 Found 861 hits with Last Name = 'berger' and Initial = 'lm'
Found 861 hits with Last Name = 'berger' and Initial = 'lm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase 4
(Homo sapiens (Human)) | BDBM50583973
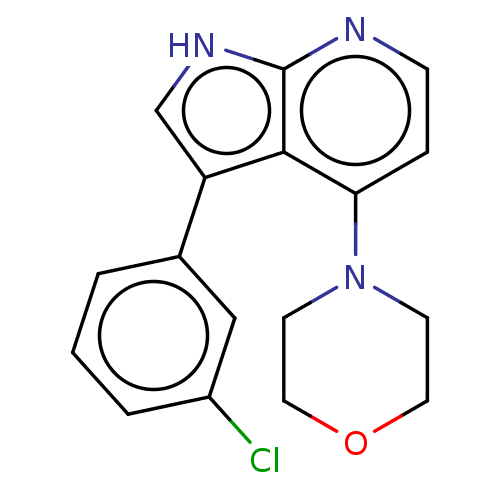
(CHEMBL5093114) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant N-terminal His6-tagged human STK4 (43 to 431 residues) expressed in Escherichia coli assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00804
BindingDB Entry DOI: 10.7270/Q2Q52TH8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 4
(Homo sapiens (Human)) | BDBM50059345
(CHEMBL3393355 | US9156845, 83)Show InChI InChI=1S/C16H15ClN4O/c17-12-3-1-2-11(8-12)13-9-18-15-14(13)16(20-10-19-15)21-4-6-22-7-5-21/h1-3,8-10H,4-7H2,(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant N-terminal His6-tagged human STK4 (43 to 431 residues) expressed in Escherichia coli assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00804
BindingDB Entry DOI: 10.7270/Q2Q52TH8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50059345

(CHEMBL3393355 | US9156845, 83)Show InChI InChI=1S/C16H15ClN4O/c17-12-3-1-2-11(8-12)13-9-18-15-14(13)16(20-10-19-15)21-4-6-22-7-5-21/h1-3,8-10H,4-7H2,(H,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant N-terminal His6-tagged human STK3 (18 to 311 residues) expressed in Escherichia coli assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00804
BindingDB Entry DOI: 10.7270/Q2Q52TH8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
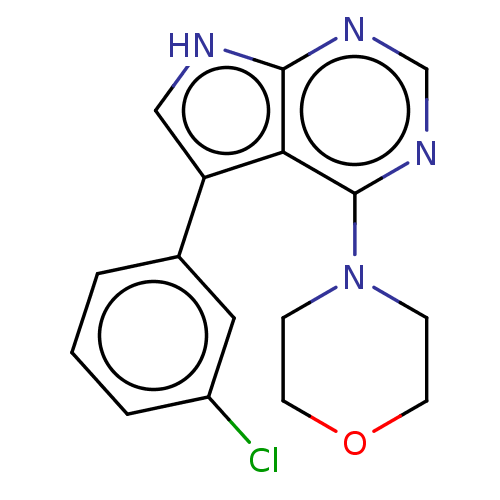
Serine/threonine-protein kinase 4
(Homo sapiens (Human)) | BDBM185556
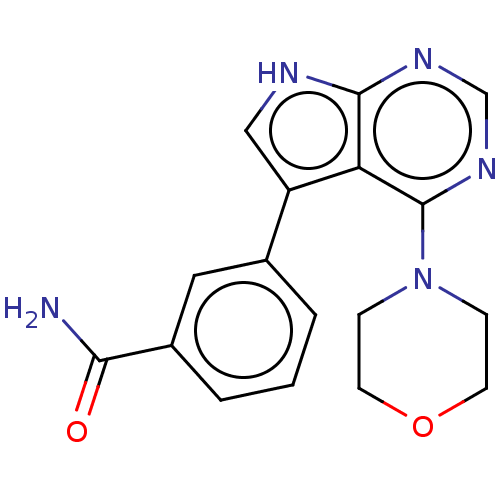
(US9156845, 215)Show SMILES NC(=O)c1cccc(c1)-c1c[nH]c2ncnc(N3CCOCC3)c12 Show InChI InChI=1S/C17H17N5O2/c18-15(23)12-3-1-2-11(8-12)13-9-19-16-14(13)17(21-10-20-16)22-4-6-24-7-5-22/h1-3,8-10H,4-7H2,(H2,18,23)(H,19,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant N-terminal His6-tagged human STK4 (43 to 431 residues) expressed in Escherichia coli assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00804
BindingDB Entry DOI: 10.7270/Q2Q52TH8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM185556

(US9156845, 215)Show SMILES NC(=O)c1cccc(c1)-c1c[nH]c2ncnc(N3CCOCC3)c12 Show InChI InChI=1S/C17H17N5O2/c18-15(23)12-3-1-2-11(8-12)13-9-19-16-14(13)17(21-10-20-16)22-4-6-24-7-5-22/h1-3,8-10H,4-7H2,(H2,18,23)(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant N-terminal His6-tagged human STK3 (18 to 311 residues) expressed in Escherichia coli assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00804
BindingDB Entry DOI: 10.7270/Q2Q52TH8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50583973

(CHEMBL5093114) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 211 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant N-terminal His6-tagged human STK3 (18 to 311 residues) expressed in Escherichia coli assessed as inhibition constant |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00804
BindingDB Entry DOI: 10.7270/Q2Q52TH8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50238177

(CHEMBL4098072)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1ccc(F)cc1 Show InChI InChI=1S/C25H22FN5O2S/c1-4-22(32)28-18-9-10-20(33-2)19(14-18)29-21-13-16(11-12-27-21)24-23(30-25(31-24)34-3)15-5-7-17(26)8-6-15/h4-14H,1H2,2-3H3,(H,27,29)(H,28,32)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase SIK2
(Homo sapiens (Human)) | BDBM50601538

