 Found 418 hits with Last Name = 'lorenzi' and Initial = 'mv'
Found 418 hits with Last Name = 'lorenzi' and Initial = 'mv' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179969
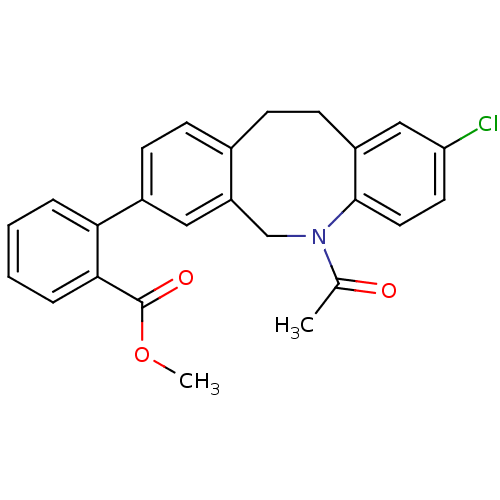
(2-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...)Show SMILES COC(=O)c1ccccc1-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22ClNO3/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)25(29)30-2)9-7-17(20)8-10-19-14-21(26)11-12-24(19)27/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179935
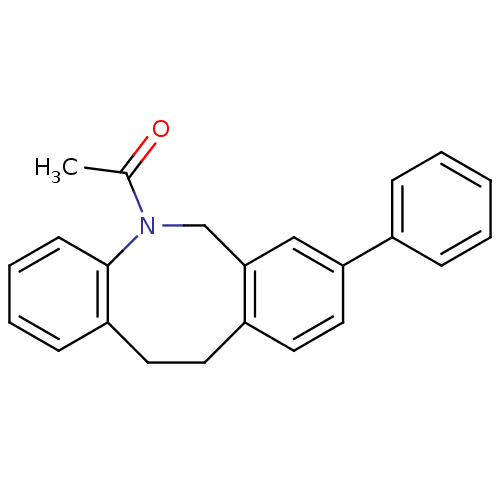
(1-(8-Phenyl-11,12-dihydro-6H-dibenzo[b,f]azocin-5-...)Show InChI InChI=1S/C23H21NO/c1-17(25)24-16-22-15-21(18-7-3-2-4-8-18)14-12-19(22)11-13-20-9-5-6-10-23(20)24/h2-10,12,14-15H,11,13,16H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179960

(1-[8-(2-acetyl-phenyl)-2-chloro-11,12-dihydro-6H-d...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1C(C)=O Show InChI InChI=1S/C25H22ClNO2/c1-16(28)23-5-3-4-6-24(23)19-9-7-18-8-10-20-14-22(26)11-12-25(20)27(17(2)29)15-21(18)13-19/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50258790
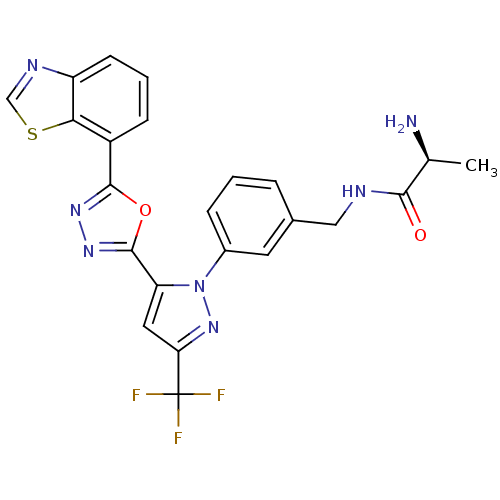
((S)-2-amino-N-(3-(5-(5-(benzo[d]thiazol-7-yl)-1,3,...)Show SMILES C[C@H](N)C(=O)NCc1cccc(c1)-n1nc(cc1-c1nnc(o1)-c1cccc2ncsc12)C(F)(F)F |r| Show InChI InChI=1S/C23H18F3N7O2S/c1-12(27)20(34)28-10-13-4-2-5-14(8-13)33-17(9-18(32-33)23(24,25)26)22-31-30-21(35-22)15-6-3-7-16-19(15)36-11-29-16/h2-9,11-12H,10,27H2,1H3,(H,28,34)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301077
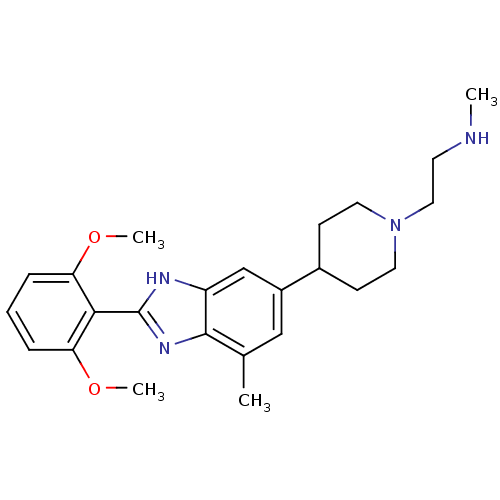
(2-(4-(2-(2,6-dimethoxyphenyl)-7-methyl-1H-benzo[d]...)Show SMILES CNCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(OC)cccc1OC Show InChI InChI=1S/C24H32N4O2/c1-16-14-18(17-8-11-28(12-9-17)13-10-25-2)15-19-23(16)27-24(26-19)22-20(29-3)6-5-7-21(22)30-4/h5-7,14-15,17,25H,8-13H2,1-4H3,(H,26,27) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301079

(2-(4-(2-(2-fluoro-6-methoxyphenyl)-7-methyl-1H-ben...)Show SMILES CNCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1c(F)cccc1OC Show InChI InChI=1S/C23H29FN4O/c1-15-13-17(16-7-10-28(11-8-16)12-9-25-2)14-19-22(15)27-23(26-19)21-18(24)5-4-6-20(21)29-3/h4-6,13-14,16,25H,7-12H2,1-3H3,(H,26,27) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301078

