,25,27,29)(H2,38,39,52)/p-4 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81575
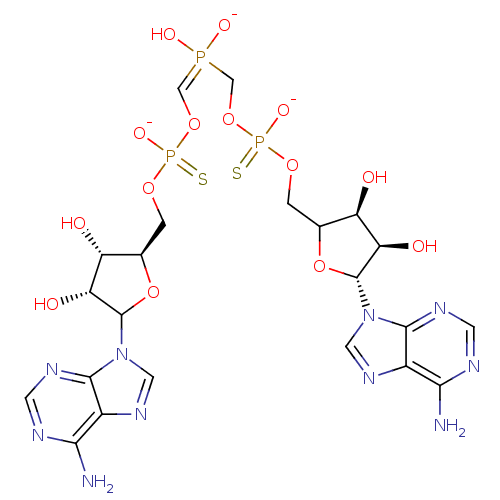
(AppppA analog, 5 (X=S))Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](COP([O-])(=S)OC=P(O)([O-])COP([S-])(=O)OCC2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H30N10O14P3S2/c23-17-11-19(27-3-25-17)31(5-29-11)21-15(35)13(33)9(45-21)1-41-48(39,50)43-7-47(37,38)8-44-49(40,51)42-2-10-14(34)16(36)22(46-10)32-6-30-12-18(24)26-4-28-20(12)32/h3-7,9-10,13-16,21-22,33-36H,1-2,8H2,(H7-,23,24,25,26,27,28,37,38,39,40,50,51)/q-1/p-2/t9-,10?,13-,14-,15-,16-,21?,22-,48?,49?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81580
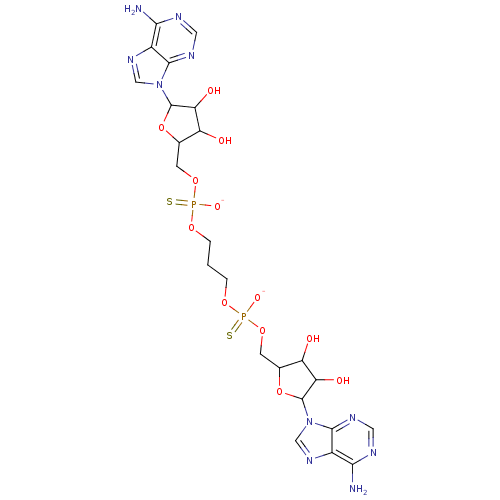
(AppppA analog, 9 (X=S))Show SMILES Nc1ncnc2n(cnc12)C1OC(COP([O-])(=S)OCCCOP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)C(O)C1O Show InChI InChI=1S/C23H32N10O12P2S2/c24-18-12-20(28-6-26-18)32(8-30-12)22-16(36)14(34)10(44-22)4-42-46(38,48)40-2-1-3-41-47(39,49)43-5-11-15(35)17(37)23(45-11)33-9-31-13-19(25)27-7-29-21(13)33/h6-11,14-17,22-23,34-37H,1-5H2,(H,38,48)(H,39,49)(H2,24,26,28)(H2,25,27,29)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81592
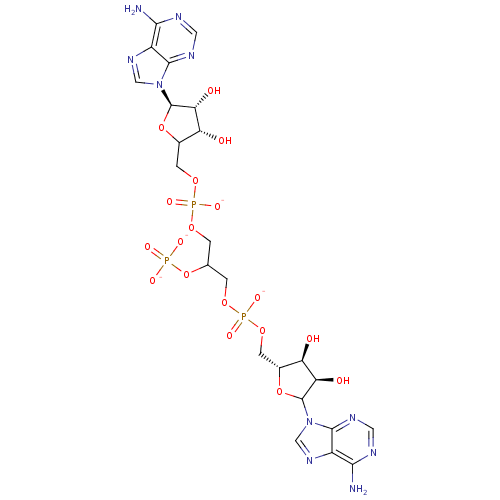
(AppppA analog, 17 (X=O))Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](COP([O-])(=O)OCC(COP([O-])(=O)OCC2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)OP([O-])([O-])=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C23H33N10O18P3/c24-18-12-20(28-5-26-18)32(7-30-12)22-16(36)14(34)10(49-22)3-47-53(41,42)45-1-9(51-52(38,39)40)2-46-54(43,44)48-4-11-15(35)17(37)23(50-11)33-8-31-13-19(25)27-6-29-21(13)33/h5-11,14-17,22-23,34-37H,1-4H2,(H,41,42)(H,43,44)(H2,24,26,28)(H2,25,27,29)(H2,38,39,40)/p-4/t9?,10-,11?,14-,15-,16-,17-,22?,23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81588
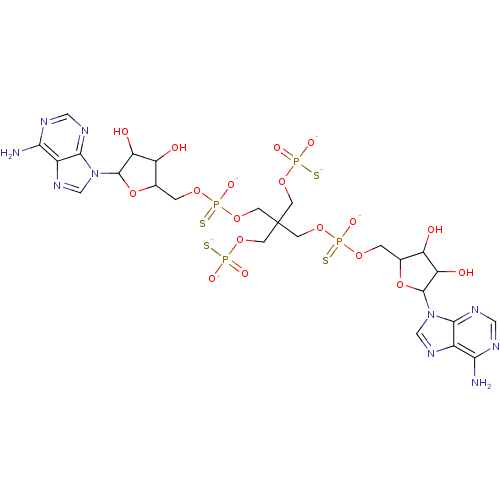
(AppppA analog, 14 (X=S))Show SMILES Nc1ncnc2n(cnc12)C1OC(COP([O-])(=S)OCC(COP([O-])([S-])=O)(COP([O-])([S-])=O)COP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)C(O)C1O Show InChI InChI=1S/C25H38N10O18P4S4/c26-19-13-21(30-7-28-19)34(9-32-13)23-17(38)15(36)11(52-23)1-46-56(44,60)50-5-25(3-48-54(40,41)58,4-49-55(42,43)59)6-51-57(45,61)47-2-12-16(37)18(39)24(53-12)35-10-33-14-20(27)29-8-31-22(14)35/h7-12,15-18,23-24,36-39H,1-6H2,(H,44,60)(H,45,61)(H2,26,28,30)(H2,27,29,31)(H2,40,41,58)(H2,42,43,59)/p-6 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81587

