 Found 131 hits with Last Name = 'arch' and Initial = 'jr'
Found 131 hits with Last Name = 'arch' and Initial = 'jr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040348
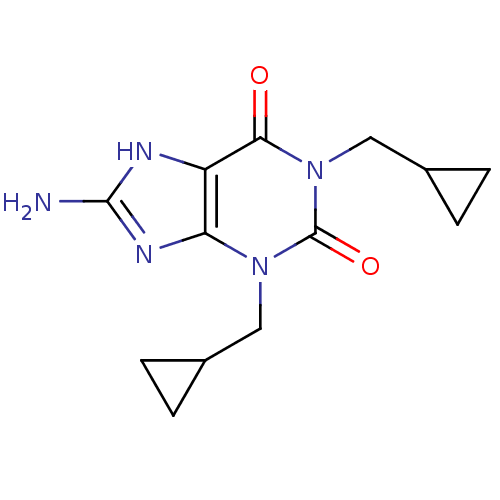
(8-Amino-1,3-bis-cyclopropylmethyl-3,7-dihydro-puri...)Show InChI InChI=1S/C13H17N5O2/c14-12-15-9-10(16-12)17(5-7-1-2-7)13(20)18(11(9)19)6-8-3-4-8/h7-8H,1-6H2,(H3,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cGMP hydrolysis by PDE 5A |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
3-dehydroquinate dehydratase
(Helicobacter pylori) | BDBM50468910
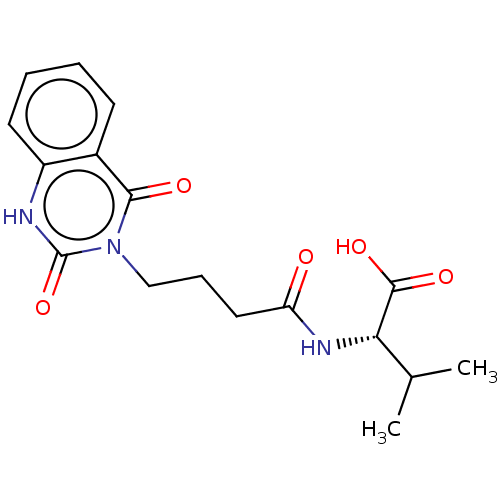
(CHEMBL4286047)Show SMILES CC(C)[C@H](NC(=O)CCCn1c(=O)[nH]c2ccccc2c1=O)C(O)=O |r| Show InChI InChI=1S/C17H21N3O5/c1-10(2)14(16(23)24)19-13(21)8-5-9-20-15(22)11-6-3-4-7-12(11)18-17(20)25/h3-4,6-7,10,14H,5,8-9H2,1-2H3,(H,18,25)(H,19,21)(H,23,24)/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of Helicobacter pylori DHQ2 |
Eur J Med Chem 156: 907-917 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.042
BindingDB Entry DOI: 10.7270/Q26W9DRP |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040371
(1,3-Bis-cyclopropylmethyl-7-(4-methoxy-benzyl)-8-[...)Show SMILES COc1ccc(Cn2c(N=C3CCCN3C)nc3n(CC4CC4)c(=O)n(CC4CC4)c(=O)c23)cc1 |w:9.8| Show InChI InChI=1S/C26H32N6O3/c1-29-13-3-4-21(29)27-25-28-23-22(30(25)14-19-9-11-20(35-2)12-10-19)24(33)32(16-18-7-8-18)26(34)31(23)15-17-5-6-17/h9-12,17-18H,3-8,13-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040346
(8-Amino-1,3-bis-cyclopropylmethyl-7-(4-methoxy-ben...)Show SMILES COc1ccc(Cn2c(N)nc3n(CC4CC4)c(=O)n(CC4CC4)c(=O)c23)cc1 Show InChI InChI=1S/C21H25N5O3/c1-29-16-8-6-15(7-9-16)10-24-17-18(23-20(24)22)25(11-13-2-3-13)21(28)26(19(17)27)12-14-4-5-14/h6-9,13-14H,2-5,10-12H2,1H3,(H2,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50040348