(2-(4-(2-(4-fluoro-2-methoxyphenyl)-7-methyl-1H-ben...)Show SMILES CNCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1ccc(F)cc1OC Show InChI InChI=1S/C23H29FN4O/c1-15-12-17(16-6-9-28(10-7-16)11-8-25-2)13-20-22(15)27-23(26-20)19-5-4-18(24)14-21(19)29-3/h4-5,12-14,16,25H,6-11H2,1-3H3,(H,26,27) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301076
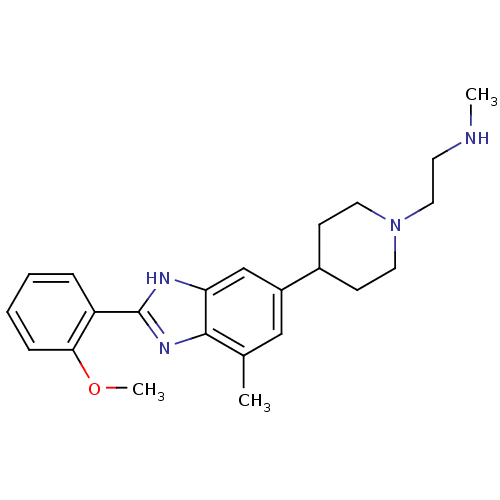
(2-(4-(2-(2-methoxyphenyl)-4-methyl-1H-benzo[d]imid...)Show SMILES CNCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1ccccc1OC Show InChI InChI=1S/C23H30N4O/c1-16-14-18(17-8-11-27(12-9-17)13-10-24-2)15-20-22(16)26-23(25-20)19-6-4-5-7-21(19)28-3/h4-7,14-15,17,24H,8-13H2,1-3H3,(H,25,26) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179969

(2-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...)Show SMILES COC(=O)c1ccccc1-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22ClNO3/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)25(29)30-2)9-7-17(20)8-10-19-14-21(26)11-12-24(19)27/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179960

(1-[8-(2-acetyl-phenyl)-2-chloro-11,12-dihydro-6H-d...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1C(C)=O Show InChI InChI=1S/C25H22ClNO2/c1-16(28)23-5-3-4-6-24(23)19-9-7-18-8-10-20-14-22(26)11-12-25(20)27(17(2)29)15-21(18)13-19/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337332
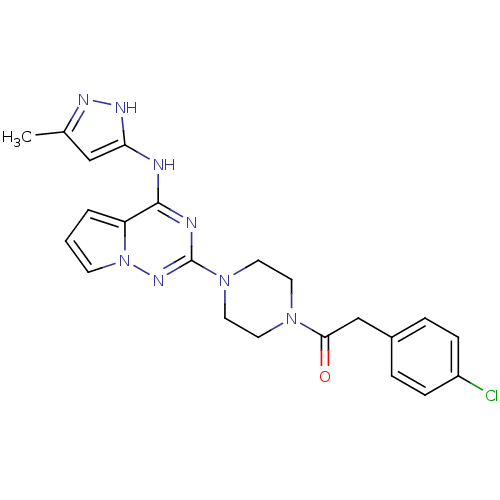
(2-(4-chlorophenyl)-1-(4-(4-(5-methyl-1H-pyrazol-3-...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2ccc(Cl)cc2)[nH]n1 Show InChI InChI=1S/C22H23ClN8O/c1-15-13-19(27-26-15)24-21-18-3-2-8-31(18)28-22(25-21)30-11-9-29(10-12-30)20(32)14-16-4-6-17(23)7-5-16/h2-8,13H,9-12,14H2,1H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50229083
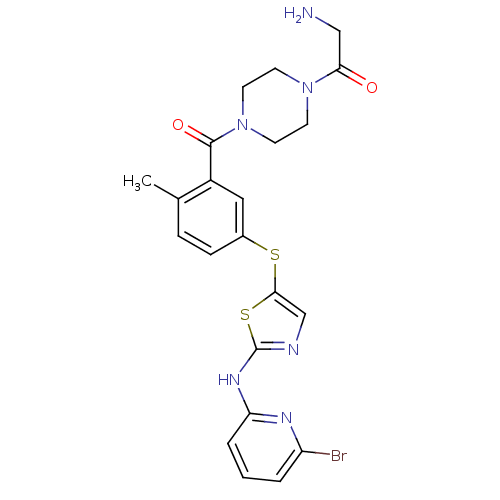
(2-amino-1-(4-(5-(2-(6-bromopyridin-2-ylamino)thiaz...)Show SMILES Cc1ccc(Sc2cnc(Nc3cccc(Br)n3)s2)cc1C(=O)N1CCN(CC1)C(=O)CN Show InChI InChI=1S/C22H23BrN6O2S2/c1-14-5-6-15(11-16(14)21(31)29-9-7-28(8-10-29)19(30)12-24)32-20-13-25-22(33-20)27-18-4-2-3-17(23)26-18/h2-6,11,13H,7-10,12,24H2,1H3,(H,25,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of TrkA |
Bioorg Med Chem Lett 18: 634-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.076
BindingDB Entry DOI: 10.7270/Q2JD4WJ5 |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301075