(AppppA analog, 14 (X=O))Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](COP([O-])(=O)OCC(COP([O-])([O-])=O)(COP([O-])([O-])=O)COP([O-])(=O)OCC2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C25H38N10O22P4/c26-19-13-21(30-7-28-19)34(9-32-13)23-17(38)15(36)11(56-23)1-50-60(46,47)54-5-25(3-52-58(40,41)42,4-53-59(43,44)45)6-55-61(48,49)51-2-12-16(37)18(39)24(57-12)35-10-33-14-20(27)29-8-31-22(14)35/h7-12,15-18,23-24,36-39H,1-6H2,(H,46,47)(H,48,49)(H2,26,28,30)(H2,27,29,31)(H2,40,41,42)(H2,43,44,45)/p-6/t11-,12?,15-,16-,17-,18-,23?,24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81589

(AppppA analog, 15 (X=O))Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](COP([O-])(=O)OCC(COP([O-])(=O)OCC2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)OP([O-])(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C33H44N15O21P3/c34-25-16-28(40-6-37-25)46(9-43-16)31-22(52)19(49)13(66-31)3-63-70(55,56)61-1-12(69-72(59,60)65-5-15-21(51)24(54)33(68-15)48-11-45-18-27(36)39-8-42-30(18)48)2-62-71(57,58)64-4-14-20(50)23(53)32(67-14)47-10-44-17-26(35)38-7-41-29(17)47/h6-15,19-24,31-33,49-54H,1-5H2,(H,55,56)(H,57,58)(H,59,60)(H2,34,37,40)(H2,35,38,41)(H2,36,39,42)/p-3/t12?,13-,14?,15-,19-,20-,21-,22-,23-,24-,31?,32-,33?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81574

(AppppA analog, 5 (X=O))Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](COP([O-])(=O)OCP(O)([O-])=COP([O-])(=O)OCC2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H30N10O16P3/c23-17-11-19(27-3-25-17)31(5-29-11)21-15(35)13(33)9(47-21)1-43-50(39,40)45-7-49(37,38)8-46-51(41,42)44-2-10-14(34)16(36)22(48-10)32-6-30-12-18(24)26-4-28-20(12)32/h3-7,9-10,13-16,21-22,33-36H,1-2,8H2,(H7-,23,24,25,26,27,28,37,38,39,40,41,42)/q-1/p-2/t9?,10-,13-,14-,15-,16-,21-,22?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81583