(8-Amino-1,3-bis-cyclopropylmethyl-3,7-dihydro-puri...)Show InChI InChI=1S/C13H17N5O2/c14-12-15-9-10(16-12)17(5-7-1-2-7)13(20)18(11(9)19)6-8-3-4-8/h7-8H,1-6H2,(H3,14,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of adenosine binding to A1 receptorof rat brain homogenates |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040379
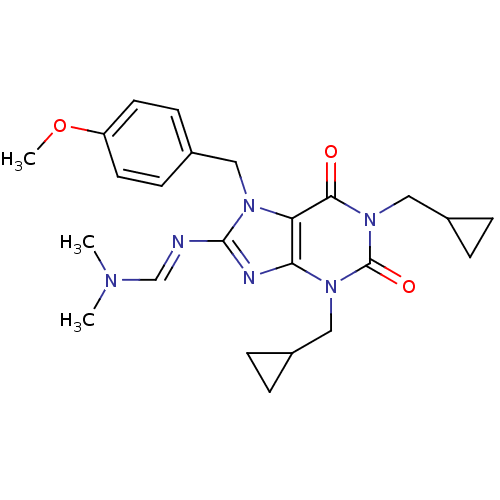
(CHEMBL423559 | N'-[1,3-Bis-cyclopropylmethyl-7-(4-...)Show SMILES COc1ccc(Cn2c(\N=C\N(C)C)nc3n(CC4CC4)c(=O)n(CC4CC4)c(=O)c23)cc1 Show InChI InChI=1S/C24H30N6O3/c1-27(2)15-25-23-26-21-20(28(23)12-18-8-10-19(33-3)11-9-18)22(31)30(14-17-6-7-17)24(32)29(21)13-16-4-5-16/h8-11,15-17H,4-7,12-14H2,1-3H3/b25-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040354
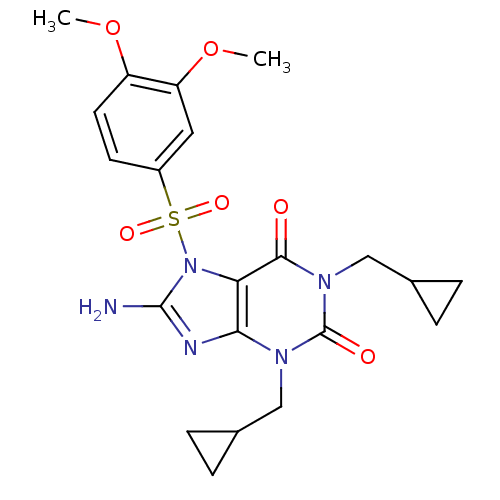
(8-Amino-1,3-bis-cyclopropylmethyl-7-(3,4-dimethoxy...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)n1c(N)nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12 Show InChI InChI=1S/C21H25N5O6S/c1-31-15-8-7-14(9-16(15)32-2)33(29,30)26-17-18(23-20(26)22)24(10-12-3-4-12)21(28)25(19(17)27)11-13-5-6-13/h7-9,12-13H,3-6,10-11H2,1-2H3,(H2,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040356
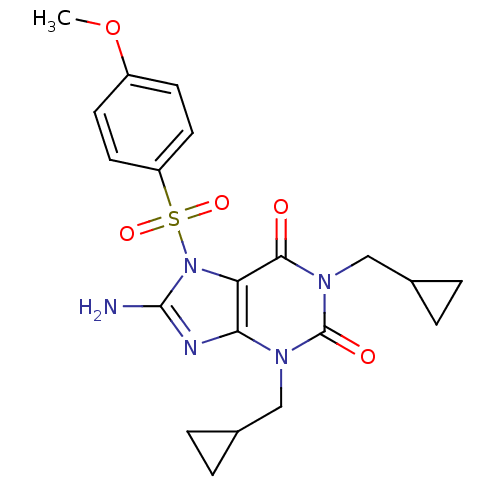
(8-Amino-1,3-bis-cyclopropylmethyl-7-(4-methoxy-ben...)Show SMILES COc1ccc(cc1)S(=O)(=O)n1c(N)nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12 Show InChI InChI=1S/C20H23N5O5S/c1-30-14-6-8-15(9-7-14)31(28,29)25-16-17(22-19(25)21)23(10-12-2-3-12)20(27)24(18(16)26)11-13-4-5-13/h6-9,12-13H,2-5,10-11H2,1H3,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50020874
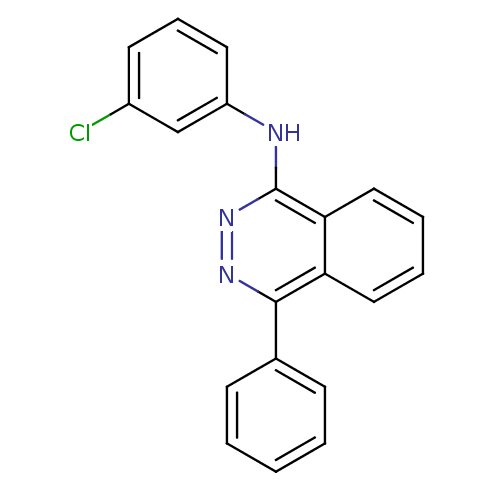
((3-Chloro-phenyl)-(4-phenyl-phthalazin-1-yl)-amine...)Show InChI InChI=1S/C20H14ClN3/c21-15-9-6-10-16(13-15)22-20-18-12-5-4-11-17(18)19(23-24-20)14-7-2-1-3-8-14/h1-13H,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5A |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50040371

(1,3-Bis-cyclopropylmethyl-7-(4-methoxy-benzyl)-8-[...)Show SMILES COc1ccc(Cn2c(N=C3CCCN3C)nc3n(CC4CC4)c(=O)n(CC4CC4)c(=O)c23)cc1 |w:9.8| Show InChI InChI=1S/C26H32N6O3/c1-29-13-3-4-21(29)27-25-28-23-22(30(25)14-19-9-11-20(35-2)12-10-19)24(33)32(16-18-7-8-18)26(34)31(23)15-17-5-6-17/h9-12,17-18H,3-8,13-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of adenosine binding to A1 receptorof rat brain homogenates |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040362
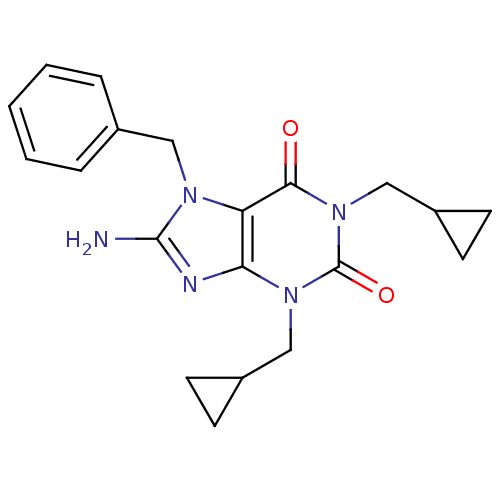
(8-Amino-7-benzyl-1,3-bis-cyclopropylmethyl-3,7-dih...)Show SMILES Nc1nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c2n1Cc1ccccc1 Show InChI InChI=1S/C20H23N5O2/c21-19-22-17-16(23(19)10-13-4-2-1-3-5-13)18(26)25(12-15-8-9-15)20(27)24(17)11-14-6-7-14/h1-5,14-15H,6-12H2,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM14363