(CHEMBL5090394)Show SMILES CNc1ncc2cc(-c3ccc(cc3Cl)-c3ncccc3F)c(=O)n(C[C@H]3OC[C@H](N)CO3)c2n1 |r,wU:29.31,wD:26.27,(3.85,-1.33,;3.08,,;1.54,,;.77,-1.33,;-.77,-1.33,;-1.54,,;-3.08,,;-3.85,1.33,;-5.39,1.33,;-6.16,2.67,;-7.7,2.67,;-8.47,1.33,;-7.7,,;-6.16,,;-5.39,-1.33,;-10.01,1.33,;-10.78,2.67,;-12.32,2.67,;-13.09,1.33,;-12.32,,;-10.78,,;-10.01,-1.33,;-3.08,2.67,;-3.85,4,;-1.54,2.67,;-.77,4,;.77,4,;1.54,2.67,;3.08,2.67,;3.85,4,;5.39,4,;3.08,5.33,;1.54,5.33,;-.77,1.33,;.77,1.33,)| | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02144
BindingDB Entry DOI: 10.7270/Q2W66QTG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50214095

(CHEMBL248713 | CHEMBL511394 | N-((5-(3-(5-fluoro-1...)Show SMILES CCNCc1cncc(c1)-c1cnc2n[nH]c(-c3nc4ccc(F)cc4[nH]3)c2c1 Show InChI InChI=1S/C21H18FN7/c1-2-23-8-12-5-13(10-24-9-12)14-6-16-19(28-29-20(16)25-11-14)21-26-17-4-3-15(22)7-18(17)27-21/h3-7,9-11,23H,2,8H2,1H3,(H,26,27)(H,25,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 4297-302 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.029
BindingDB Entry DOI: 10.7270/Q20C4VGJ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro antagonist effect on estrogen receptor alpha transcriptional activation in MCF-7 cells against 10 pM 17-beta-estradiol |
J Med Chem 44: 1654-7 (2001)
BindingDB Entry DOI: 10.7270/Q2F47NDX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50214115
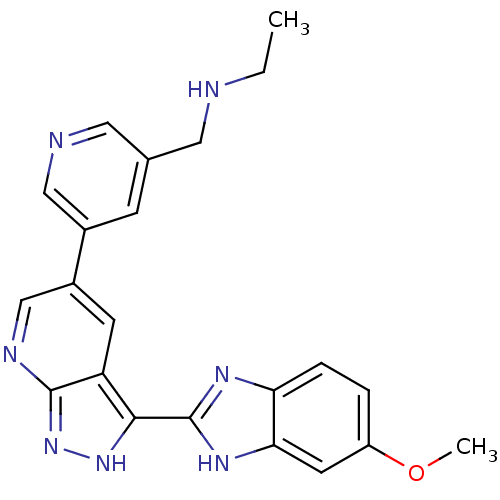
(CHEMBL429478 | N-((5-(3-(5-methoxy-1H-benzo[d]imid...)Show SMILES CCNCc1cncc(c1)-c1cnc2n[nH]c(-c3nc4ccc(OC)cc4[nH]3)c2c1 Show InChI InChI=1S/C22H21N7O/c1-3-23-9-13-6-14(11-24-10-13)15-7-17-20(28-29-21(17)25-12-15)22-26-18-5-4-16(30-2)8-19(18)27-22/h4-8,10-12,23H,3,9H2,1-2H3,(H,26,27)(H,25,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 4297-302 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.029
BindingDB Entry DOI: 10.7270/Q20C4VGJ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase SIK2
(Homo sapiens (Human)) | BDBM50601534
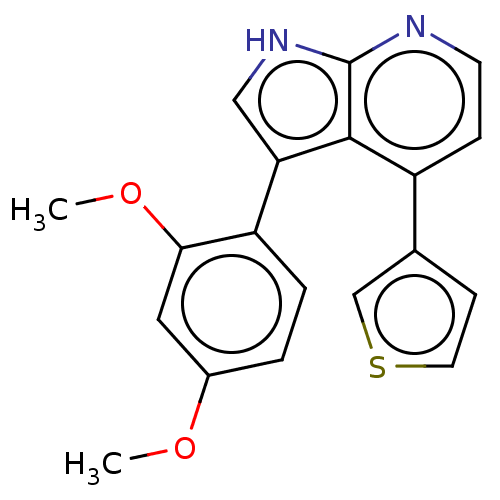
(CHEMBL4539742)Show SMILES COc1ccc(-c2c[nH]c3nccc(-c4ccsc4)c23)c(OC)c1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02144
BindingDB Entry DOI: 10.7270/Q2W66QTG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50214108

