

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 1 (RAT) | BDBM50102298 (4-(4-Formyl-5-hydroxy-6-methyl-3-phosphonomethyl-p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102295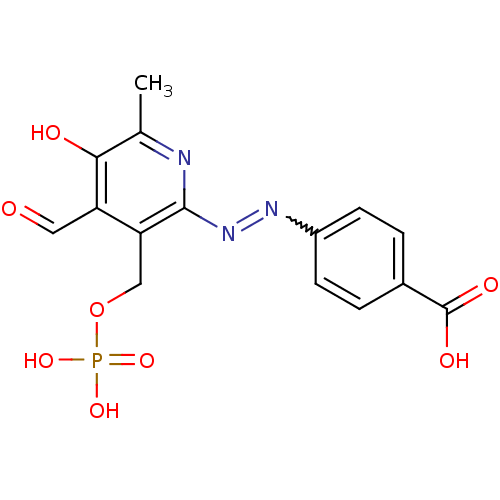 (4-(4-Formyl-5-hydroxy-6-methyl-3-phosphonooxymethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102299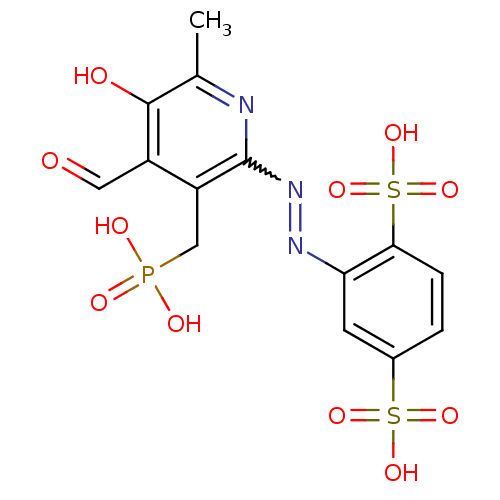 (2-(4-Formyl-5-hydroxy-6-methyl-3-phosphonomethyl-p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371580 (CHEMBL1162175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102300 (CHEMBL331250 | [4-(4-Formyl-5-hydroxy-6-methyl-3-p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371581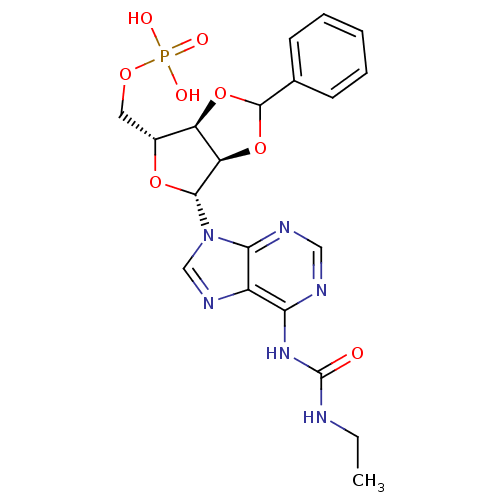 (CHEMBL1162179) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50374555 (REGRELOR DISODIUM) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as platelet aggregation by washed platelet assay | Bioorg Med Chem Lett 18: 2167-71 (2008) Article DOI: 10.1016/j.bmcl.2008.01.038 BindingDB Entry DOI: 10.7270/Q2GM885R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371580 (CHEMBL1162175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102301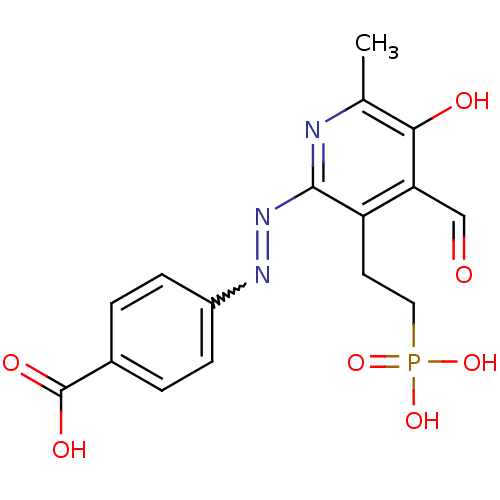 (4-[4-Formyl-5-hydroxy-6-methyl-3-(2-phosphono-ethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50102299 (2-(4-Formyl-5-hydroxy-6-methyl-3-phosphonomethyl-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP was determined at recombinant P2X3 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50102300 (CHEMBL331250 | [4-(4-Formyl-5-hydroxy-6-methyl-3-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP was determined at recombinant P2X3 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371582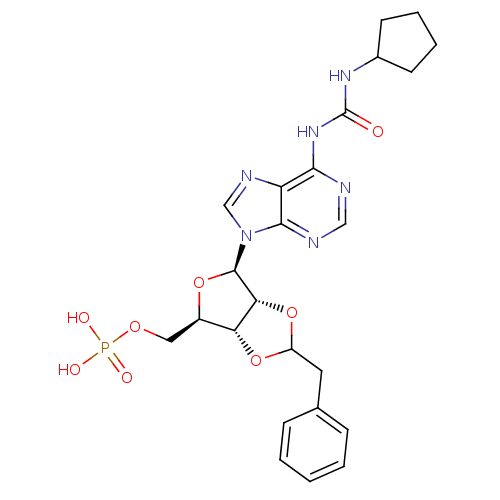 (CHEMBL1162182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102296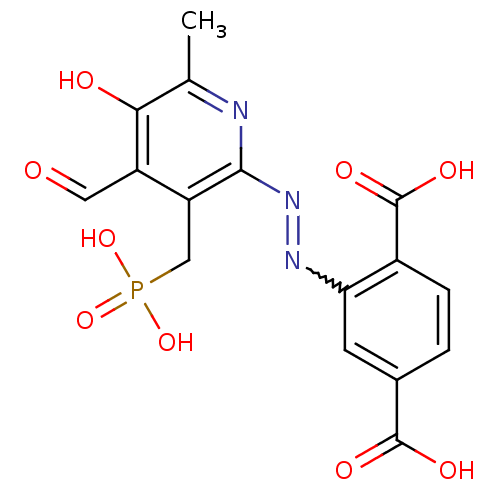 (2-(4-Formyl-5-hydroxy-6-methyl-3-phosphonomethyl-p...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50374558 (CHEMBL255350) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as platelet aggregation by washed platelet assay | Bioorg Med Chem Lett 18: 2167-71 (2008) Article DOI: 10.1016/j.bmcl.2008.01.038 BindingDB Entry DOI: 10.7270/Q2GM885R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371583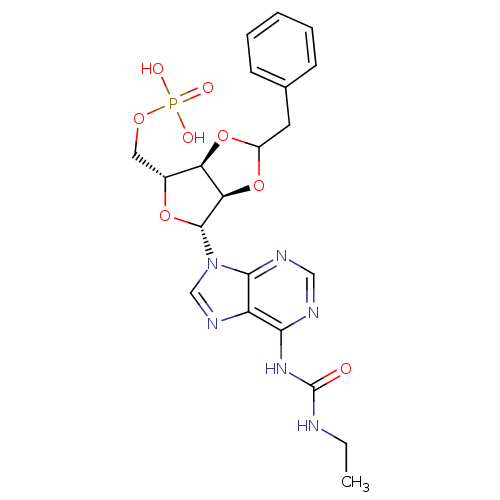 (CHEMBL1162184) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102304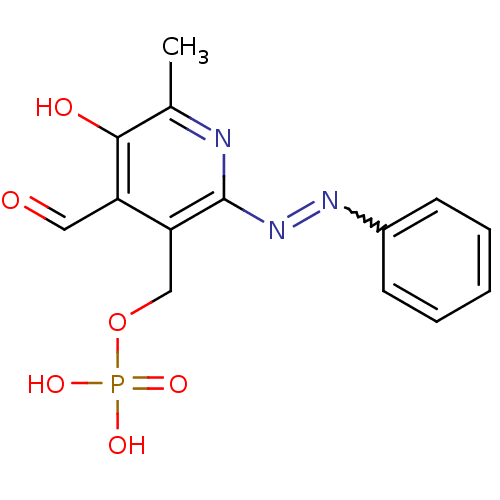 (CHEMBL116926 | Phosphoric acid mono-(4-formyl-5-hy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50102297 (CHEMBL118007 | [2-(3,5-Bis-phosphonomethyl-phenyla...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM50064800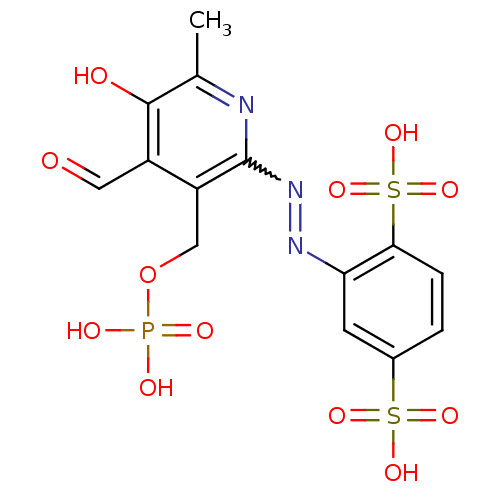 (2-(4-Formyl-5-hydroxy-6-methyl-3-phosphonooxymethy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371589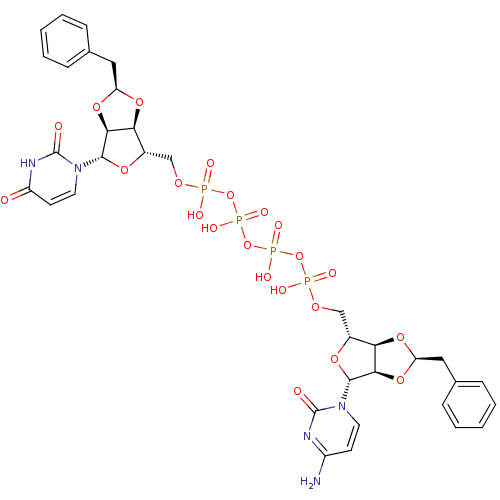 (CHEMBL1162196) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50118238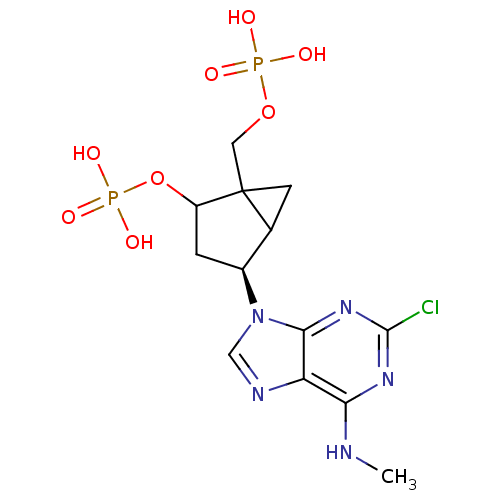 (CHEMBL339873 | MRS 2279) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51.6 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Inhibition of 30 nM 2-MeSADP stimulation of 2PY1-mediated phospholipase C (PLC) activity in Turkey erythocyte ghosts | J Med Chem 43: 829-42 (2000) BindingDB Entry DOI: 10.7270/Q2474BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371594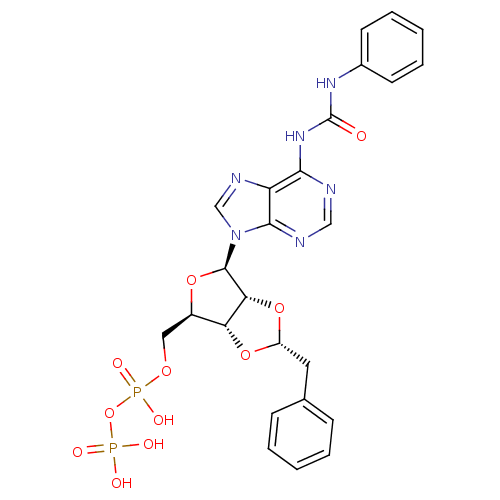 (CHEMBL1162161) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50118238 (CHEMBL339873 | MRS 2279) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against Turkey erythrocyte P2Y purinoceptor 1 (P2Y1) by the compound is measured | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371584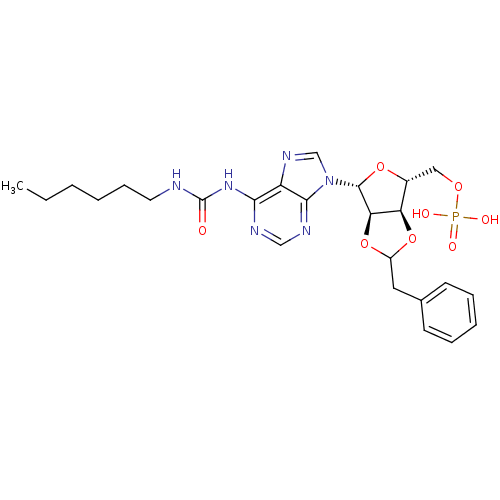 (CHEMBL1162185) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50064800 (2-(4-Formyl-5-hydroxy-6-methyl-3-phosphonooxymethy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP was determined at recombinant P2X3 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 1 (RAT) | BDBM85043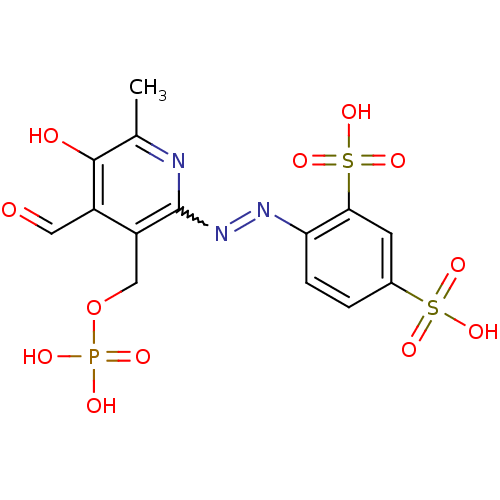 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X1 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50102298 (4-(4-Formyl-5-hydroxy-6-methyl-3-phosphonomethyl-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP was determined at recombinant P2X3 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50102295 (4-(4-Formyl-5-hydroxy-6-methyl-3-phosphonooxymethy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP was determined at recombinant P2X3 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50102301 (4-[4-Formyl-5-hydroxy-6-methyl-3-(2-phosphono-ethy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP was determined at recombinant P2X3 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50085314 (CHEMBL350828 | Phosphoric acid mono-[4-(6-methylam...