(3-(2-propoxyphenyl)-2,4,7,8,9-pentazabicyclo[4.3.0...)Show InChI InChI=1S/C13H13N5O2/c1-2-7-20-9-6-4-3-5-8(9)11-14-12-10(13(19)15-11)16-18-17-12/h3-6H,2,7H2,1H3,(H2,14,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040372
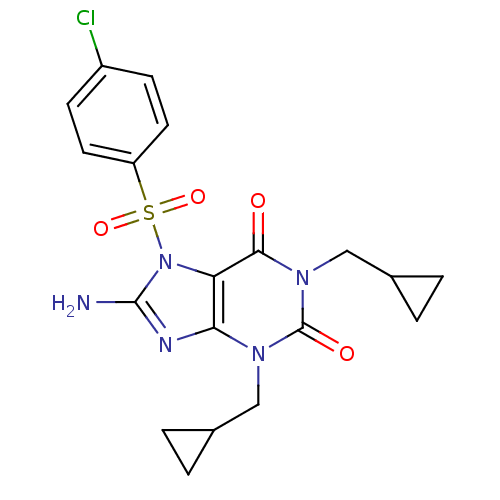
(8-Amino-7-(4-chloro-benzenesulfonyl)-1,3-bis-cyclo...)Show SMILES Nc1nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c2n1S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C19H20ClN5O4S/c20-13-5-7-14(8-6-13)30(28,29)25-15-16(22-18(25)21)23(9-11-1-2-11)19(27)24(17(15)26)10-12-3-4-12/h5-8,11-12H,1-4,9-10H2,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040377
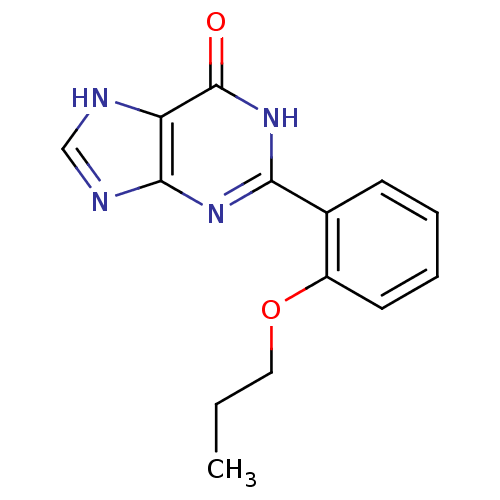
(2-(2-Propoxy-phenyl)-1,7-dihydro-purin-6-one | 2-(...)Show InChI InChI=1S/C14H14N4O2/c1-2-7-20-10-6-4-3-5-9(10)12-17-13-11(14(19)18-12)15-8-16-13/h3-6,8H,2,7H2,1H3,(H2,15,16,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5A |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50040359
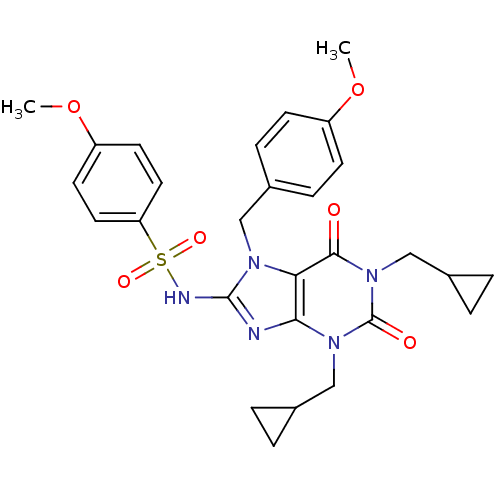
(CHEMBL145159 | N-[1,3-Bis-cyclopropylmethyl-7-(4-m...)Show SMILES COc1ccc(Cn2c(NS(=O)(=O)c3ccc(OC)cc3)nc3n(CC4CC4)c(=O)n(CC4CC4)c(=O)c23)cc1 Show InChI InChI=1S/C28H31N5O6S/c1-38-21-9-7-20(8-10-21)15-31-24-25(29-27(31)30-40(36,37)23-13-11-22(39-2)12-14-23)32(16-18-3-4-18)28(35)33(26(24)34)17-19-5-6-19/h7-14,18-19H,3-6,15-17H2,1-2H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of adenosine binding to A1 receptorof rat brain homogenates |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040349
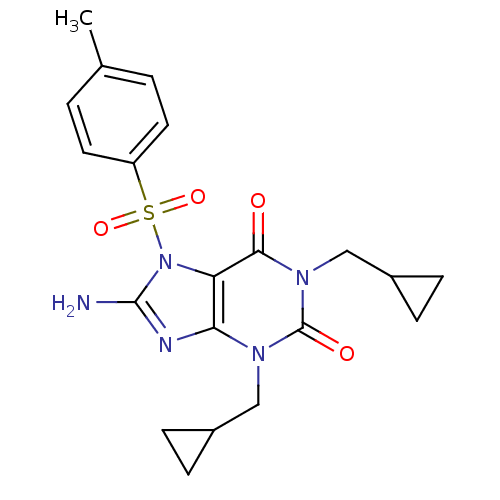
(8-Amino-1,3-bis-cyclopropylmethyl-7-(toluene-4-sul...)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1c(N)nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12 Show InChI InChI=1S/C20H23N5O4S/c1-12-2-8-15(9-3-12)30(28,29)25-16-17(22-19(25)21)23(10-13-4-5-13)20(27)24(18(16)26)11-14-6-7-14/h2-3,8-9,13-14H,4-7,10-11H2,1H3,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50040345