(AppppA analog, 12 (X=O))Show SMILES Nc1ncnc2n(cnc12)C1OC(COP([O-])(=O)OCC(COP(O)([O-])=O)(COP([O-])(=O)OCC2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)COP([O-])(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C35H49N15O25P4/c36-26-17-29(42-8-39-26)48(11-45-17)32-23(54)20(51)14(73-32)1-66-77(60,61)70-5-35(4-69-76(57,58)59,6-71-78(62,63)67-2-15-21(52)24(55)33(74-15)49-12-46-18-27(37)40-9-43-30(18)49)7-72-79(64,65)68-3-16-22(53)25(56)34(75-16)50-13-47-19-28(38)41-10-44-31(19)50/h8-16,20-25,32-34,51-56H,1-7H2,(H,60,61)(H,62,63)(H,64,65)(H2,36,39,42)(H2,37,40,43)(H2,38,41,44)(H2,57,58,59)/p-4/t14-,15?,16?,20-,21-,22+,23-,24-,25+,32?,33-,34?,35?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81570
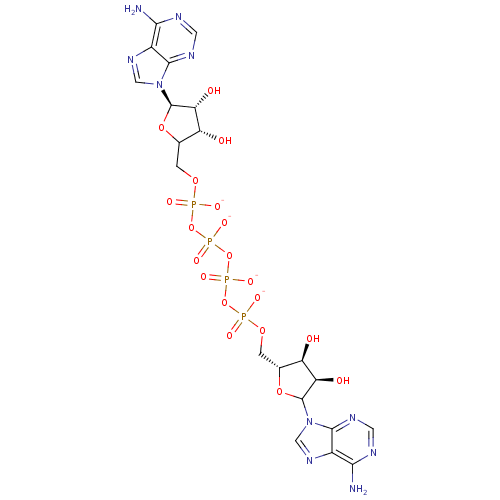
(AppppA analog, 1 (X=O))Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](COP([O-])(=O)OP([O-])(=O)OP([O-])(=O)OP([O-])(=O)OCC2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C20H28N10O19P4/c21-15-9-17(25-3-23-15)29(5-27-9)19-13(33)11(31)7(45-19)1-43-50(35,36)47-52(39,40)49-53(41,42)48-51(37,38)44-2-8-12(32)14(34)20(46-8)30-6-28-10-16(22)24-4-26-18(10)30/h3-8,11-14,19-20,31-34H,1-2H2,(H,35,36)(H,37,38)(H,39,40)(H,41,42)(H2,21,23,25)(H2,22,24,26)/p-4/t7-,8?,11-,12-,13-,14-,19?,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81571
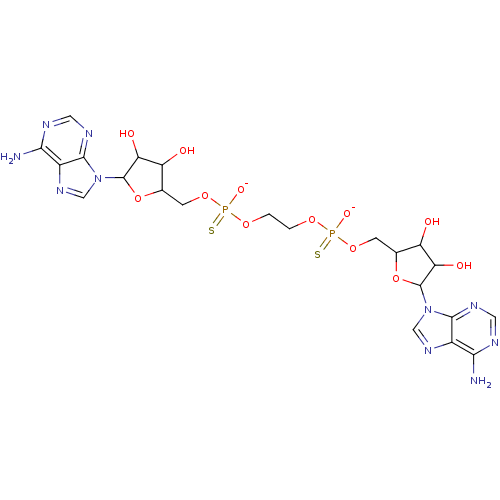
(AppppA analog, 2 (X=S))Show SMILES Nc1ncnc2n(cnc12)C1OC(COP([O-])(=S)OCCOP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)C(O)C1O Show InChI InChI=1S/C22H30N10O12P2S2/c23-17-11-19(27-5-25-17)31(7-29-11)21-15(35)13(33)9(43-21)3-41-45(37,47)39-1-2-40-46(38,48)42-4-10-14(34)16(36)22(44-10)32-8-30-12-18(24)26-6-28-20(12)32/h5-10,13-16,21-22,33-36H,1-4H2,(H,37,47)(H,38,48)(H2,23,25,27)(H2,24,26,28)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81591
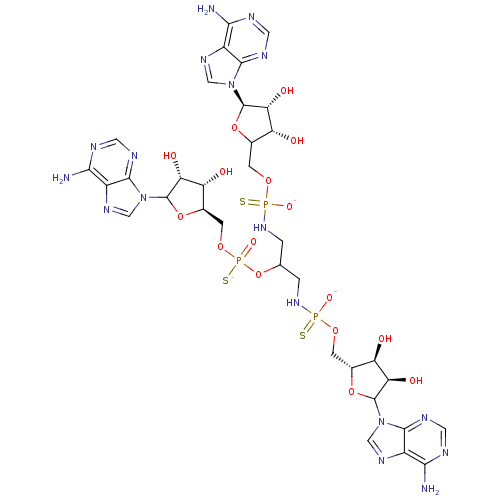
(AppppA analog, 16 (X=S))Show SMILES Nc1ncnc2n(cnc12)[C@@H]1OC(COP([O-])(=S)NCC(CNP([S-])(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)OP([S-])(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C33H46N17O16P3S3/c34-25-16-28(40-6-37-25)48(9-43-16)31-22(54)19(51)13(63-31)3-60-67(57,70)46-1-12(66-69(59,72)62-5-15-21(53)24(56)33(65-15)50-11-45-18-27(36)39-8-42-30(18)50)2-47-68(58,71)61-4-14-20(52)23(55)32(64-14)49-10-44-17-26(35)38-7-41-29(17)49/h6-15,19-24,31-33,51-56H,1-5H2,(H,59,72)(H2,34,37,40)(H2,35,38,41)(H2,36,39,42)(H2,46,57,70)(H2,47,58,71)/p-3/t12?,13-,14?,15-,19-,20-,21-,22-,23-,24-,31?,32-,33?,67?,68?,69?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81579