(2-(4-(2-(2-methoxypyridin-3-yl)-3H-imidazo[4,5-b]p...)Show SMILES CNCCN1CCC(CC1)c1cnc2nc([nH]c2c1)-c1cccnc1OC Show InChI InChI=1S/C20H26N6O/c1-21-8-11-26-9-5-14(6-10-26)15-12-17-19(23-13-15)25-18(24-17)16-4-3-7-22-20(16)27-2/h3-4,7,12-14,21H,5-6,8-11H2,1-2H3,(H,23,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122323
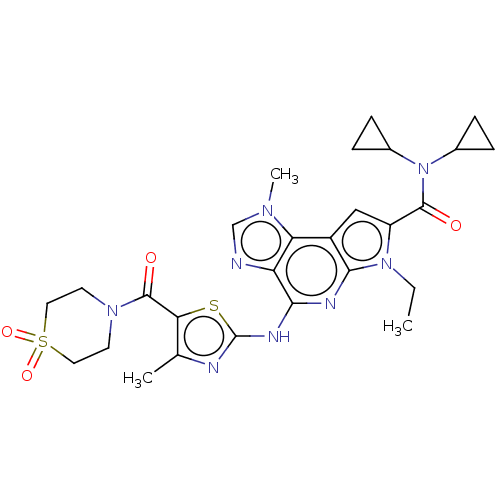
(CHEMBL3622146)Show SMILES CCn1c(cc2c1nc(Nc1nc(C)c(s1)C(=O)N1CCS(=O)(=O)CC1)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C27H32N8O4S2/c1-4-34-19(25(36)35(16-5-6-16)17-7-8-17)13-18-21-20(28-14-32(21)3)23(30-24(18)34)31-27-29-15(2)22(40-27)26(37)33-9-11-41(38,39)12-10-33/h13-14,16-17H,4-12H2,1-3H3,(H,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179940

(2-(5-acetyl-2-bromo-5,6,11,12-tetrahydro-dibenzo[b...)Show SMILES COC(=O)c1ccccc1-c1ccc2CCc3cc(Br)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22BrNO3/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)25(29)30-2)9-7-17(20)8-10-19-14-21(26)11-12-24(19)27/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301069

(2-(4-(2-(2-methoxypyridin-3-yl)-4-methyl-1H-benzo[...)Show SMILES CNCCN1CCC(CC1)c1cc(C)c2nc([nH]c2c1)-c1cccnc1OC Show InChI InChI=1S/C22H29N5O/c1-15-13-17(16-6-10-27(11-7-16)12-9-23-2)14-19-20(15)26-21(25-19)18-5-4-8-24-22(18)28-3/h4-5,8,13-14,16,23H,6-7,9-12H2,1-3H3,(H,25,26) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179964
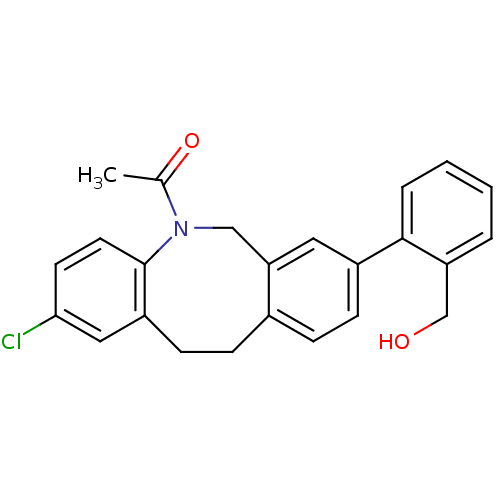
(1-[2-chloro-8-(2-hydroxymethyl-phenyl)-11,12-dihyd...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1CO Show InChI InChI=1S/C24H22ClNO2/c1-16(28)26-14-21-12-18(23-5-3-2-4-20(23)15-27)8-6-17(21)7-9-19-13-22(25)10-11-24(19)26/h2-6,8,10-13,27H,7,9,14-15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337333
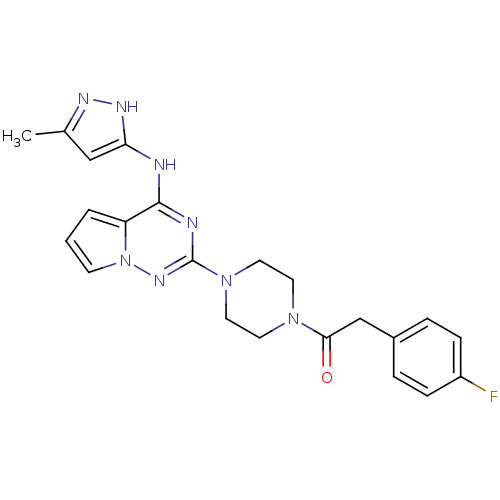
(2-(4-fluorophenyl)-1-(4-(4-(5-methyl-1H-pyrazol-3-...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2ccc(F)cc2)[nH]n1 Show InChI InChI=1S/C22H23FN8O/c1-15-13-19(27-26-15)24-21-18-3-2-8-31(18)28-22(25-21)30-11-9-29(10-12-30)20(32)14-16-4-6-17(23)7-5-16/h2-8,13H,9-12,14H2,1H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122326
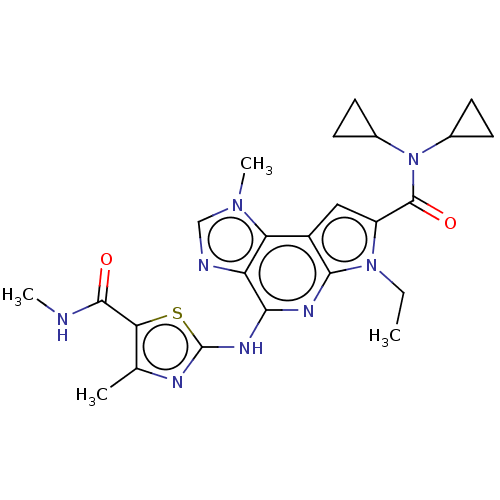
(CHEMBL3622143)Show SMILES CCn1c(cc2c1nc(Nc1nc(C)c(s1)C(=O)NC)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C24H28N8O2S/c1-5-31-16(23(34)32(13-6-7-13)14-8-9-14)10-15-18-17(26-11-30(18)4)20(28-21(15)31)29-24-27-12(2)19(35-24)22(33)25-3/h10-11,13-14H,5-9H2,1-4H3,(H,25,33)(H,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179940