(CHEMBL146165 | N-(1,3-Bis-cyclopropylmethyl-2,6-di...)Show SMILES COc1ccc(cc1)S(=O)(=O)Nc1nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c2[nH]1 Show InChI InChI=1S/C20H23N5O5S/c1-30-14-6-8-15(9-7-14)31(28,29)23-19-21-16-17(22-19)24(10-12-2-3-12)20(27)25(18(16)26)11-13-4-5-13/h6-9,12-13H,2-5,10-11H2,1H3,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of adenosine binding to A1 receptorof rat brain homogenates |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50040351
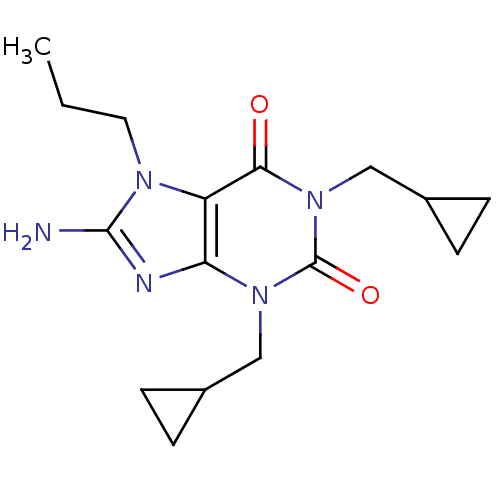
(8-Amino-1,3-bis-cyclopropylmethyl-7-propyl-3,7-dih...)Show SMILES CCCn1c(N)nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12 Show InChI InChI=1S/C16H23N5O2/c1-2-7-19-12-13(18-15(19)17)20(8-10-3-4-10)16(23)21(14(12)22)9-11-5-6-11/h10-11H,2-9H2,1H3,(H2,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of adenosine binding to A1 receptorof rat brain homogenates |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040359

(CHEMBL145159 | N-[1,3-Bis-cyclopropylmethyl-7-(4-m...)Show SMILES COc1ccc(Cn2c(NS(=O)(=O)c3ccc(OC)cc3)nc3n(CC4CC4)c(=O)n(CC4CC4)c(=O)c23)cc1 Show InChI InChI=1S/C28H31N5O6S/c1-38-21-9-7-20(8-10-21)15-31-24-25(29-27(31)30-40(36,37)23-13-11-22(39-2)12-14-23)32(16-18-3-4-18)28(35)33(26(24)34)17-19-5-6-19/h7-14,18-19H,3-6,15-17H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50040355
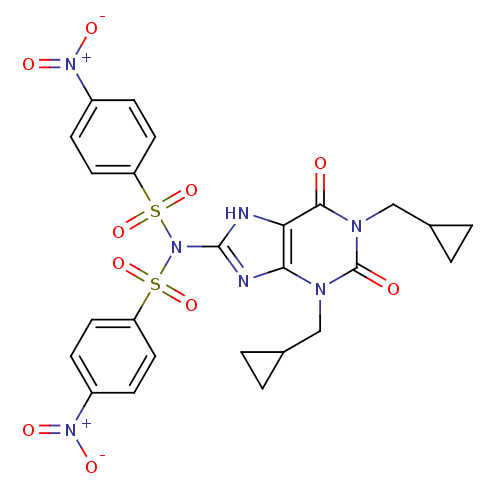
(1,3-Bis(cyclopropylmethyl)-8-bis[(4-nitrophenylsul...)Show SMILES [O-][N+](=O)c1ccc(cc1)S(=O)(=O)N(c1nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c2[nH]1)S(=O)(=O)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C25H23N7O10S2/c33-23-21-22(28(13-15-1-2-15)25(34)29(23)14-16-3-4-16)27-24(26-21)32(43(39,40)19-9-5-17(6-10-19)30(35)36)44(41,42)20-11-7-18(8-12-20)31(37)38/h5-12,15-16H,1-4,13-14H2,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of adenosine binding to A1 receptorof rat brain homogenates |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50040344
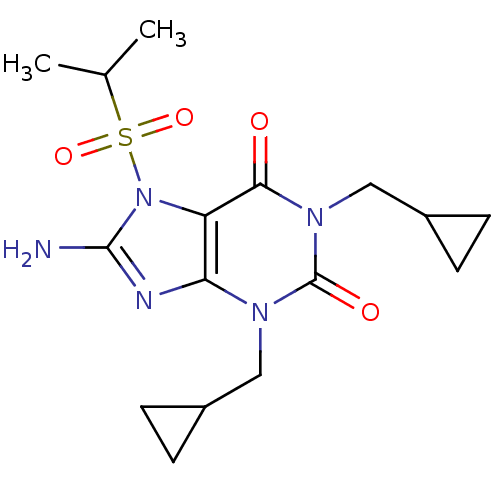
(8-Amino-1,3-bis-cyclopropylmethyl-7-(propane-2-sul...)Show SMILES CC(C)S(=O)(=O)n1c(N)nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12 Show InChI InChI=1S/C16H23N5O4S/c1-9(2)26(24,25)21-12-13(18-15(21)17)19(7-10-3-4-10)16(23)20(14(12)22)8-11-5-6-11/h9-11H,3-8H2,1-2H3,(H2,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of adenosine binding to A1 receptorof rat brain homogenates |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50040362