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Inhibition of 30 nM 2-MeSADP stimulation of 2PY1-mediated phospholipase C (PLC) activity in Turkey erythocyte ghosts | J Med Chem 43: 829-42 (2000) BindingDB Entry DOI: 10.7270/Q2474BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 2 (RAT) | BDBM50102298 (4-(4-Formyl-5-hydroxy-6-methyl-3-phosphonomethyl-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X2 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50085821 (CHEMBL168427 | Phosphoric acid mono-[4-(6-methylam...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Inhibition of 30 nM 2-MeSADP stimulation of 2PY1-mediated phospholipase C (PLC) activity in Turkey erythocyte ghosts | J Med Chem 43: 829-42 (2000) BindingDB Entry DOI: 10.7270/Q2474BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50076466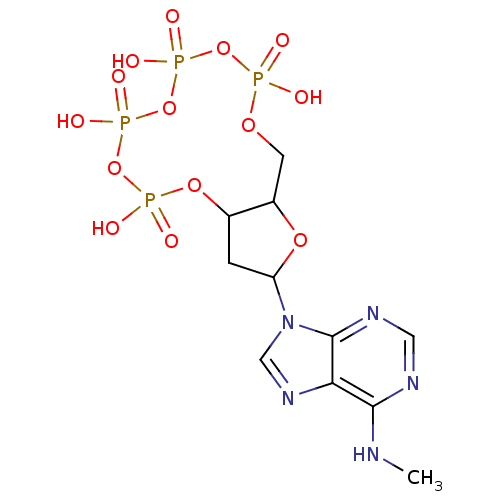 (2-(6-Methylamino-purin-9-yl)-5,7,9,11-tetraoxo-tet...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description In vitro antagonist activity at P2Y1 receptor in turkey erythrocyte membranes. | J Med Chem 42: 1625-38 (1999) Article DOI: 10.1021/jm980657j BindingDB Entry DOI: 10.7270/Q28C9WZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371585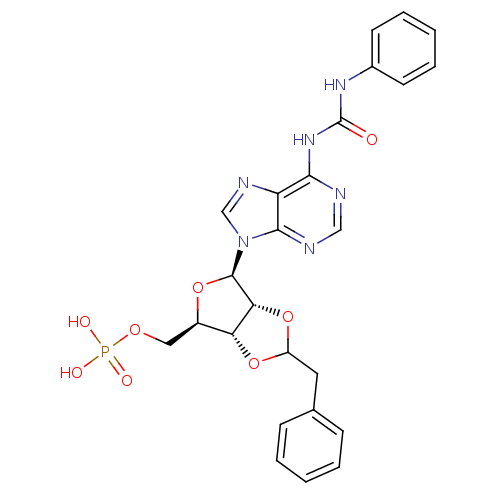 (CHEMBL1162188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50374572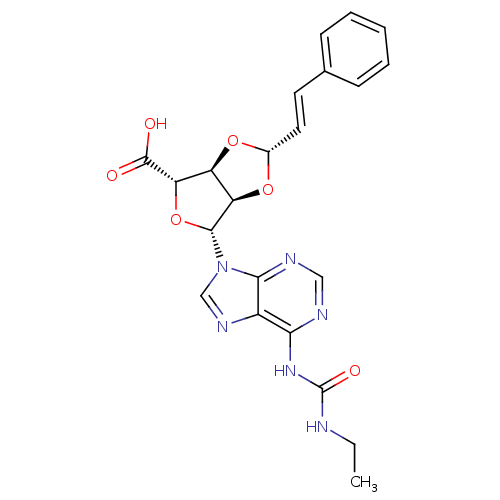 (CHEMBL403010) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as platelet aggregation by washed platelet assay | Bioorg Med Chem Lett 18: 2167-71 (2008) Article