(CHEMBL248712 | N-((5-(3-(5,6-difluoro-1H-benzo[d]i...)Show SMILES CCNCc1cncc(c1)-c1cnc2n[nH]c(-c3nc4cc(F)c(F)cc4[nH]3)c2c1 Show InChI InChI=1S/C21H17F2N7/c1-2-24-7-11-3-12(9-25-8-11)13-4-14-19(29-30-20(14)26-10-13)21-27-17-5-15(22)16(23)6-18(17)28-21/h3-6,8-10,24H,2,7H2,1H3,(H,27,28)(H,26,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 4297-302 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.029
BindingDB Entry DOI: 10.7270/Q20C4VGJ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50613306
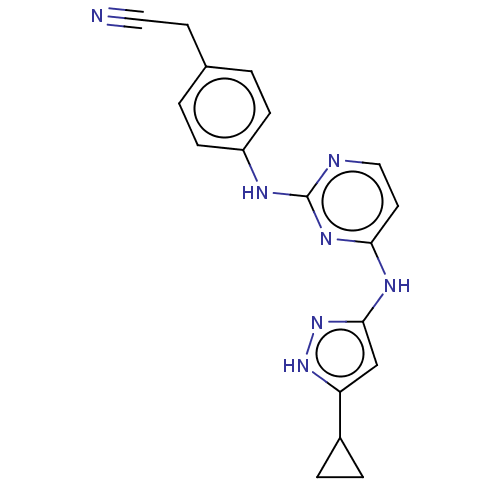
(CHEMBL1235645)Show SMILES N#CCc1ccc(Nc2nccc(Nc3cc([nH]n3)C3CC3)n2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50602487
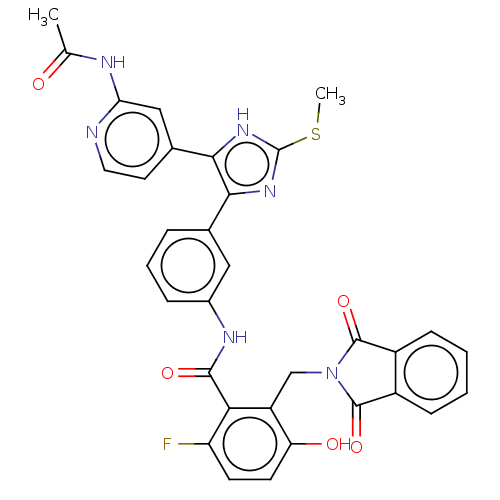
(CHEMBL5183286)Show SMILES CSc1nc(c([nH]1)-c1ccnc(NC(C)=O)c1)-c1cccc(NC(=O)c2c(F)ccc(O)c2CN2C(=O)c3ccccc3C2=O)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50099587
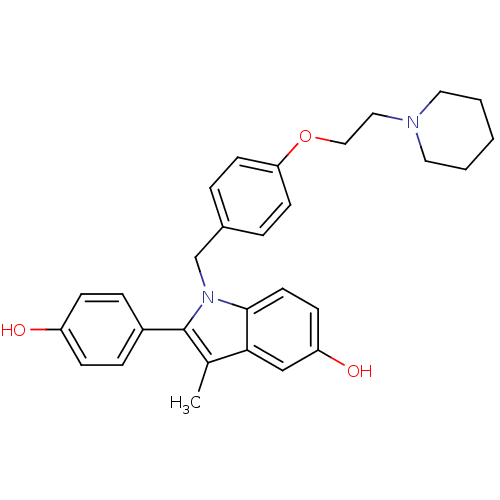
(2-(4-Hydroxy-phenyl)-3-methyl-1-[4-(2-piperidin-1-...)Show SMILES Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCN3CCCCC3)cc2)c2ccc(O)cc12 Show InChI InChI=1S/C29H32N2O3/c1-21-27-19-25(33)11-14-28(27)31(29(21)23-7-9-24(32)10-8-23)20-22-5-12-26(13-6-22)34-18-17-30-15-3-2-4-16-30/h5-14,19,32-33H,2-4,15-18,20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro antagonist effect on estrogen receptor alpha transcriptional activation in MCF-7 cells against 10 pM 17-beta-estradiol |
J Med Chem 44: 1654-7 (2001)
BindingDB Entry DOI: 10.7270/Q2F47NDX |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50338221
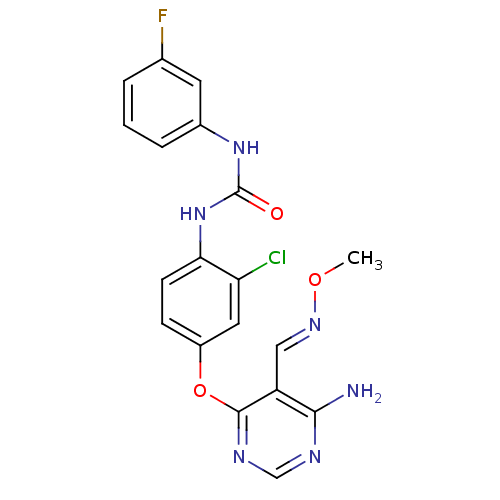
(1-(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-...)Show SMILES CO\N=C\c1c(N)ncnc1Oc1ccc(NC(=O)Nc2cccc(F)c2)c(Cl)c1 Show InChI InChI=1S/C19H16ClFN6O3/c1-29-25-9-14-17(22)23-10-24-18(14)30-13-5-6-16(15(20)8-13)27-19(28)26-12-4-2-3-11(21)7-12/h2-10H,1H3,(H2,22,23,24)(H2,26,27,28)/b25-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 21: 1815-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.053
BindingDB Entry DOI: 10.7270/Q2ZP46DK |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50214100
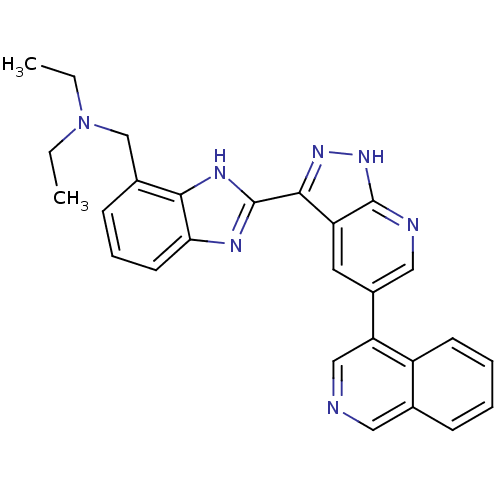
(CHEMBL249303 | N-ethyl-N-((2-(5-(isoquinolin-4-yl)...)Show SMILES CCN(CC)Cc1cccc2nc([nH]c12)-c1n[nH]c2ncc(cc12)-c1cncc2ccccc12 Show InChI InChI=1S/C27H25N7/c1-3-34(4-2)16-18-9-7-11-23-24(18)31-27(30-23)25-21-12-19(14-29-26(21)33-32-25)22-15-28-13-17-8-5-6-10-20(17)22/h5-15H,3-4,16H2,1-2H3,(H,30,31)(H,29,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 4297-302 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.029
BindingDB Entry DOI: 10.7270/Q20C4VGJ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141149
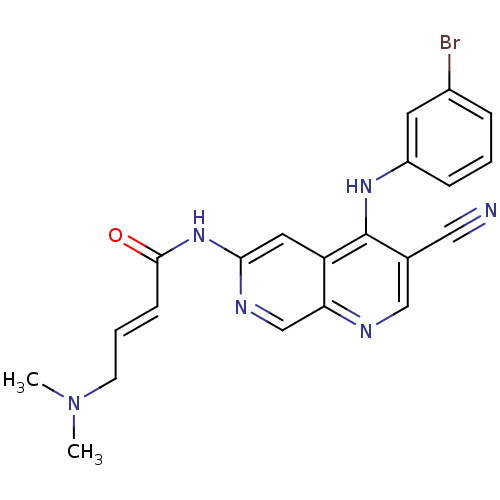
((E)-4-Dimethylamino-but-2-enoic acid [4-(3-bromo-p...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3cccc(Br)c3)c(cnc2cn1)C#N Show InChI InChI=1S/C21H19BrN6O/c1-28(2)8-4-7-20(29)27-19-10-17-18(13-25-19)24-12-14(11-23)21(17)26-16-6-3-5-15(22)9-16/h3-7,9-10,12-13H,8H2,1-2H3,(H,24,26)(H,25,27,29)/b7-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of peptide substrate phosphorylation by epidermal growth factor receptor |
Bioorg Med Chem Lett 14: 1411-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.034
BindingDB Entry DOI: 10.7270/Q2G161DC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50141149