(8-Amino-7-benzyl-1,3-bis-cyclopropylmethyl-3,7-dih...)Show SMILES Nc1nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c2n1Cc1ccccc1 Show InChI InChI=1S/C20H23N5O2/c21-19-22-17-16(23(19)10-13-4-2-1-3-5-13)18(26)25(12-15-8-9-15)20(27)24(17)11-14-6-7-14/h1-5,14-15H,6-12H2,(H2,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of adenosine binding to A1 receptorof rat brain homogenates |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50040350
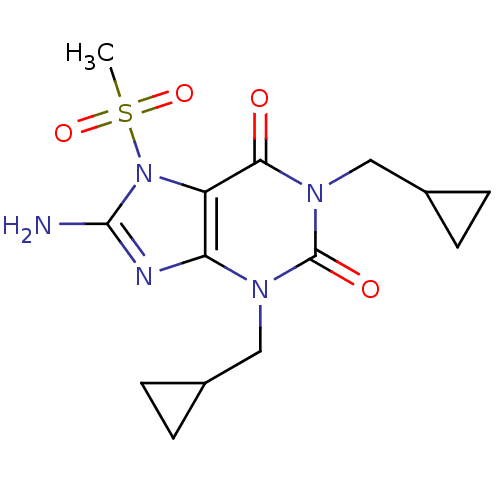
(8-Amino-1,3-bis-cyclopropylmethyl-7-methanesulfony...)Show SMILES CS(=O)(=O)n1c(N)nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12 Show InChI InChI=1S/C14H19N5O4S/c1-24(22,23)19-10-11(16-13(19)15)17(6-8-2-3-8)14(21)18(12(10)20)7-9-4-5-9/h8-9H,2-7H2,1H3,(H2,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of adenosine binding to A1 receptorof rat brain homogenates |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040352
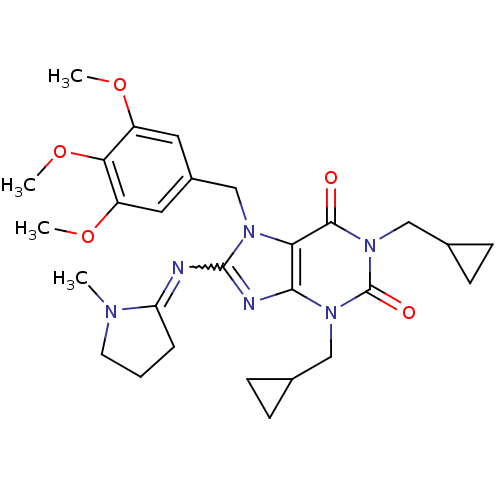
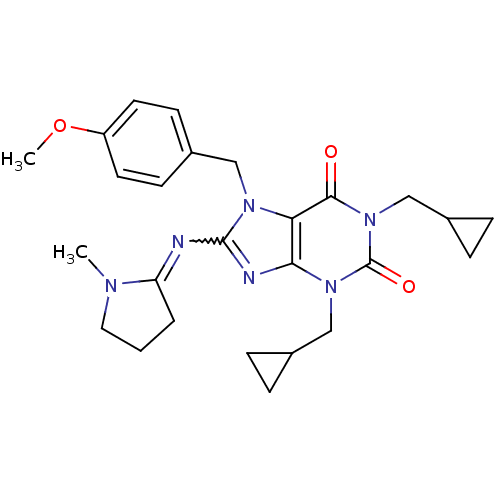
(1,3-Bis-cyclopropylmethyl-8-[1-methyl-pyrrolidin-(...)Show SMILES COc1cc(Cn2c(N=C3CCCN3C)nc3n(CC4CC4)c(=O)n(CC4CC4)c(=O)c23)cc(OC)c1OC |w:8.7| Show InChI InChI=1S/C28H36N6O5/c1-31-11-5-6-22(31)29-27-30-25-23(32(27)16-19-12-20(37-2)24(39-4)21(13-19)38-3)26(35)34(15-18-9-10-18)28(36)33(25)14-17-7-8-17/h12-13,17-18H,5-11,14-16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50040343
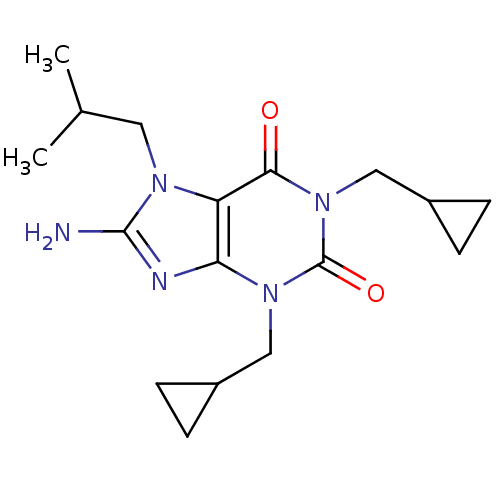
(8-Amino-1,3-bis-cyclopropylmethyl-7-isobutyl-3,7-d...)Show SMILES CC(C)Cn1c(N)nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12 Show InChI InChI=1S/C17H25N5O2/c1-10(2)7-20-13-14(19-16(20)18)21(8-11-3-4-11)17(24)22(15(13)23)9-12-5-6-12/h10-12H,3-9H2,1-2H3,(H2,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of adenosine binding to A1 receptorof rat brain homogenates |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040369
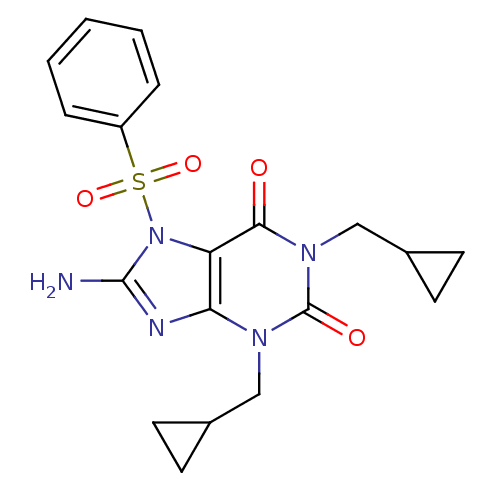
(8-Amino-7-benzenesulfonyl-1,3-bis-cyclopropylmethy...)Show SMILES Nc1nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c2n1S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H21N5O4S/c20-18-21-16-15(24(18)29(27,28)14-4-2-1-3-5-14)17(25)23(11-13-8-9-13)19(26)22(16)10-12-6-7-12/h1-5,12-13H,6-11H2,(H2,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50040349