(2-(5-acetyl-2-bromo-5,6,11,12-tetrahydro-dibenzo[b...)Show SMILES COC(=O)c1ccccc1-c1ccc2CCc3cc(Br)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22BrNO3/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)25(29)30-2)9-7-17(20)8-10-19-14-21(26)11-12-24(19)27/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337334
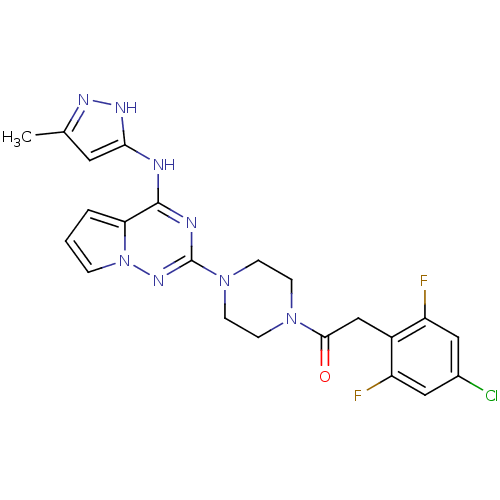
(2-(4-chloro-2,6-difluorophenyl)-1-(4-(4-(5-methyl-...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2c(F)cc(Cl)cc2F)[nH]n1 Show InChI InChI=1S/C22H21ClF2N8O/c1-13-9-19(29-28-13)26-21-18-3-2-4-33(18)30-22(27-21)32-7-5-31(6-8-32)20(34)12-15-16(24)10-14(23)11-17(15)25/h2-4,9-11H,5-8,12H2,1H3,(H2,26,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50229085
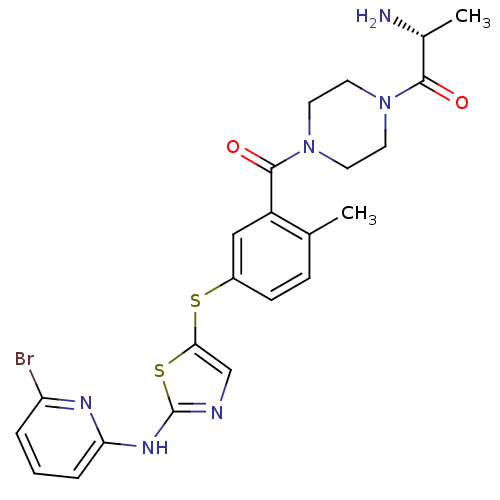
((R)-2-amino-1-(4-(5-(2-(6-bromopyridin-2-ylamino)t...)Show SMILES C[C@@H](N)C(=O)N1CCN(CC1)C(=O)c1cc(Sc2cnc(Nc3cccc(Br)n3)s2)ccc1C Show InChI InChI=1S/C23H25BrN6O2S2/c1-14-6-7-16(33-20-13-26-23(34-20)28-19-5-3-4-18(24)27-19)12-17(14)22(32)30-10-8-29(9-11-30)21(31)15(2)25/h3-7,12-13,15H,8-11,25H2,1-2H3,(H,26,27,28)/t15-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of TrkA |
Bioorg Med Chem Lett 18: 634-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.076
BindingDB Entry DOI: 10.7270/Q2JD4WJ5 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50229082
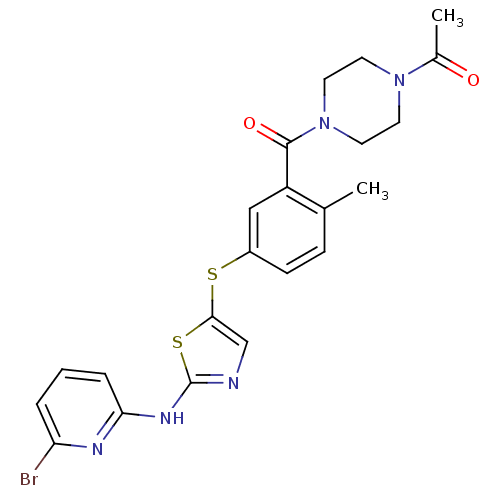
(1-(4-(5-(2-(6-bromopyridin-2-ylamino)thiazol-5-ylt...)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cc(Sc2cnc(Nc3cccc(Br)n3)s2)ccc1C Show InChI InChI=1S/C22H22BrN5O2S2/c1-14-6-7-16(12-17(14)21(30)28-10-8-27(9-11-28)15(2)29)31-20-13-24-22(32-20)26-19-5-3-4-18(23)25-19/h3-7,12-13H,8-11H2,1-2H3,(H,24,25,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of TrkA |
Bioorg Med Chem Lett 18: 634-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.076
BindingDB Entry DOI: 10.7270/Q2JD4WJ5 |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50229087
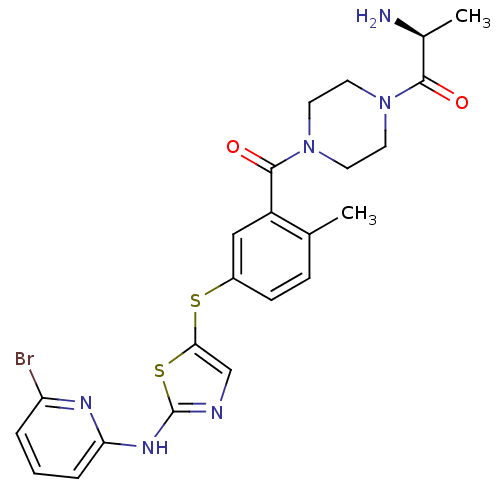
((S)-2-amino-1-(4-(5-(2-(6-bromopyridin-2-ylamino)t...)Show SMILES C[C@H](N)C(=O)N1CCN(CC1)C(=O)c1cc(Sc2cnc(Nc3cccc(Br)n3)s2)ccc1C Show InChI InChI=1S/C23H25BrN6O2S2/c1-14-6-7-16(33-20-13-26-23(34-20)28-19-5-3-4-18(24)27-19)12-17(14)22(32)30-10-8-29(9-11-30)21(31)15(2)25/h3-7,12-13,15H,8-11,25H2,1-2H3,(H,26,27,28)/t15-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of TrkA |
Bioorg Med Chem Lett 18: 634-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.076
BindingDB Entry DOI: 10.7270/Q2JD4WJ5 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337336
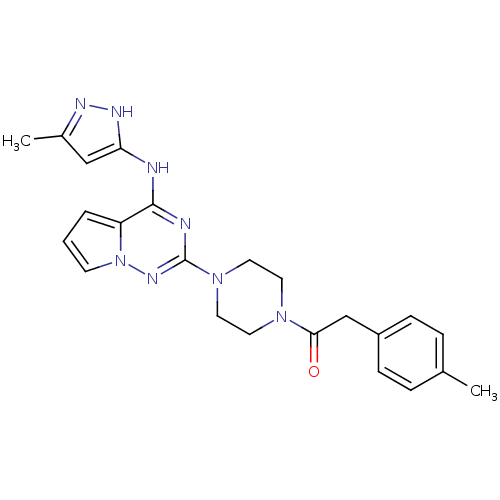
(1-(4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2ccc(C)cc2)[nH]n1 Show InChI InChI=1S/C23H26N8O/c1-16-5-7-18(8-6-16)15-21(32)29-10-12-30(13-11-29)23-25-22(19-4-3-9-31(19)28-23)24-20-14-17(2)26-27-20/h3-9,14H,10-13,15H2,1-2H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337335