((E)-4-Dimethylamino-but-2-enoic acid [4-(3-bromo-p...)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3cccc(Br)c3)c(cnc2cn1)C#N Show InChI InChI=1S/C21H19BrN6O/c1-28(2)8-4-7-20(29)27-19-10-17-18(13-25-19)24-12-14(11-23)21(17)26-16-6-3-5-15(22)9-16/h3-7,9-10,12-13H,8H2,1-2H3,(H,24,26)(H,25,27,29)/b7-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of peptide substrate phosphorylation by epidermal growth factor receptor |
Bioorg Med Chem Lett 14: 1411-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.034
BindingDB Entry DOI: 10.7270/Q2G161DC |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth-Ayerst Research
| Assay Description
The EGF-R kinase autophosphorylation activity was measured by DELFIA/time-resolved fluorometry with excitation at 340 nm and emission at 615 nm. Po... |
J Med Chem 44: 2719-34 (2001)
Article DOI: 10.1021/jm0005555
BindingDB Entry DOI: 10.7270/Q2PN93TS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50602490
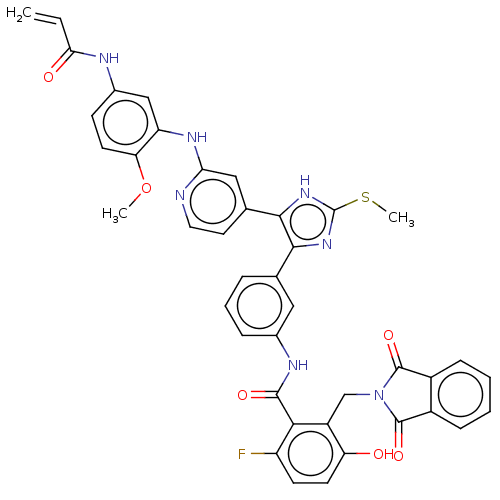
(CHEMBL5188399)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1cccc(NC(=O)c2c(F)ccc(O)c2CN2C(=O)c3ccccc3C2=O)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4567

(4-anilinoquinazoline deriv. 2 | BMC163482 Compound...)Show InChI InChI=1S/C17H13BrN4O/c1-2-16(23)21-13-6-7-15-14(9-13)17(20-10-19-15)22-12-5-3-4-11(18)8-12/h2-10H,1H2,(H,21,23)(H,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of autophosphorylation of cytoplasmic domain of epidermal growth factor receptor |
J Med Chem 46: 49-63 (2002)
Article DOI: 10.1021/jm020241c
BindingDB Entry DOI: 10.7270/Q2PV6M3H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50338215

(1-(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-...)Show SMILES CO\N=C\c1c(N)ncnc1Oc1ccc(NC(=O)Nc2cc(C)on2)c(Cl)c1 Show InChI InChI=1S/C17H16ClN7O4/c1-9-5-14(25-29-9)24-17(26)23-13-4-3-10(6-12(13)18)28-16-11(7-22-27-2)15(19)20-8-21-16/h3-8H,1-2H3,(H2,19,20,21)(H2,23,24,25,26)/b22-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 21: 1815-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.053
BindingDB Entry DOI: 10.7270/Q2ZP46DK |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50338214
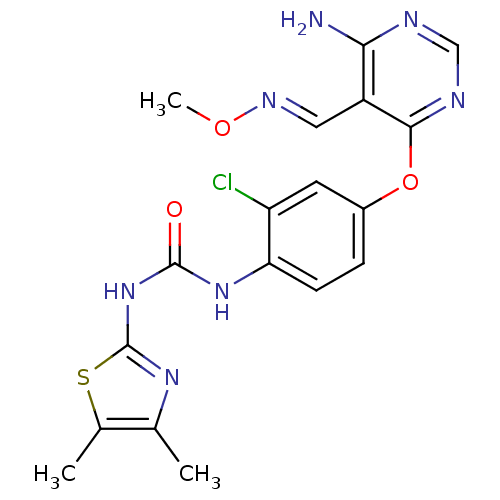
(1-(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-...)Show SMILES CO\N=C\c1c(N)ncnc1Oc1ccc(NC(=O)Nc2nc(C)c(C)s2)c(Cl)c1 Show InChI InChI=1S/C18H18ClN7O3S/c1-9-10(2)30-18(24-9)26-17(27)25-14-5-4-11(6-13(14)19)29-16-12(7-23-28-3)15(20)21-8-22-16/h4-8H,1-3H3,(H2,20,21,22)(H2,24,25,26,27)/b23-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 21: 1815-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.053
BindingDB Entry DOI: 10.7270/Q2ZP46DK |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4582