(8-Amino-1,3-bis-cyclopropylmethyl-7-(toluene-4-sul...)Show SMILES Cc1ccc(cc1)S(=O)(=O)n1c(N)nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12 Show InChI InChI=1S/C20H23N5O4S/c1-12-2-8-15(9-3-12)30(28,29)25-16-17(22-19(25)21)23(10-13-4-5-13)20(27)24(18(16)26)11-14-6-7-14/h2-3,8-9,13-14H,4-7,10-11H2,1H3,(H2,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of adenosine binding to A1 receptorof rat brain homogenates |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Bromodomain adjacent to zinc finger domain protein 2A
(Homo sapiens (Human)) | BDBM50601335

(CHEMBL5178939)Show SMILES CCc1c([nH]c(C)c1C(C)=O)-c1csc(n1)N1CCNCC1C(=O)NCCc1ccno1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00173
BindingDB Entry DOI: 10.7270/Q27M0D0Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50040372

(8-Amino-7-(4-chloro-benzenesulfonyl)-1,3-bis-cyclo...)Show SMILES Nc1nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c2n1S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C19H20ClN5O4S/c20-13-5-7-14(8-6-13)30(28,29)25-15-16(22-18(25)21)23(9-11-1-2-11)19(27)24(17(15)26)10-12-3-4-12/h5-8,11-12H,1-4,9-10H2,(H2,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of adenosine binding to A1 receptorof rat brain homogenates |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Bos taurus) | BDBM50040375
(1,3-Bis(cyclopropylmethyl)-8-bis[(4-nitrophenylsul...)Show SMILES Cn1c(nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12)N(S(=O)(=O)c1ccc(cc1)[N+]([O-])=O)S(=O)(=O)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C26H25N7O10S2/c1-28-22-23(29(14-16-2-3-16)26(35)30(24(22)34)15-17-4-5-17)27-25(28)33(44(40,41)20-10-6-18(7-11-20)31(36)37)45(42,43)21-12-8-19(9-13-21)32(38)39/h6-13,16-17H,2-5,14-15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 2 at 100 uM |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040376
(1,3-Bis(cyclopropylmethyl)-8-bis[(phenylsulfonyl)a...)Show SMILES COc1ccc(Cn2c(nc3n(CC4CC4)c(=O)n(CC4CC4)c(=O)c23)N(S(=O)(=O)c2ccccc2)S(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C33H33N5O7S2/c1-45-26-18-16-25(17-19-26)20-35-29-30(36(21-23-12-13-23)33(40)37(31(29)39)22-24-14-15-24)34-32(35)38(46(41,42)27-8-4-2-5-9-27)47(43,44)28-10-6-3-7-11-28/h2-11,16-19,23-24H,12-15,20-22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50320629
(2-chloro-5-(5-((2-(4-(4-fluorophenylamino)-6-morph...)Show SMILES OC(=O)c1cc(ccc1Cl)-c1ccc(CN=Nc2nc(Nc3ccc(F)cc3)nc(n2)N2CCOCC2)o1 |w:16.17| Show InChI InChI=1S/C25H21ClFN7O4/c26-20-7-1-15(13-19(20)22(35)36)21-8-6-18(38-21)14-28-33-24-30-23(29-17-4-2-16(27)3-5-17)31-25(32-24)34-9-11-37-12-10-34/h1-8,13H,9-12,14H2,(H,35,36)(H,29,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Z£rich
Curated by ChEMBL
| Assay Description
Inhibition of human BACE-1 after 30 mins by FRET assay |
Eur J Med Chem 156: 907-917 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.042
BindingDB Entry DOI: 10.7270/Q26W9DRP |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040366
(8-Amino-1,3-bis-cyclopropylmethyl-7-(4-nitro-benzy...)Show SMILES Nc1nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c2n1Cc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C20H22N6O4/c21-19-22-17-16(23(19)9-14-5-7-15(8-6-14)26(29)30)18(27)25(11-13-3-4-13)20(28)24(17)10-12-1-2-12/h5-8,12-13H,1-4,9-11H2,(H2,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040365
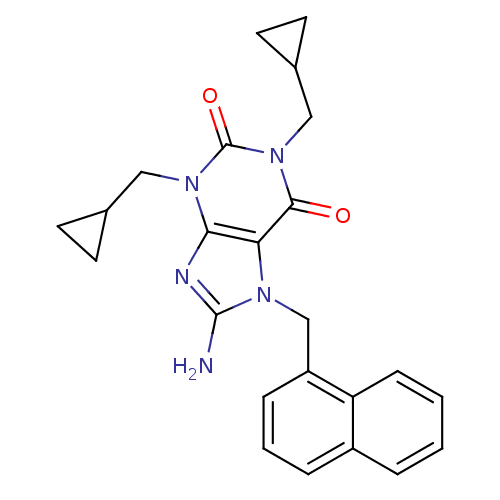
(8-Amino-1,3-bis-cyclopropylmethyl-7-naphthalen-1-y...)Show SMILES Nc1nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c2n1Cc1cccc2ccccc12 Show InChI InChI=1S/C24H25N5O2/c25-23-26-21-20(27(23)14-18-6-3-5-17-4-1-2-7-19(17)18)22(30)29(13-16-10-11-16)24(31)28(21)12-15-8-9-15/h1-7,15-16H,8-14H2,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50040358
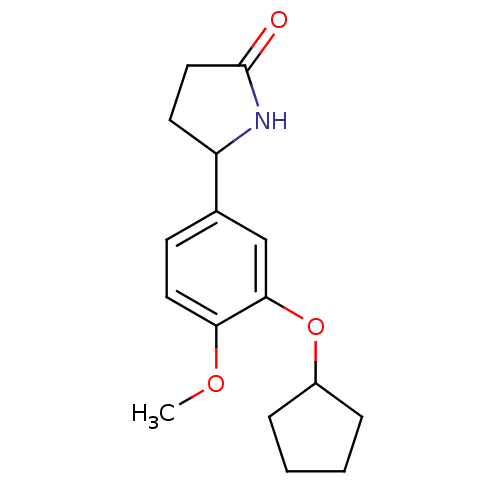
(5-(3-Cyclopentyloxy-4-methoxy-phenyl)-pyrrolidin-2...)Show InChI InChI=1S/C16H21NO3/c1-19-14-8-6-11(13-7-9-16(18)17-13)10-15(14)20-12-4-2-3-5-12/h6,8,10,12-13H,2-5,7,9H2,1H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of adenosine binding to A1 receptorof rat brain homogenates |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Bromodomain adjacent to zinc finger domain protein 2A
(Homo sapiens (Human)) | BDBM50601334