((4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2-f...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)c2ccccc2S(C)(=O)=O)[nH]n1 Show InChI InChI=1S/C22H24N8O3S/c1-15-14-19(26-25-15)23-20-17-7-5-9-30(17)27-22(24-20)29-12-10-28(11-13-29)21(31)16-6-3-4-8-18(16)34(2,32)33/h3-9,14H,10-13H2,1-2H3,(H2,23,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122318
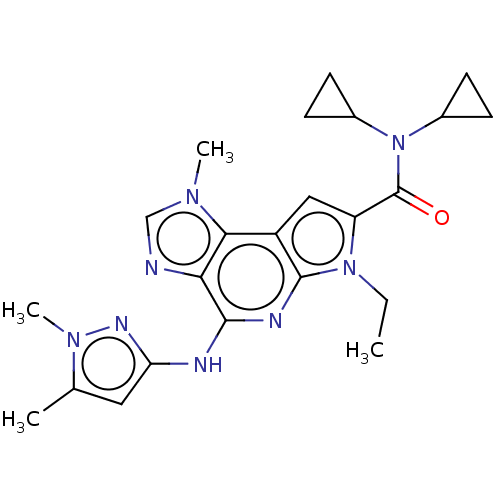
(BMS-911543)Show SMILES CCn1c(cc2c1nc(Nc1cc(C)n(C)n1)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C23H28N8O/c1-5-30-17(23(32)31(14-6-7-14)15-8-9-15)11-16-20-19(24-12-28(20)3)21(26-22(16)30)25-18-10-13(2)29(4)27-18/h10-12,14-15H,5-9H2,1-4H3,(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122319
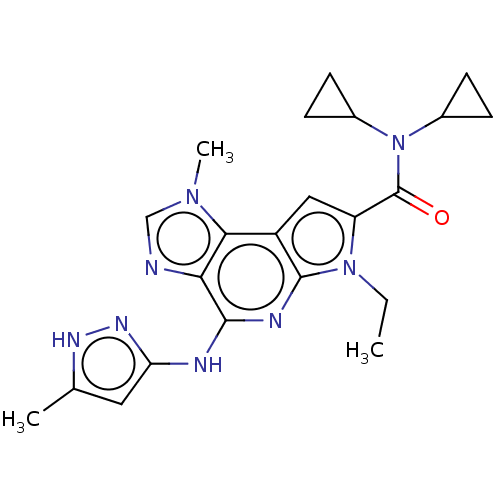
(CHEMBL3622150)Show SMILES CCn1c(cc2c1nc(Nc1cc(C)[nH]n1)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C22H26N8O/c1-4-29-16(22(31)30(13-5-6-13)14-7-8-14)10-15-19-18(23-11-28(19)3)20(25-21(15)29)24-17-9-12(2)26-27-17/h9-11,13-14H,4-8H2,1-3H3,(H2,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM130257
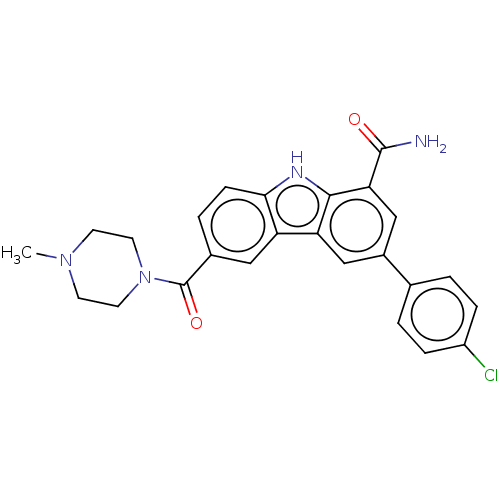
(US8815840, 17)Show SMILES CN1CCN(CC1)C(=O)c1ccc2[nH]c3c(cc(cc3c2c1)-c1ccc(Cl)cc1)C(N)=O Show InChI InChI=1S/C25H23ClN4O2/c1-29-8-10-30(11-9-29)25(32)16-4-7-22-19(12-16)20-13-17(15-2-5-18(26)6-3-15)14-21(24(27)31)23(20)28-22/h2-7,12-14,28H,8-11H2,1H3,(H2,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 25: 2809-12 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.101
BindingDB Entry DOI: 10.7270/Q2BC419G |
More data for this
Ligand-Target Pair | |
Histone-arginine methyltransferase CARM1
(Homo sapiens (Human)) | BDBM50301073