(AppppA analog, 8 (X=S))Show SMILES NC(COP([O-])(=S)OCC1OC(C(O)C1O)n1cnc2c(N)ncnc12)COP([S-])(=O)OCC1OC(C(O)C1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C23H33N11O12P2S2/c24-9(1-41-47(39,49)43-3-10-14(35)16(37)22(45-10)33-7-31-12-18(25)27-5-29-20(12)33)2-42-48(40,50)44-4-11-15(36)17(38)23(46-11)34-8-32-13-19(26)28-6-30-21(13)34/h5-11,14-17,22-23,35-38H,1-4,24H2,(H,39,49)(H,40,50)(H2,25,27,29)(H2,26,28,30)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81585

(AppppA analog, 13 (X=O))Show SMILES Nc1ncnc2n(cnc12)C1OC(COP([O-])(=O)OCC(COP([O-])(=O)OCC2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)(COP([O-])(=O)OCC2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)COP([O-])(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C45H60N20O28P4/c46-33-21-37(54-9-50-33)62(13-58-21)41-29(70)25(66)17(90-41)1-82-94(74,75)86-5-45(6-87-95(76,77)83-2-18-26(67)30(71)42(91-18)63-14-59-22-34(47)51-10-55-38(22)63,7-88-96(78,79)84-3-19-27(68)31(72)43(92-19)64-15-60-23-35(48)52-11-56-39(23)64)8-89-97(80,81)85-4-20-28(69)32(73)44(93-20)65-16-61-24-36(49)53-12-57-40(24)65/h9-20,25-32,41-44,66-73H,1-8H2,(H,74,75)(H,76,77)(H,78,79)(H,80,81)(H2,46,50,54)(H2,47,51,55)(H2,48,52,56)(H2,49,53,57)/p-4/t17-,18?,19?,20?,25-,26-,27-,28+,29-,30-,31-,32+,41?,42-,43-,44?,45?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81581

(AppppA analog, 10 (X=S))Show SMILES Nc1ncnc2n(cnc12)[C@@H]1OC(COP([O-])(=S)NCCCNP([S-])(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C23H34N12O10P2S2/c24-18-12-20(28-6-26-18)34(8-30-12)22-16(38)14(36)10(44-22)4-42-46(40,48)32-2-1-3-33-47(41,49)43-5-11-15(37)17(39)23(45-11)35-9-31-13-19(25)27-7-29-21(13)35/h6-11,14-17,22-23,36-39H,1-5H2,(H2,24,26,28)(H2,25,27,29)(H2,32,40,48)(H2,33,41,49)/p-2/t10-,11?,14-,15-,16-,17-,22?,23-,46?,47?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81577