(CHEMBL5172246)Show SMILES CCc1c([nH]c(C)c1C(C)=O)-c1csc(n1)N1CCNCC1C(=O)NC1(Cc2ccccc2Br)CC1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00173
BindingDB Entry DOI: 10.7270/Q27M0D0Z |
More data for this
Ligand-Target Pair | |
Bromodomain adjacent to zinc finger domain protein 2A
(Homo sapiens (Human)) | BDBM50601332

(CHEMBL5174553)Show SMILES Cc1[nH]c(-c2csc(n2)N2CCN(Cc3c[nH]c4ncncc34)CC2)c2CCCC(=O)c12 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00173
BindingDB Entry DOI: 10.7270/Q27M0D0Z |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040343

(8-Amino-1,3-bis-cyclopropylmethyl-7-isobutyl-3,7-d...)Show SMILES CC(C)Cn1c(N)nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12 Show InChI InChI=1S/C17H25N5O2/c1-10(2)7-20-13-14(19-16(20)18)21(8-11-3-4-11)17(24)22(15(13)23)9-12-5-6-12/h10-12H,3-9H2,1-2H3,(H2,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Bromodomain adjacent to zinc finger domain protein 2A
(Homo sapiens (Human)) | BDBM50601322

(CHEMBL5192710) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00173
BindingDB Entry DOI: 10.7270/Q27M0D0Z |
More data for this
Ligand-Target Pair | |
Bromodomain adjacent to zinc finger domain protein 2A
(Homo sapiens (Human)) | BDBM50601341

(CHEMBL5205271)Show SMILES CCc1c([nH]c(C)c1C(C)=O)-c1csc(n1)N1CCNCC1C(=O)NCCC(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00173
BindingDB Entry DOI: 10.7270/Q27M0D0Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain adjacent to zinc finger domain protein 2A
(Homo sapiens (Human)) | BDBM50601319

(CHEMBL5186561) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00173
BindingDB Entry DOI: 10.7270/Q27M0D0Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain adjacent to zinc finger domain protein 2A
(Homo sapiens (Human)) | BDBM50601320
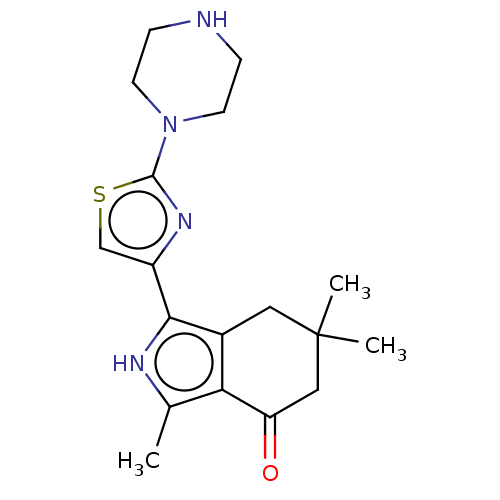
(CHEMBL5170762)Show SMILES Cc1[nH]c(-c2csc(n2)N2CCNCC2)c2CC(C)(C)CC(=O)c12 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00173
BindingDB Entry DOI: 10.7270/Q27M0D0Z |
More data for this
Ligand-Target Pair | |
Bromodomain adjacent to zinc finger domain protein 2A
(Homo sapiens (Human)) | BDBM50601318

(CHEMBL5172147)Show SMILES CC(C)CCc1c([nH]c(C)c1C(C)=O)-c1csc(n1)N1CCNCC1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00173
BindingDB Entry DOI: 10.7270/Q27M0D0Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain adjacent to zinc finger domain protein 2A
(Homo sapiens (Human)) | BDBM50601331