(2-(4-(2-(2-methoxypyridin-3-yl)-1H-benzo[d]imidazo...)Show SMILES CNCCN1CCC(CC1)c1ccc2nc([nH]c2c1)-c1cccnc1OC Show InChI InChI=1S/C21H27N5O/c1-22-10-13-26-11-7-15(8-12-26)16-5-6-18-19(14-16)25-20(24-18)17-4-3-9-23-21(17)27-2/h3-6,9,14-15,22H,7-8,10-13H2,1-2H3,(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CARM1 assessed as inhibition of histone3 methylation |
Bioorg Med Chem Lett 19: 5063-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.040
BindingDB Entry DOI: 10.7270/Q2KW5G3W |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337337
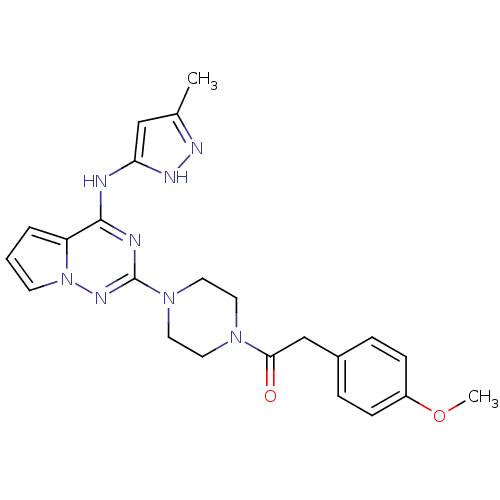
(2-(4-methoxyphenyl)-1-(4-(4-(5-methyl-1H-pyrazol-3...)Show SMILES COc1ccc(CC(=O)N2CCN(CC2)c2nc(Nc3cc(C)n[nH]3)c3cccn3n2)cc1 Show InChI InChI=1S/C23H26N8O2/c1-16-14-20(27-26-16)24-22-19-4-3-9-31(19)28-23(25-22)30-12-10-29(11-13-30)21(32)15-17-5-7-18(33-2)8-6-17/h3-9,14H,10-13,15H2,1-2H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122327
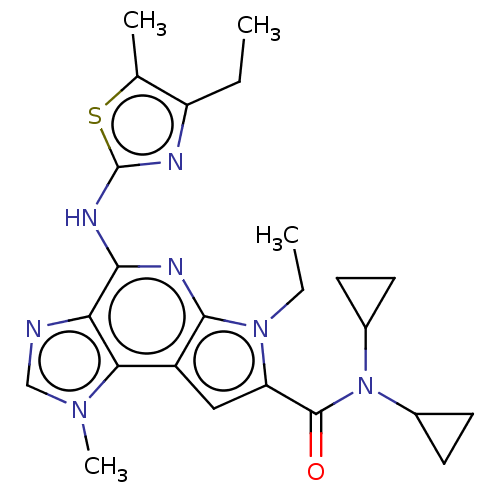
(CHEMBL3622142)Show SMILES CCc1nc(Nc2nc3n(CC)c(cc3c3n(C)cnc23)C(=O)N(C2CC2)C2CC2)sc1C Show InChI InChI=1S/C24H29N7OS/c1-5-17-13(3)33-24(26-17)28-21-19-20(29(4)12-25-19)16-11-18(30(6-2)22(16)27-21)23(32)31(14-7-8-14)15-9-10-15/h11-12,14-15H,5-10H2,1-4H3,(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122324