DOI: 10.1016/j.bmcl.2008.01.038 BindingDB Entry DOI: 10.7270/Q2GM885R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50076460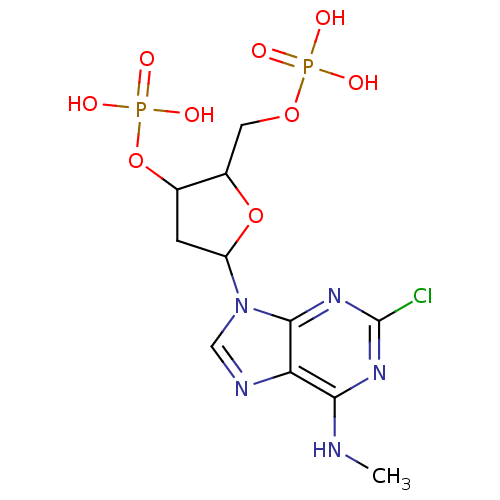 (CHEMBL288798 | Phosphoric acid mono-[5-(2-chloro-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity against Turkey erythrocyte P2Y purinoceptor 1 (P2Y1) by the compound is measured | J Med Chem 45: 208-18 (2001) BindingDB Entry DOI: 10.7270/Q2JS9R58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50076460 (CHEMBL288798 | Phosphoric acid mono-[5-(2-chloro-6...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description In vitro antagonist activity at P2Y1 receptor in turkey erythrocyte membranes. | J Med Chem 42: 1625-38 (1999) Article DOI: 10.1021/jm980657j BindingDB Entry DOI: 10.7270/Q28C9WZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50085322 (CHEMBL164811 | Phosphoric acid mono-[5-(2-chloro-6...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Inhibition of 30 nM 2-MeSADP stimulation of 2PY1-mediated phospholipase C (PLC) activity in Turkey erythocyte ghosts | J Med Chem 43: 829-42 (2000) BindingDB Entry DOI: 10.7270/Q2474BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371578 (CHEMBL1162172) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371577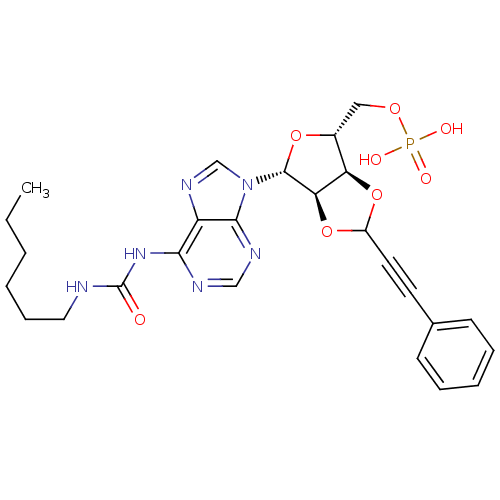 (CHEMBL1162173) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 211 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50104022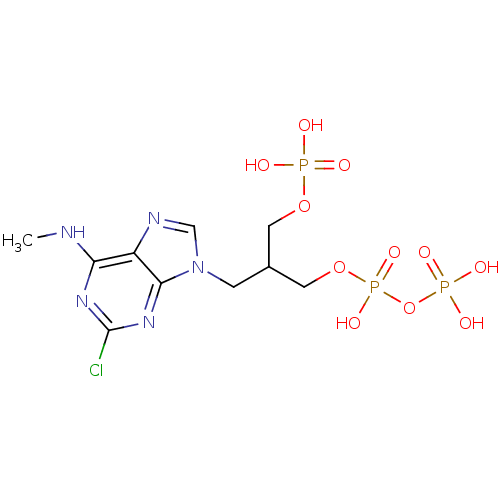 (3-[2-chloro-6-(methylamino)-9H-purin-9-yl]-2-[(pho...