(AppppA analog, 6 (X=O))Show SMILES Nc1ncnc2n(cnc12)C1O[C@H](COP([O-])(=O)OCC(O)COP([O-])(=O)OCC2O[C@H]([C@H](O)C2O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H31N10O16P2/c23-16-12-18(27-5-25-16)31(7-29-12)20-15(35)14(34)10(46-20)3-44-49(38,39)42-1-9(33)2-43-50(40,41)45-4-11-47-21(22(36)48(11)37)32-8-30-13-17(24)26-6-28-19(13)32/h5-11,14-15,20-22,33-37H,1-4H2,(H,38,39)(H,40,41)(H2,23,25,27)(H2,24,26,28)/p-2/t9?,10-,11?,14-,15-,20?,21-,22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81582

(AppppA analog, 11 (X=S))Show SMILES Nc1ncnc2n(cnc12)C1OC(COP([O-])(=S)SCCCSP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)C(O)C1O Show InChI InChI=1S/C23H32N10O10P2S4/c24-18-12-20(28-6-26-18)32(8-30-12)22-16(36)14(34)10(42-22)4-40-44(38,46)48-2-1-3-49-45(39,47)41-5-11-15(35)17(37)23(43-11)33-9-31-13-19(25)27-7-29-21(13)33/h6-11,14-17,22-23,34-37H,1-5H2,(H,38,46)(H,39,47)(H2,24,26,28)(H2,25,27,29)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81578

(AppppA analog, 7 (X=S))Show SMILES CC(COP([S-])(=O)OCC1OC(C(O)C1O)n1cnc2c(N)ncnc12)COP([O-])(=S)OCC1OC(C(O)C1O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C24H34N10O12P2S2/c1-10(2-41-47(39,49)43-4-11-15(35)17(37)23(45-11)33-8-31-13-19(25)27-6-29-21(13)33)3-42-48(40,50)44-5-12-16(36)18(38)24(46-12)34-9-32-14-20(26)28-7-30-22(14)34/h6-12,15-18,23-24,35-38H,2-5H2,1H3,(H,39,49)(H,40,50)(H2,25,27,29)(H2,26,28,30)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81573

(AppppA analog, 4 (X=S))Show SMILES Nc1ncnc2n(cnc12)C1OC(COP([O-])(=S)OCC(O)C(O)COP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)C(O)C1O Show InChI InChI=1S/C24H34N10O14P2S2/c25-19-13-21(29-5-27-19)33(7-31-13)23-17(39)15(37)11(47-23)3-45-49(41,51)43-1-9(35)10(36)2-44-50(42,52)46-4-12-16(38)18(40)24(48-12)34-8-32-14-20(26)28-6-30-22(14)34/h5-12,15-18,23-24,35-40H,1-4H2,(H,41,51)(H,42,52)(H2,25,27,29)(H2,26,28,30)/p-2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Bis(5'-adenosyl)-triphosphatase
(Homo sapiens (Human)) | BDBM81572
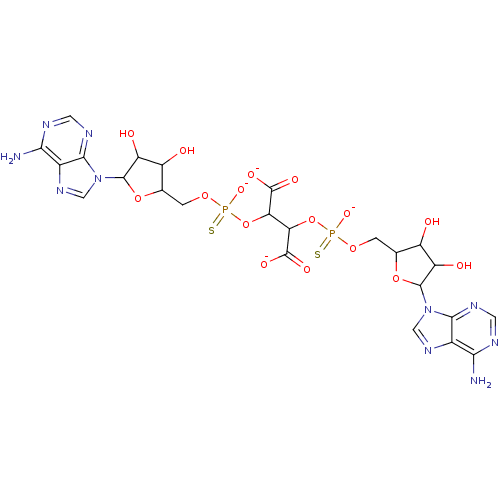
(AppppA analog, 3 (X=S))Show SMILES Nc1ncnc2n(cnc12)C1OC(COP([O-])(=S)OC(C(OP([S-])(=O)OCC2OC(C(O)C2O)n2cnc3c(N)ncnc23)C([O-])=O)C([O-])=O)C(O)C1O Show InChI InChI=1S/C24H30N10O16P2S2/c25-17-9-19(29-3-27-17)33(5-31-9)21-13(37)11(35)7(47-21)1-45-51(43,53)49-15(23(39)40)16(24(41)42)50-52(44,54)46-2-8-12(36)14(38)22(48-8)34-6-32-10-18(26)28-4-30-20(10)34/h3-8,11-16,21-22,35-38H,1-2H2,(H,39,40)(H,41,42)(H,43,53)(H,44,54)(H2,25,27,29)(H2,26,28,30)/p-4 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kimmel Cancer Center