(4-anilinoquinazoline deriv. 10o | N-{4-[(3-bromoph...)Show InChI InChI=1S/C18H13BrN4O2/c19-12-3-1-4-13(9-12)23-18-15-10-14(22-17(25)5-2-8-24)6-7-16(15)20-11-21-18/h1,3-4,6-7,9-11,24H,8H2,(H,22,25)(H,20,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth-Ayerst Research
| Assay Description
The EGF-R kinase autophosphorylation activity was measured by DELFIA/time-resolved fluorometry with excitation at 340 nm and emission at 615 nm. Po... |
J Med Chem 44: 2719-34 (2001)
Article DOI: 10.1021/jm0005555
BindingDB Entry DOI: 10.7270/Q2PN93TS |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50338219
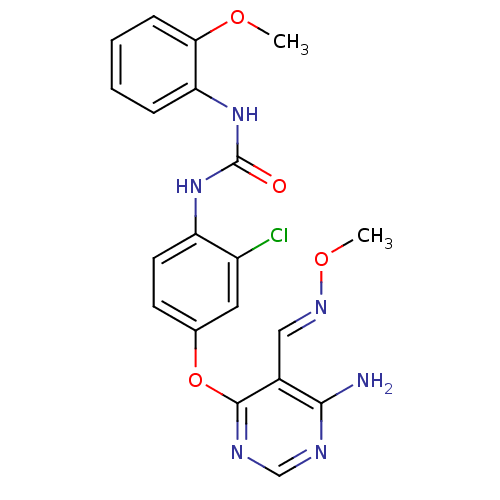
(1-(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-...)Show SMILES CO\N=C\c1c(N)ncnc1Oc1ccc(NC(=O)Nc2ccccc2OC)c(Cl)c1 Show InChI InChI=1S/C20H19ClN6O4/c1-29-17-6-4-3-5-16(17)27-20(28)26-15-8-7-12(9-14(15)21)31-19-13(10-25-30-2)18(22)23-11-24-19/h3-11H,1-2H3,(H2,22,23,24)(H2,26,27,28)/b25-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 21: 1815-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.053
BindingDB Entry DOI: 10.7270/Q2ZP46DK |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50214107

((5-(3-(4-(methoxymethyl)-1H-benzo[d]imidazol-2-yl)...)Show SMILES CNCc1cncc(c1)-c1cnc2n[nH]c(-c3nc4cccc(COC)c4[nH]3)c2c1 Show InChI InChI=1S/C22H21N7O/c1-23-8-13-6-15(10-24-9-13)16-7-17-20(28-29-21(17)25-11-16)22-26-18-5-3-4-14(12-30-2)19(18)27-22/h3-7,9-11,23H,8,12H2,1-2H3,(H,26,27)(H,25,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 4297-302 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.029
BindingDB Entry DOI: 10.7270/Q20C4VGJ |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50338217
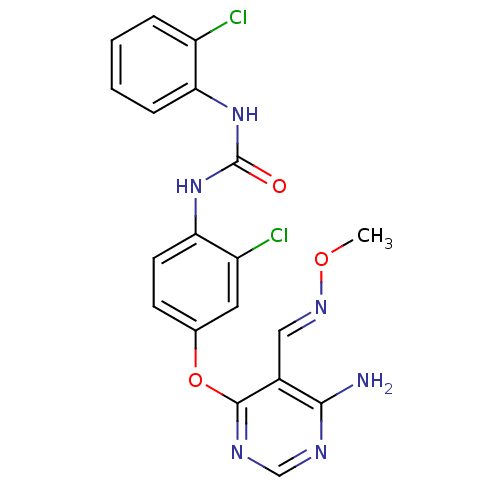
(1-(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-...)Show SMILES CO\N=C\c1c(N)ncnc1Oc1ccc(NC(=O)Nc2ccccc2Cl)c(Cl)c1 Show InChI InChI=1S/C19H16Cl2N6O3/c1-29-25-9-12-17(22)23-10-24-18(12)30-11-6-7-16(14(21)8-11)27-19(28)26-15-5-3-2-4-13(15)20/h2-10H,1H3,(H2,22,23,24)(H2,26,27,28)/b25-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 21: 1815-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.053
BindingDB Entry DOI: 10.7270/Q2ZP46DK |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50338222
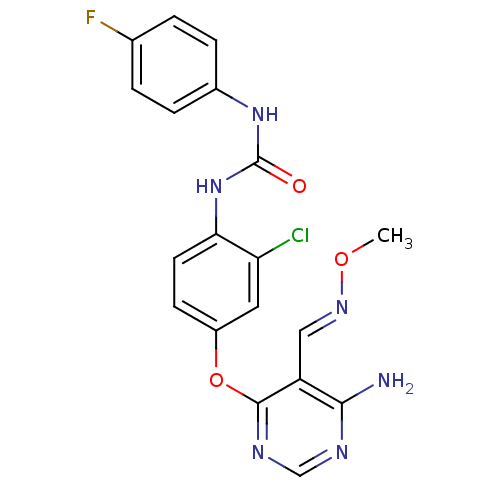
(1-(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-...)Show SMILES CO\N=C\c1c(N)ncnc1Oc1ccc(NC(=O)Nc2ccc(F)cc2)c(Cl)c1 Show InChI InChI=1S/C19H16ClFN6O3/c1-29-25-9-14-17(22)23-10-24-18(14)30-13-6-7-16(15(20)8-13)27-19(28)26-12-4-2-11(21)3-5-12/h2-10H,1H3,(H2,22,23,24)(H2,26,27,28)/b25-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 21: 1815-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.053
BindingDB Entry DOI: 10.7270/Q2ZP46DK |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50214099
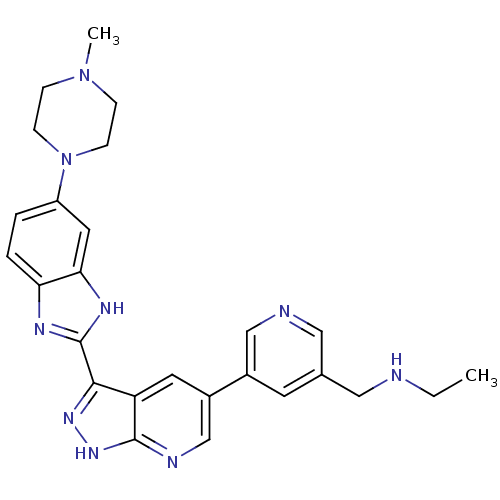
(CHEMBL400569 | N-((5-(3-(5-(4-methylpiperazin-1-yl...)Show SMILES CCNCc1cncc(c1)-c1cnc2[nH]nc(-c3nc4ccc(cc4[nH]3)N3CCN(C)CC3)c2c1 Show InChI InChI=1S/C26H29N9/c1-3-27-13-17-10-18(15-28-14-17)19-11-21-24(32-33-25(21)29-16-19)26-30-22-5-4-20(12-23(22)31-26)35-8-6-34(2)7-9-35/h4-5,10-12,14-16,27H,3,6-9,13H2,1-2H3,(H,30,31)(H,29,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 4297-302 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.029
BindingDB Entry DOI: 10.7270/Q20C4VGJ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20625
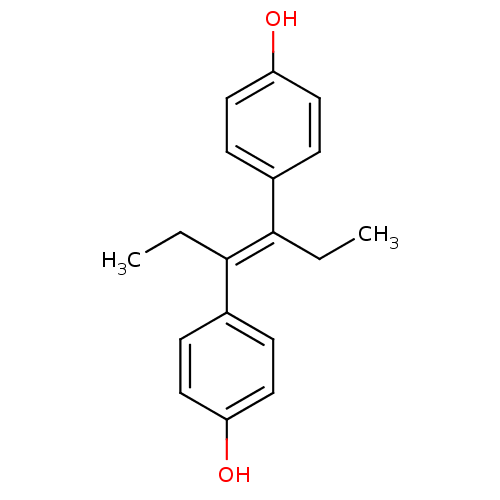
(4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...)Show InChI InChI=1S/C18H20O2/c1-3-17(13-5-9-15(19)10-6-13)18(4-2)14-7-11-16(20)12-8-14/h5-12,19-20H,3-4H2,1-2H3/b18-17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-17-beta-estradiol binding to human estrogen receptor alpha |
J Med Chem 44: 1654-7 (2001)
BindingDB Entry DOI: 10.7270/Q2F47NDX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50338216
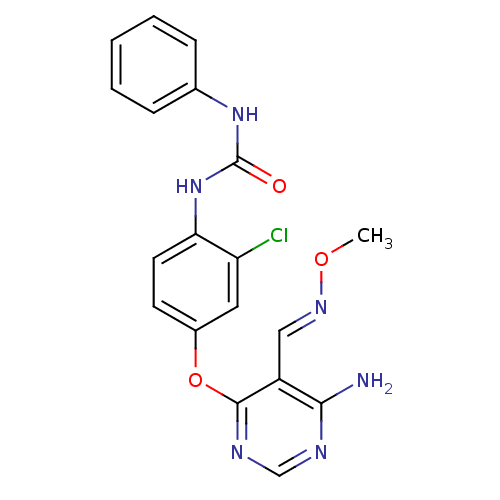
(1-(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-...)Show SMILES CO\N=C\c1c(N)ncnc1Oc1ccc(NC(=O)Nc2ccccc2)c(Cl)c1 Show InChI InChI=1S/C19H17ClN6O3/c1-28-24-10-14-17(21)22-11-23-18(14)29-13-7-8-16(15(20)9-13)26-19(27)25-12-5-3-2-4-6-12/h2-11H,1H3,(H2,21,22,23)(H2,25,26,27)/b24-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 21: 1815-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.053
BindingDB Entry DOI: 10.7270/Q2ZP46DK |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50338223