(CHEMBL3622145)Show SMILES CCn1c(cc2c1nc(Nc1nc(C)c(s1)S(C)(=O)=O)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-5-29-16(21(31)30(13-6-7-13)14-8-9-14)10-15-18-17(24-11-28(18)3)19(26-20(15)29)27-23-25-12(2)22(34-23)35(4,32)33/h10-11,13-14H,5-9H2,1-4H3,(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50121379
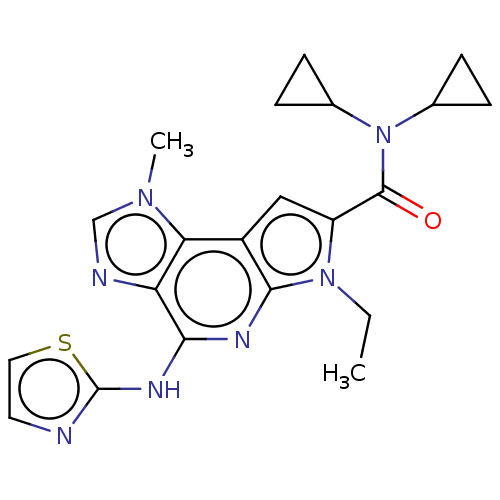
(CHEMBL3622134)Show SMILES CCn1c(cc2c1nc(Nc1nccs1)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C21H23N7OS/c1-3-27-15(20(29)28(12-4-5-12)13-6-7-13)10-14-17-16(23-11-26(17)2)18(24-19(14)27)25-21-22-8-9-30-21/h8-13H,3-7H2,1-2H3,(H,22,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using 5-FAMKKKKEEIYFFFG-OH as substrate after 60 mins by HTRF assay |
ACS Med Chem Lett 6: 845-9 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00225
BindingDB Entry DOI: 10.7270/Q2Q2421M |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179967
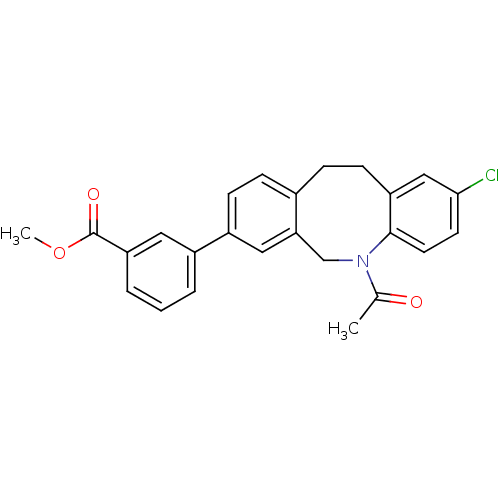
(3-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...)Show SMILES COC(=O)c1cccc(c1)-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22ClNO3/c1-16(28)27-15-22-13-19(18-4-3-5-21(12-18)25(29)30-2)8-6-17(22)7-9-20-14-23(26)10-11-24(20)27/h3-6,8,10-14H,7,9,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50122321
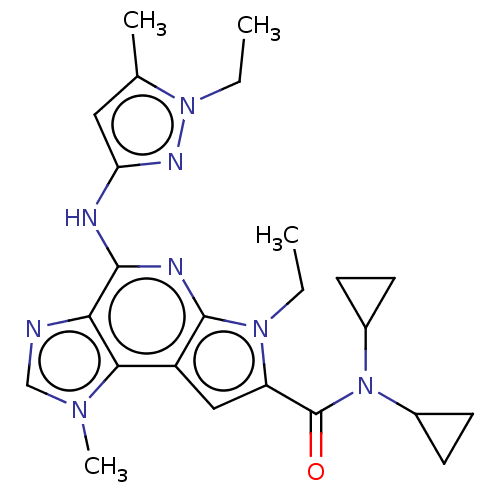
(CHEMBL3622148)Show SMILES CCn1nc(Nc2nc3n(CC)c(cc3c3n(C)cnc23)C(=O)N(C2CC2)C2CC2)cc1C Show InChI InChI=1S/C24H30N8O/c1-5-30-18(24(33)32(15-7-8-15)16-9-10-16)12-17-21-20(25-13-29(21)4)22(27-23(17)30)26-19-11-14(3)31(6-2)28-19/h11-13,15-16H,5-10H2,1-4H3,(H,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant JAK2 (unknown origin) using 5-FAM-KKKKEEIYFFFG-OH substrate and ATP incubated for 180 mins by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337338
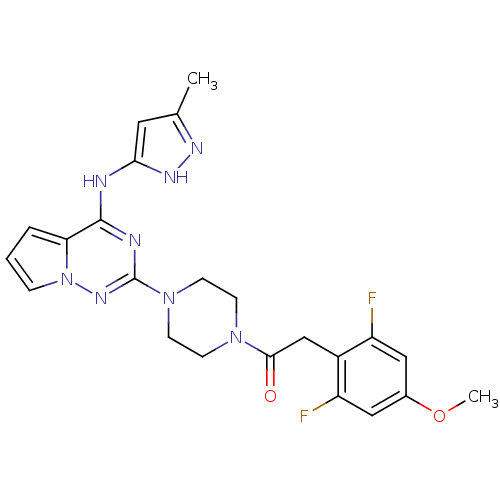
(2-(2,6-difluoro-4-methoxyphenyl)-1-(4-(4-(5-methyl...)Show SMILES COc1cc(F)c(CC(=O)N2CCN(CC2)c2nc(Nc3cc(C)n[nH]3)c3cccn3n2)c(F)c1 Show InChI InChI=1S/C23H24F2N8O2/c1-14-10-20(29-28-14)26-22-19-4-3-5-33(19)30-23(27-22)32-8-6-31(7-9-32)21(34)13-16-17(24)11-15(35-2)12-18(16)25/h3-5,10-12H,6-9,13H2,1-2H3,(H2,26,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50121378
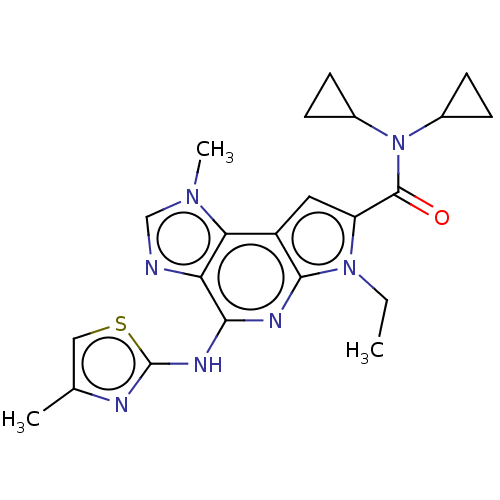
(CHEMBL3622135)Show SMILES CCn1c(cc2c1nc(Nc1nc(C)cs1)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C22H25N7OS/c1-4-28-16(21(30)29(13-5-6-13)14-7-8-14)9-15-18-17(23-11-27(18)3)19(25-20(15)28)26-22-24-12(2)10-31-22/h9-11,13-14H,4-8H2,1-3H3,(H,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using 5-FAMKKKKEEIYFFFG-OH as substrate after 60 mins by HTRF assay |
ACS Med Chem Lett 6: 845-9 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00225
BindingDB Entry DOI: 10.7270/Q2Q2421M |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50122319