| Assay Description
Inhibition assay using Fhit with ApppBODIPY. |
BMC Chem Biol 1: 3 (2001)
Article DOI: 10.1186/1472-6769-1-3
BindingDB Entry DOI: 10.7270/Q2445JZ9 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM50174768
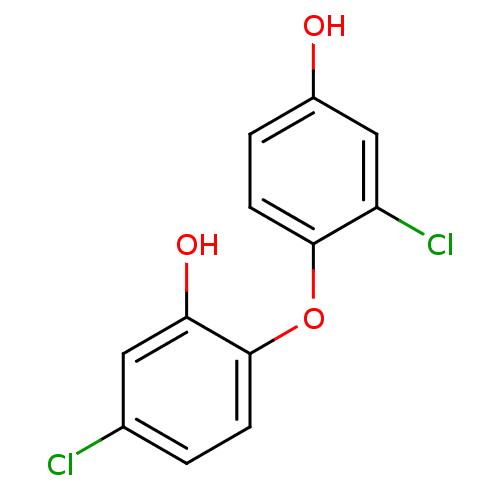
(5‐chloro‐2‐(2‐chloro‐...)Show InChI InChI=1S/C12H8Cl2O3/c13-7-1-3-12(10(16)5-7)17-11-4-2-8(15)6-9(11)14/h1-6,15-16H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM25407
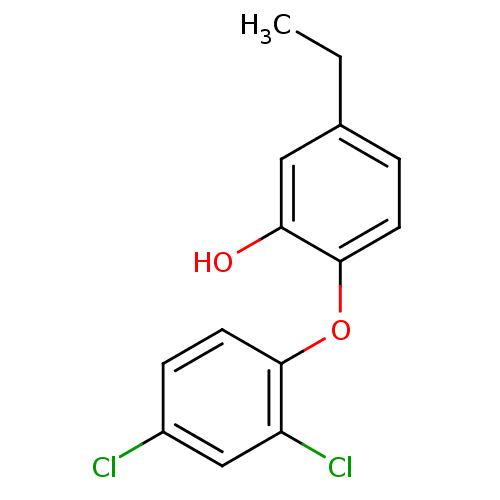
(2‐(2,4‐dichlorophenoxy)‐5‐...)Show InChI InChI=1S/C14H12Cl2O2/c1-2-9-3-5-14(12(17)7-9)18-13-6-4-10(15)8-11(13)16/h3-8,17H,2H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM50174760
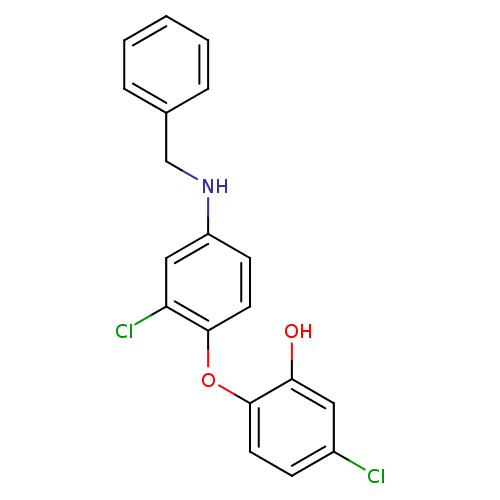
(2‐[4‐(benzylamino)‐2‐chlor...)Show InChI InChI=1S/C19H15Cl2NO2/c20-14-6-8-19(17(23)10-14)24-18-9-7-15(11-16(18)21)22-12-13-4-2-1-3-5-13/h1-11,22-23H,12H2 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii enoyl acyl-carrier protein reductase |
Bioorg Med Chem Lett 23: 3551-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.035
BindingDB Entry DOI: 10.7270/Q2XK8GZ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM50174774
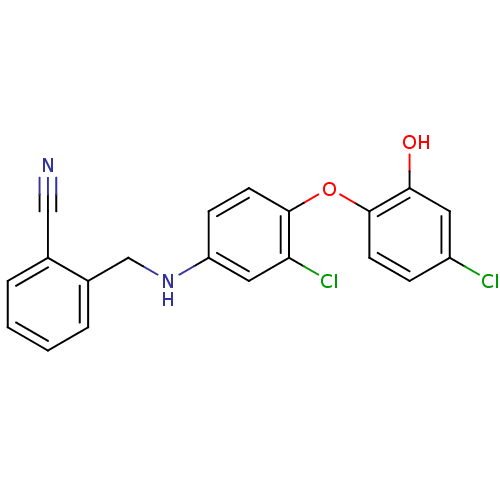
(2‐({[3‐chloro‐4‐(4‐c...)Show InChI InChI=1S/C20H14Cl2N2O2/c21-15-5-7-20(18(25)9-15)26-19-8-6-16(10-17(19)22)24-12-14-4-2-1-3-13(14)11-23/h1-10,24-25H,12H2 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM119865

(6‐chloro‐3‐(2,4‐dichloroph...)Show InChI InChI=1S/C13H6Cl3NO2/c14-7-1-3-11(10(16)5-7)19-12-4-2-9(15)8(6-17)13(12)18/h1-5,18H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM50435717
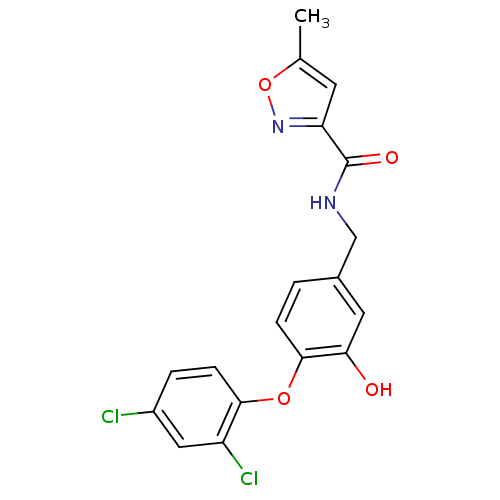
(CHEMBL2392450 | N‐{[4‐(2,4‐dichl...)Show SMILES Cc1cc(no1)C(=O)NCc1ccc(Oc2ccc(Cl)cc2Cl)c(O)c1 Show InChI InChI=1S/C18H14Cl2N2O4/c1-10-6-14(22-26-10)18(24)21-9-11-2-4-17(15(23)7-11)25-16-5-3-12(19)8-13(16)20/h2-8,23H,9H2,1H3,(H,21,24) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii enoyl acyl-carrier protein reductase |