(CHEMBL5200427)Show SMILES Cc1c[nH]nc1CN1CCN(CC1)c1nc(cs1)-c1[nH]c(C)c2c1CCCC2=O | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00173
BindingDB Entry DOI: 10.7270/Q27M0D0Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bromodomain adjacent to zinc finger domain protein 2A
(Homo sapiens (Human)) | BDBM50601328

(CHEMBL5205483)Show SMILES CCc1c([nH]c(C)c1C(C)=O)-c1csc(n1)N1CCN(Cc2c[nH]c3ncncc23)CC1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00173
BindingDB Entry DOI: 10.7270/Q27M0D0Z |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040374
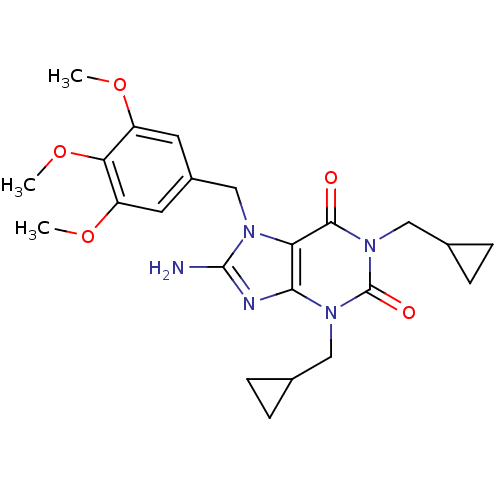
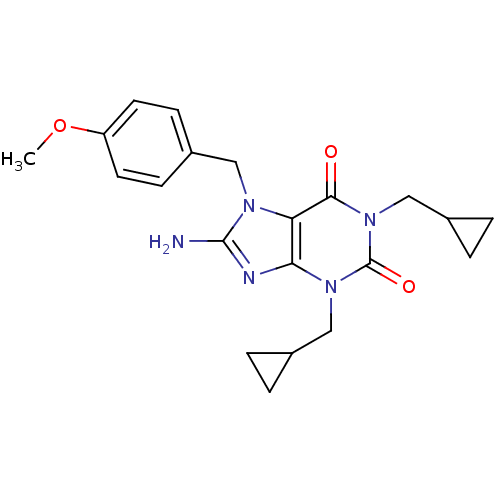
(8-Amino-1,3-bis-cyclopropylmethyl-7-(3,4,5-trimeth...)Show SMILES COc1cc(Cn2c(N)nc3n(CC4CC4)c(=O)n(CC4CC4)c(=O)c23)cc(OC)c1OC Show InChI InChI=1S/C23H29N5O5/c1-31-16-8-15(9-17(32-2)19(16)33-3)12-26-18-20(25-22(26)24)27(10-13-4-5-13)23(30)28(21(18)29)11-14-6-7-14/h8-9,13-14H,4-7,10-12H2,1-3H3,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1C
(Homo sapiens (Human)) | BDBM14363

(3-(2-propoxyphenyl)-2,4,7,8,9-pentazabicyclo[4.3.0...)Show InChI InChI=1S/C13H13N5O2/c1-2-7-20-9-6-4-3-5-8(9)11-14-12-10(13(19)15-11)16-18-17-12/h3-6H,2,7H2,1H3,(H2,14,15,16,17,18,19) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 1C at 100 uM |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Bromodomain adjacent to zinc finger domain protein 2A
(Homo sapiens (Human)) | BDBM50601327

(CHEMBL5171471)Show SMILES CCc1c([nH]c(C)c1C(C)=O)-c1csc(n1)N1CCN(Cc2n[nH]cc2C)CC1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00173
BindingDB Entry DOI: 10.7270/Q27M0D0Z |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50040351

(8-Amino-1,3-bis-cyclopropylmethyl-7-propyl-3,7-dih...)Show SMILES CCCn1c(N)nc2n(CC3CC3)c(=O)n(CC3CC3)c(=O)c12 Show InChI InChI=1S/C16H23N5O2/c1-2-7-19-12-13(18-15(19)17)20(8-10-3-4-10)16(23)21(14(12)22)9-11-5-6-11/h10-11H,2-9H2,1H3,(H2,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Concentration required to inhibit 50% activity of phosphodiesterase VA isoenzyme at 100 microM. |
J Med Chem 37: 476-85 (1994)
BindingDB Entry DOI: 10.7270/Q2P26X6N |
More data for this
Ligand-Target Pair | |
Bromodomain adjacent to zinc finger domain protein 2A
(Homo sapiens (Human)) | BDBM50601343
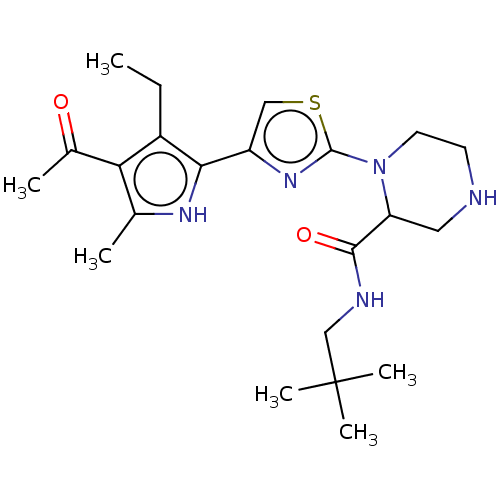
(CHEMBL5190134)Show SMILES CCc1c([nH]c(C)c1C(C)=O)-c1csc(n1)N1CCNCC1C(=O)NCC(C)(C)C | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00173
BindingDB Entry DOI: 10.7270/Q27M0D0Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data