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Inhibitory concentration against P2Y1 receptor in turkey erythrocyte membranes | J Med Chem 44: 3092-108 (2001) BindingDB Entry DOI: 10.7270/Q20864KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371579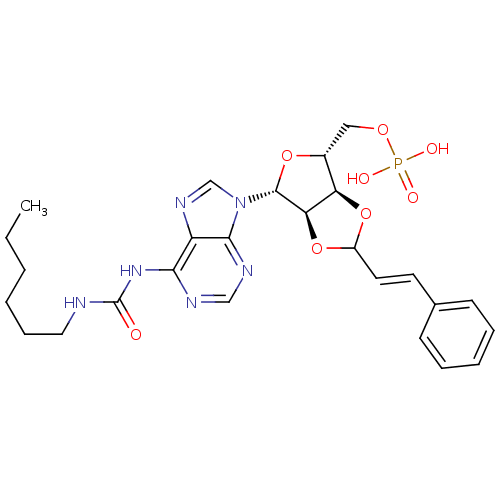 (CHEMBL1162171) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 237 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM85043 (CAS_149017-66-3 | CHEMBL69234 | NSC_6093163 | PPAD...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP was determined at recombinant P2X3 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50374559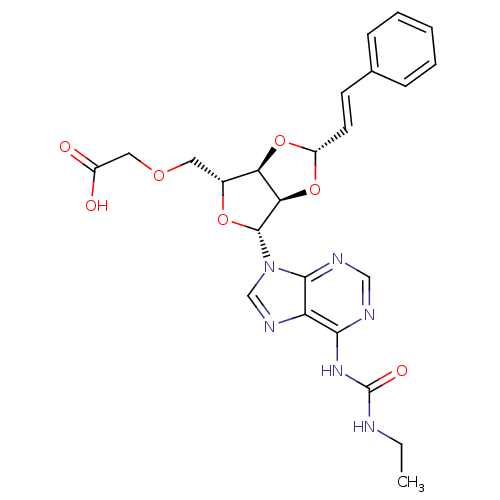 (CHEMBL255349) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 243 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as platelet aggregation by washed platelet assay | Bioorg Med Chem Lett 18: 2167-71 (2008) Article DOI: 10.1016/j.bmcl.2008.01.038 BindingDB Entry DOI: 10.7270/Q2GM885R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 2 (RAT) | BDBM50102299 (2-(4-Formyl-5-hydroxy-6-methyl-3-phosphonomethyl-p...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP at P2X2 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50371576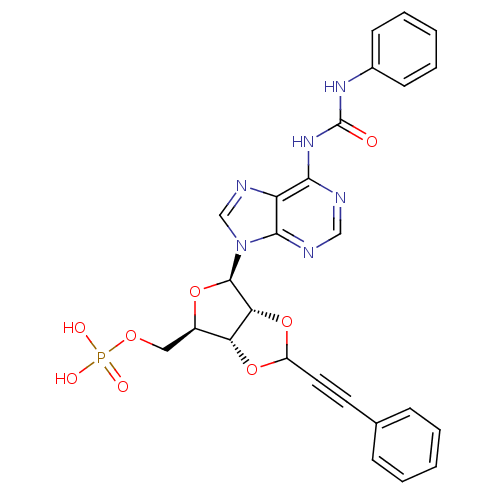 (CHEMBL1162164) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
Inspire Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Antagonist activity at P2Y12 receptor assessed as inhibition of ADP-induced human platelet aggregation by washed platelet assay | J Med Chem 51: 1007-25 (2008) Article DOI: 10.1021/jm701348d BindingDB Entry DOI: 10.7270/Q2P55PB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50062291 (CHEMBL54116 | Phosphoric acid mono-[2-(6-methylami...) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (RAT) | BDBM50102296 (2-(4-Formyl-5-hydroxy-6-methyl-3-phosphonomethyl-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of inward ion current elicited by ATP was determined at recombinant P2X3 receptor expressed in Xenopus oocytes | J Med Chem 44: 340-9 (2001) BindingDB Entry DOI: 10.7270/Q2FN15HC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50118229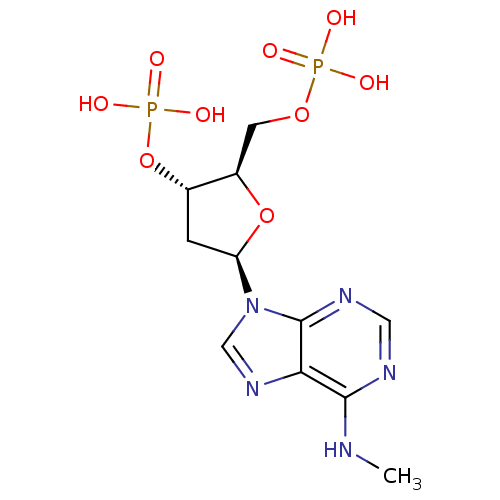 (CHEMBL129841 | MRS 2179) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Antagonist activity at P2Y1 receptor measured as capacity to inhibit 50% of phospholipase C stimulation elicited by 10 nM 2-MeSATP | J Med Chem 41: 183-90 (1998) Article DOI: 10.1021/jm970433l BindingDB Entry DOI: 10.7270/Q2SX6DWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50118229 (CHEMBL129841 | MRS 2179) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description In vitro antagonist activity at P2Y1 receptor in turkey erythrocyte membranes. | J Med Chem 42: 1625-38 (1999) Article DOI: 10.1021/jm980657j BindingDB Entry DOI: 10.7270/Q28C9WZM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50118229 (CHEMBL129841 | MRS 2179) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Inhibition of 30 nM 2-MeSADP stimulation of 2PY1-mediated phospholipase C (PLC) activity in Turkey erythocyte ghosts | J Med Chem 43: 829-42 (2000) BindingDB Entry DOI: 10.7270/Q2474BJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 350 total ) | Next | Last >> |