Bioorg Med Chem Lett 23: 3551-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.035
BindingDB Entry DOI: 10.7270/Q2XK8GZ7 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM50435717

(CHEMBL2392450 | N‐{[4‐(2,4‐dichl...)Show SMILES Cc1cc(no1)C(=O)NCc1ccc(Oc2ccc(Cl)cc2Cl)c(O)c1 Show InChI InChI=1S/C18H14Cl2N2O4/c1-10-6-14(22-26-10)18(24)21-9-11-2-4-17(15(23)7-11)25-16-5-3-12(19)8-13(16)20/h2-8,23H,9H2,1H3,(H,21,24) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM119866
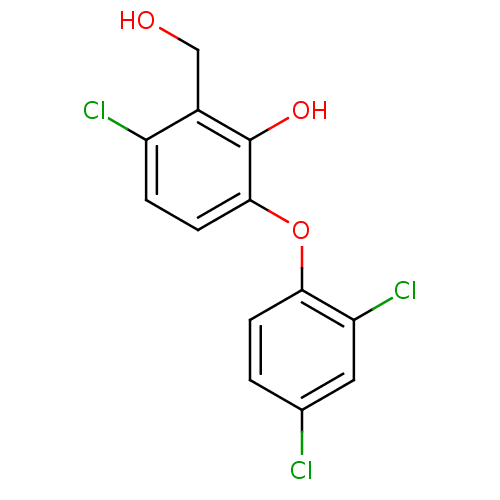
(3‐chloro‐6‐(2,4‐dichloroph...)Show InChI InChI=1S/C13H9Cl3O3/c14-7-1-3-11(10(16)5-7)19-12-4-2-9(15)8(6-17)13(12)18/h1-5,17-18H,6H2 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM25403
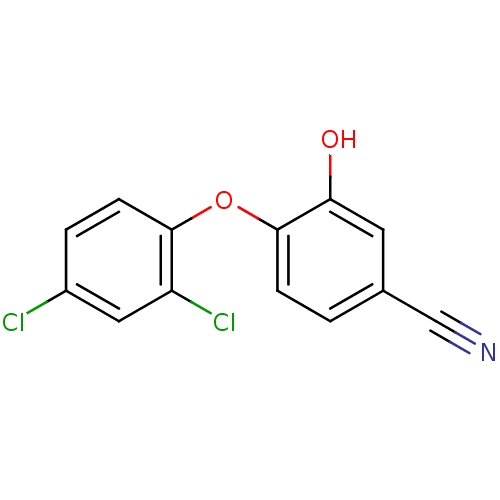
(4‐(2,4‐dichlorophenoxy)‐3‐...)Show InChI InChI=1S/C13H7Cl2NO2/c14-9-2-4-12(10(15)6-9)18-13-3-1-8(7-16)5-11(13)17/h1-6,17H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM119867
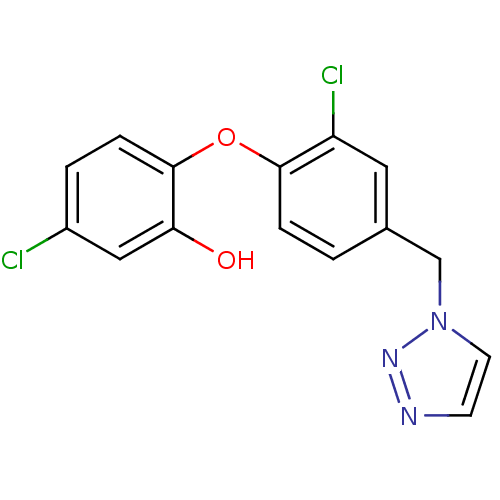
(5‐chloro‐2‐[2‐chloro‐...)Show InChI InChI=1S/C15H11Cl2N3O2/c16-11-2-4-15(13(21)8-11)22-14-3-1-10(7-12(14)17)9-20-6-5-18-19-20/h1-8,21H,9H2 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
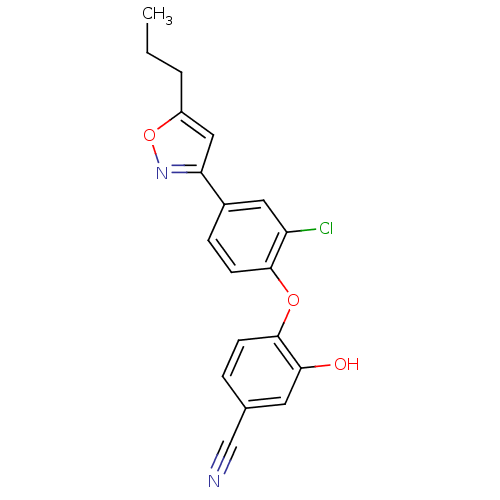
(Toxoplasma gondii) | BDBM50435720
(CHEMBL2392447)Show InChI InChI=1S/C19H15ClN2O3/c1-2-3-14-10-16(22-25-14)13-5-7-18(15(20)9-13)24-19-6-4-12(11-21)8-17(19)23/h4-10,23H,2-3H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii enoyl acyl-carrier protein reductase |
Bioorg Med Chem Lett 23: 3551-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.035
BindingDB Entry DOI: 10.7270/Q2XK8GZ7 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
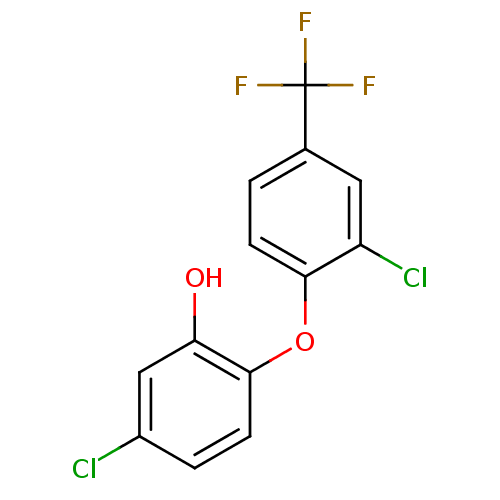
(Toxoplasma gondii) | BDBM50431922
(5‐chloro‐2‐[2‐chloro‐...)Show InChI InChI=1S/C13H7Cl2F3O2/c14-8-2-4-12(10(19)6-8)20-11-3-1-7(5-9(11)15)13(16,17)18/h1-6,19H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
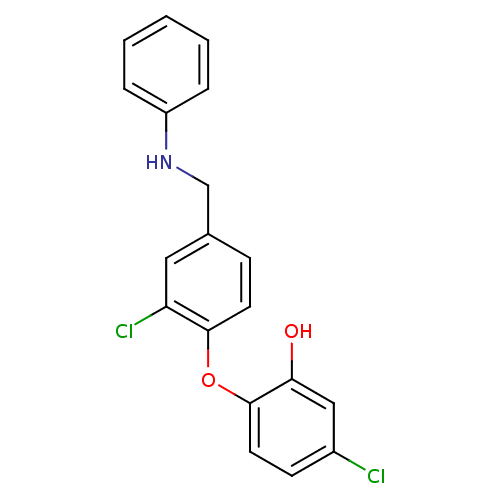
(Toxoplasma gondii) | BDBM119869
(5‐chloro‐2‐{2‐chloro‐...)Show InChI InChI=1S/C19H15Cl2NO2/c20-14-7-9-19(17(23)11-14)24-18-8-6-13(10-16(18)21)12-22-15-4-2-1-3-5-15/h1-11,22-23H,12H2 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM119870
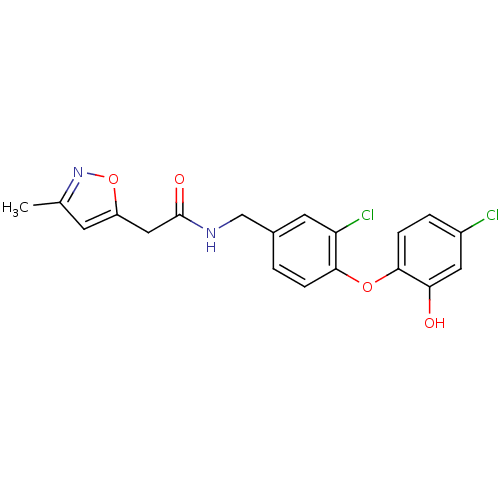