(1-(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-...)Show SMILES CO\N=C\c1c(N)ncnc1Oc1ccc(NC(=O)Nc2ccc(F)cc2F)c(Cl)c1 Show InChI InChI=1S/C19H15ClF2N6O3/c1-30-26-8-12-17(23)24-9-25-18(12)31-11-3-5-15(13(20)7-11)27-19(29)28-16-4-2-10(21)6-14(16)22/h2-9H,1H3,(H2,23,24,25)(H2,27,28,29)/b26-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 21: 1815-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.053
BindingDB Entry DOI: 10.7270/Q2ZP46DK |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50338220
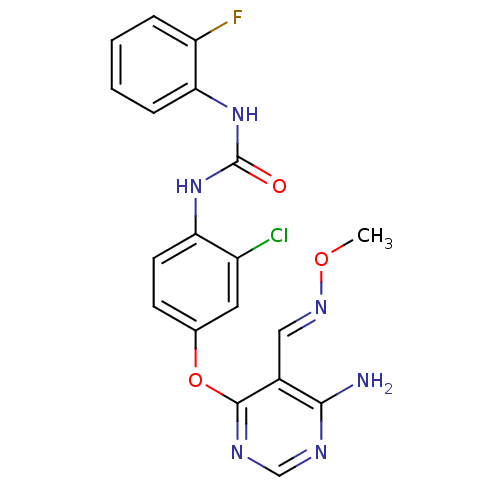
(1-(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-...)Show SMILES CO\N=C\c1c(N)ncnc1Oc1ccc(NC(=O)Nc2ccccc2F)c(Cl)c1 Show InChI InChI=1S/C19H16ClFN6O3/c1-29-25-9-12-17(22)23-10-24-18(12)30-11-6-7-15(13(20)8-11)26-19(28)27-16-5-3-2-4-14(16)21/h2-10H,1H3,(H2,22,23,24)(H2,26,27,28)/b25-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 21: 1815-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.053
BindingDB Entry DOI: 10.7270/Q2ZP46DK |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50338213
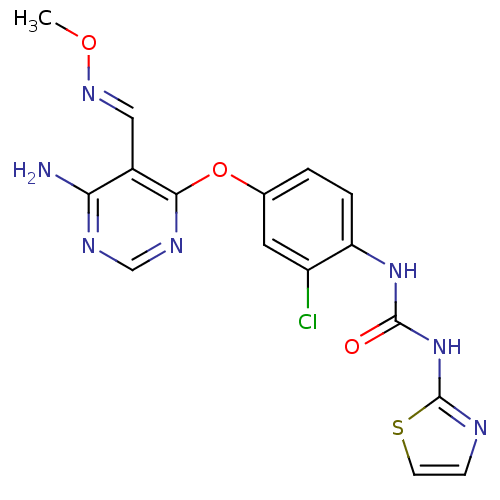
(1-(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-...)Show SMILES CO\N=C\c1c(N)ncnc1Oc1ccc(NC(=O)Nc2nccs2)c(Cl)c1 Show InChI InChI=1S/C16H14ClN7O3S/c1-26-22-7-10-13(18)20-8-21-14(10)27-9-2-3-12(11(17)6-9)23-15(25)24-16-19-4-5-28-16/h2-8H,1H3,(H2,18,20,21)(H2,19,23,24,25)/b22-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 |
Bioorg Med Chem Lett 21: 1815-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.053
BindingDB Entry DOI: 10.7270/Q2ZP46DK |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50099585
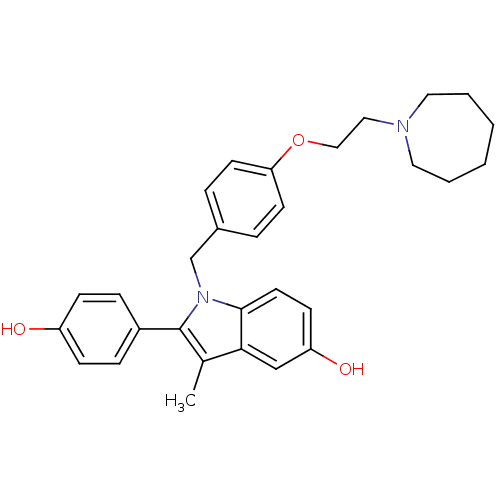
(1-(4-(2-(azepan-1-yl)ethoxy)benzyl)-2-(4-hydroxyph...)Show SMILES Cc1c(-c2ccc(O)cc2)n(Cc2ccc(OCCN3CCCCCC3)cc2)c2ccc(O)cc12 Show InChI InChI=1S/C30H34N2O3/c1-22-28-20-26(34)12-15-29(28)32(30(22)24-8-10-25(33)11-9-24)21-23-6-13-27(14-7-23)35-19-18-31-16-4-2-3-5-17-31/h6-15,20,33-34H,2-5,16-19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro antagonist effect on estrogen receptor alpha transcriptional activation in MCF-7 cells against 10 pM 17-beta-estradiol |
J Med Chem 44: 1654-7 (2001)
BindingDB Entry DOI: 10.7270/Q2F47NDX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50613306