(N‐{[3‐chloro‐4‐(4‐ch...)Show SMILES Cc1cc(CC(=O)NCc2ccc(Oc3ccc(Cl)cc3O)c(Cl)c2)on1 Show InChI InChI=1S/C19H16Cl2N2O4/c1-11-6-14(27-23-11)9-19(25)22-10-12-2-4-17(15(21)7-12)26-18-5-3-13(20)8-16(18)24/h2-8,24H,9-10H2,1H3,(H,22,25) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM50435719
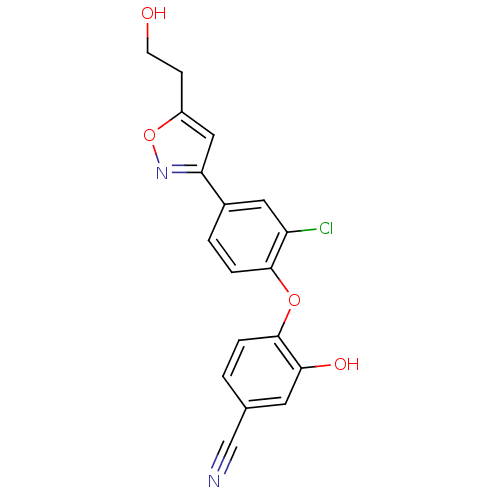
(CHEMBL2392448)Show InChI InChI=1S/C18H13ClN2O4/c19-14-8-12(15-9-13(5-6-22)25-21-15)2-4-17(14)24-18-3-1-11(10-20)7-16(18)23/h1-4,7-9,22-23H,5-6H2 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii enoyl acyl-carrier protein reductase |
Bioorg Med Chem Lett 23: 3551-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.035
BindingDB Entry DOI: 10.7270/Q2XK8GZ7 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM50431923
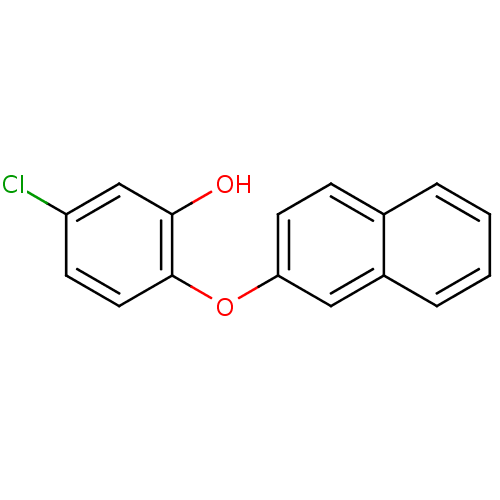
(5‐chloro‐2‐(naphthalen‐2&#...)Show InChI InChI=1S/C16H11ClO2/c17-13-6-8-16(15(18)10-13)19-14-7-5-11-3-1-2-4-12(11)9-14/h1-10,18H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM119868
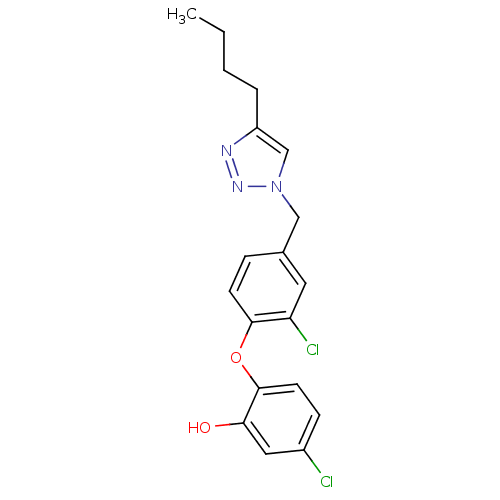
(2‐{4‐[(4‐butyl‐1,2,3‐...)Show InChI InChI=1S/C19H19Cl2N3O2/c1-2-3-4-15-12-24(23-22-15)11-13-5-7-18(16(21)9-13)26-19-8-6-14(20)10-17(19)25/h5-10,12,25H,2-4,11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM50431920
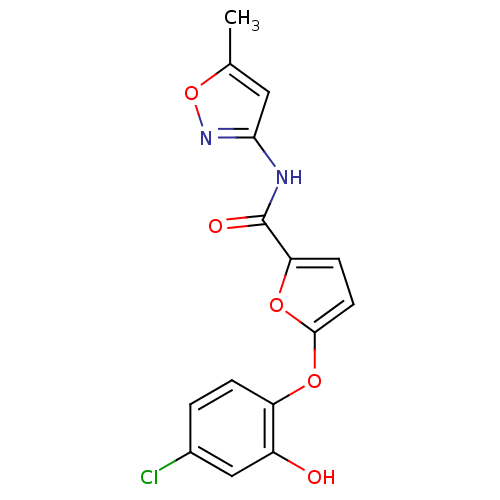
(5‐(4‐chloro‐2‐hydroxypheno...)Show InChI InChI=1S/C15H11ClN2O5/c1-8-6-13(18-23-8)17-15(20)12-4-5-14(22-12)21-11-3-2-9(16)7-10(11)19/h2-7,19H,1H3,(H,17,18,20) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM119871
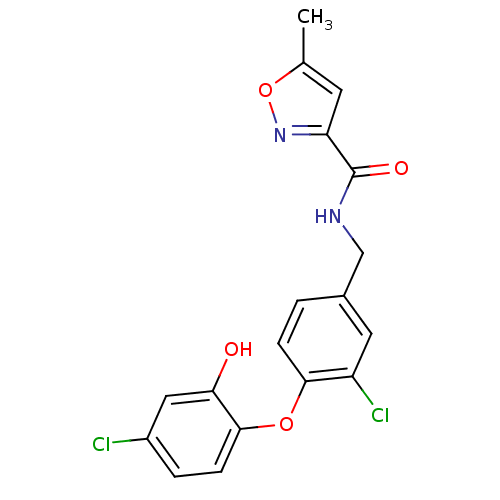
(N‐{[3‐chloro‐4‐(4‐ch...)Show SMILES Cc1cc(no1)C(=O)NCc1ccc(Oc2ccc(Cl)cc2O)c(Cl)c1 Show InChI InChI=1S/C18H14Cl2N2O4/c1-10-6-14(22-26-10)18(24)21-9-11-2-4-16(13(20)7-11)25-17-5-3-12(19)8-15(17)23/h2-8,23H,9H2,1H3,(H,21,24) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Johns Hopkins Bloomberg School of Public Health
| Assay Description
The reaction mixture (100 µL) contained 100 µM crotonyl-CoA, 1 µL DMSO (or compounds dissolved in DMSO), 5 nM TgENR, 100 mM sodium/pot... |
Biochemistry 52: 9155-66 (2013)
Article DOI: 10.1021/bi400945y
BindingDB Entry DOI: 10.7270/Q2PV6J27 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data