(CHEMBL1235645)Show SMILES N#CCc1ccc(Nc2nccc(Nc3cc([nH]n3)C3CC3)n2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM50613306

(CHEMBL1235645)Show SMILES N#CCc1ccc(Nc2nccc(Nc3cc([nH]n3)C3CC3)n2)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-17-beta-estradiol binding to human estrogen receptor alpha |
J Med Chem 44: 1654-7 (2001)
BindingDB Entry DOI: 10.7270/Q2F47NDX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM20625

(4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...)Show InChI InChI=1S/C18H20O2/c1-3-17(13-5-9-15(19)10-6-13)18(4-2)14-7-11-16(20)12-8-14/h5-12,19-20H,3-4H2,1-2H3/b18-17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against [3H]-17-beta-estradiol binding to human estrogen receptor 2 |
J Med Chem 44: 1654-7 (2001)
BindingDB Entry DOI: 10.7270/Q2F47NDX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase SIK2
(Homo sapiens (Human)) | BDBM50148921

(CHEMBL3770443)Show SMILES CNc1ncc2cc(-c3ccc(cc3Cl)-c3cccc(C)n3)c(=O)n(C[C@H]3OC[C@H](N)CO3)c2n1 |r,wU:26.27,wD:29.31,(-5.08,.92,;-4.02,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.66,-.77,;4,-1.54,;3.99,-3.08,;5.33,-3.85,;6.66,-3.08,;6.66,-1.54,;5.33,-.77,;5.33,.46,;7.99,-3.86,;9.33,-3.09,;10.66,-3.86,;10.66,-5.4,;9.32,-6.17,;9.32,-7.4,;7.99,-5.4,;2.66,.77,;3.73,1.38,;1.33,1.54,;1.33,3.08,;2.67,3.85,;2.68,5.39,;4.01,6.15,;5.34,5.38,;6.41,5.99,;5.34,3.84,;4,3.07,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C25H25ClN6O3/c1-14-4-3-5-21(30-14)15-6-7-18(20(26)9-15)19-8-16-10-29-25(28-2)31-23(16)32(24(19)33)11-22-34-12-17(27)13-35-22/h3-10,17,22H,11-13,27H2,1-2H3,(H,28,29,31)/t17-,22- | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02144
BindingDB Entry DOI: 10.7270/Q2W66QTG |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50602490

(CHEMBL5188399)Show SMILES COc1ccc(NC(=O)C=C)cc1Nc1cc(ccn1)-c1[nH]c(SC)nc1-c1cccc(NC(=O)c2c(F)ccc(O)c2CN2C(=O)c3ccccc3C2=O)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50377274
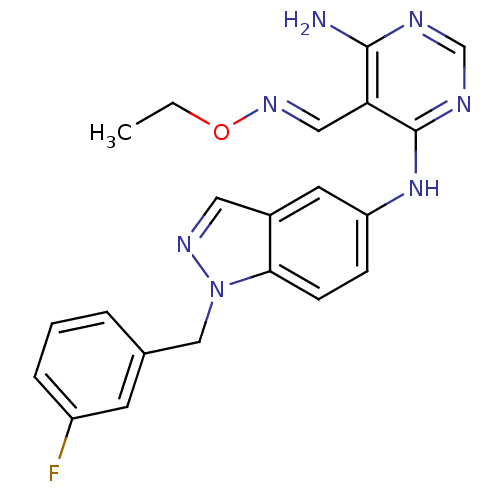
(CHEMBL402294)Show SMILES CCO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 Show InChI InChI=1S/C21H20FN7O/c1-2-30-27-11-18-20(23)24-13-25-21(18)28-17-6-7-19-15(9-17)10-26-29(19)12-14-4-3-5-16(22)8-14/h3-11,13H,2,12H2,1H3,(H3,23,24,25,28)/b27-11+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of EGFR |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50602489
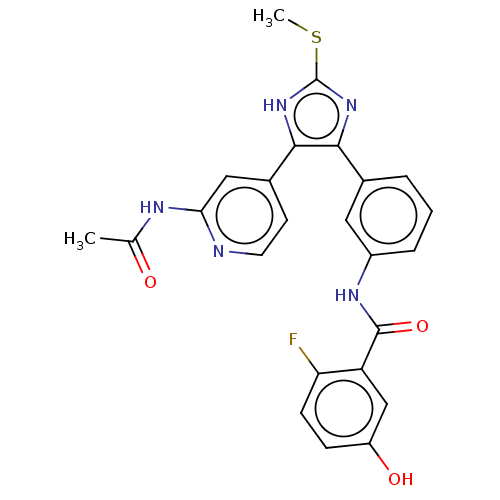
(CHEMBL5188250)Show SMILES CSc1nc(c([nH]1)-c1ccnc(NC(C)=O)c1)-c1cccc(NC(=O)c2cc(O)ccc2F)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00848
BindingDB Entry DOI: 10.7270/Q2Q52TQZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50214111
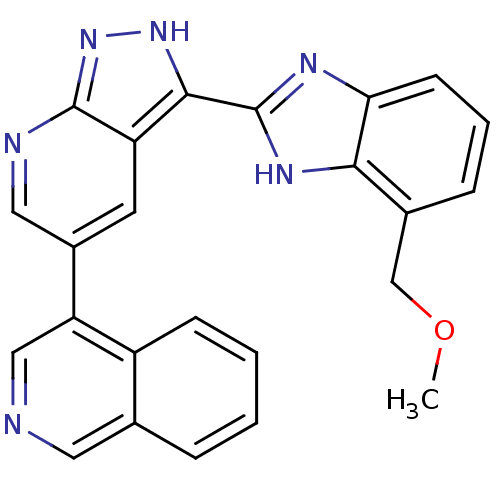
(4-(3-(4-(methoxymethyl)-1H-benzo[d]imidazol-2-yl)-...)Show SMILES COCc1cccc2nc([nH]c12)-c1[nH]nc2ncc(cc12)-c1cncc2ccccc12 Show InChI InChI=1S/C24H18N6O/c1-31-13-15-6-4-8-20-21(15)28-24(27-20)22-18-9-16(11-26-23(18)30-29-22)19-12-25-10-14-5-2-3-7-17(14)19/h2-12H,13H2,1H3,(H,27,28)(H,26,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C.
Curated by ChEMBL
| Assay Description
Inhibition of CDK1 |
Bioorg Med Chem Lett 17: 4297-302 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.029
BindingDB Entry DOI: 10.7270/Q20C4VGJ |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50377285
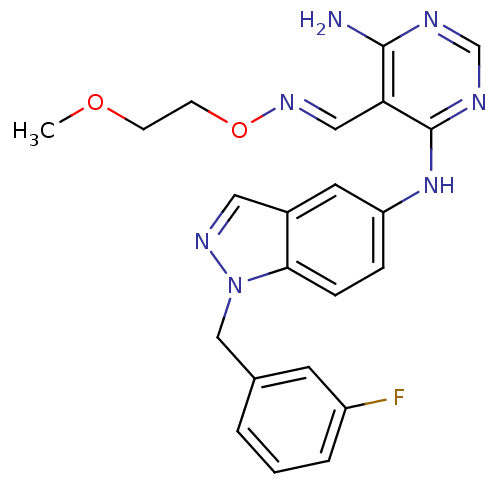
(CHEMBL255237)Show SMILES COCCO\N=C\c1c(N)ncnc1Nc1ccc2n(Cc3cccc(F)c3)ncc2c1 Show InChI InChI=1S/C22H22FN7O2/c1-31-7-8-32-28-12-19-21(24)25-14-26-22(19)29-18-5-6-20-16(10-18)11-27-30(20)13-15-3-2-4-17(23)9-15/h2-6,9-12,14H,7-8,13H2,1H3,(H3,24,25,26,29)/b28-12+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of ErbB2 |
Bioorg Med Chem Lett 18: 3495-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.024
BindingDB Entry DOI: 10.7270/Q21G0N5V |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4568

(4-anilinoquinazoline deriv. 10a | N-{4-[(3-bromoph...)Show SMILES CN(C)CC#CC(=O)Nc1ccc2ncnc(Nc3cccc(Br)c3)c2c1 Show InChI InChI=1S/C20H18BrN5O/c1-26(2)10-4-7-19(27)24-16-8-9-18-17(12-16)20(23-13-22-18)25-15-6-3-5-14(21)11-15/h3,5-6,8-9,11-13H,10H2,1-2H3,(H,24,27)(H,22,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth-Ayerst Research
| Assay Description
The EGF-R kinase autophosphorylation activity was measured by DELFIA/time-resolved fluorometry with excitation at 340 nm and emission at 615 nm. Po... |
J Med Chem 44: 2719-34 (2001)
Article DOI: 10.1021/jm0005555
BindingDB Entry DOI: 10.7270/Q2PN93TS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase SIK3
(Homo sapiens) | BDBM50601534

(CHEMBL4539742)Show SMILES COc1ccc(-c2c[nH]c3nccc(-c4ccsc4)c23)c(OC)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02144
BindingDB Entry DOI: 10.7270/Q2W66QTG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data