(CHEMBL3622150)Show SMILES CCn1c(cc2c1nc(Nc1cc(C)[nH]n1)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C22H26N8O/c1-4-29-16(22(31)30(13-5-6-13)14-7-8-14)10-15-19-18(23-11-28(19)3)20(25-21(15)29)24-17-9-12(2)26-27-17/h9-11,13-14H,4-8H2,1-3H3,(H2,24,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of recombinant TYK2 (unknown origin) by scintillation counting method |
ACS Med Chem Lett 6: 850-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00226
BindingDB Entry DOI: 10.7270/Q2CJ8G8Z |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179936
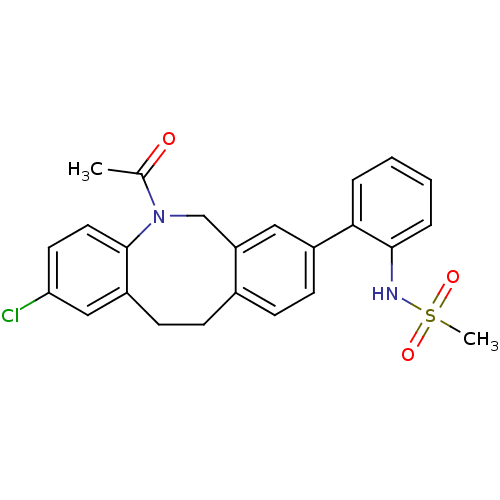
(CHEMBL382563 | N-[2-(5-acetyl-2-chloro-5,6,11,12-t...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1NS(C)(=O)=O Show InChI InChI=1S/C24H23ClN2O3S/c1-16(28)27-15-20-13-18(22-5-3-4-6-23(22)26-31(2,29)30)9-7-17(20)8-10-19-14-21(25)11-12-24(19)27/h3-7,9,11-14,26H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337339
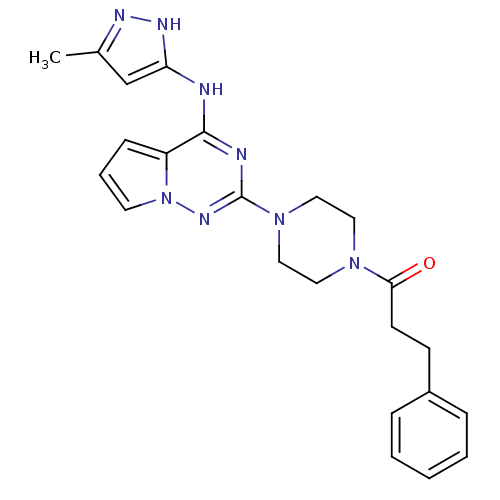
(1-(4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)CCc2ccccc2)[nH]n1 Show InChI InChI=1S/C23H26N8O/c1-17-16-20(27-26-17)24-22-19-8-5-11-31(19)28-23(25-22)30-14-12-29(13-15-30)21(32)10-9-18-6-3-2-4-7-18/h2-8,11,16H,9-10,12-15H2,1H3,(H2,24,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179964

(1-[2-chloro-8-(2-hydroxymethyl-phenyl)-11,12-dihyd...)Show SMILES CC(=O)N1Cc2cc(ccc2CCc2cc(Cl)ccc12)-c1ccccc1CO Show InChI InChI=1S/C24H22ClNO2/c1-16(28)26-14-21-12-18(23-5-3-2-4-20(23)15-27)8-6-17(21)7-9-19-13-22(25)10-11-24(19)26/h2-6,8,10-13,27H,7,9,14-15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179967

(3-(5-acetyl-2-chloro-5,6,11,12-tetrahydro-dibenzo[...)Show SMILES COC(=O)c1cccc(c1)-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C25H22ClNO3/c1-16(28)27-15-22-13-19(18-4-3-5-21(12-18)25(29)30-2)8-6-17(22)7-9-20-14-23(26)10-11-24(20)27/h3-6,8,10-14H,7,9,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 by SEAP assay |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50229086
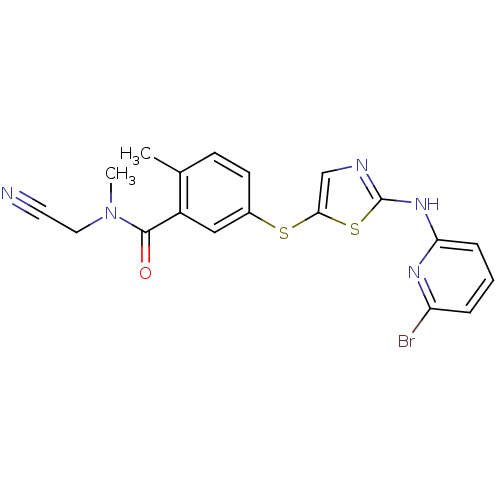
(5-(2-(6-bromopyridin-2-ylamino)thiazol-5-ylthio)-N...)Show SMILES CN(CC#N)C(=O)c1cc(Sc2cnc(Nc3cccc(Br)n3)s2)ccc1C Show InChI InChI=1S/C19H16BrN5OS2/c1-12-6-7-13(10-14(12)18(26)25(2)9-8-21)27-17-11-22-19(28-17)24-16-5-3-4-15(20)23-16/h3-7,10-11H,9H2,1-2H3,(H,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of TrkA |
Bioorg Med Chem Lett 18: 634-9 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.076
BindingDB Entry DOI: 10.7270/Q2JD4WJ5 |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50179932

(1-[2-chloro-8-(2-methoxy-phenyl)-11,12-dihydro-6H-...)Show SMILES COc1ccccc1-c1ccc2CCc3cc(Cl)ccc3N(Cc2c1)C(C)=O Show InChI InChI=1S/C24H22ClNO2/c1-16(27)26-15-20-13-18(22-5-3-4-6-24(22)28-2)9-7-17(20)8-10-19-14-21(25)11-12-23(19)26/h3-7,9,11-14H,8,10,15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of 17beta-HSD3 |
Bioorg Med Chem Lett 16: 1532-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.039
BindingDB Entry DOI: 10.7270/Q2CV4HBV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337340

((2-bromophenyl)(4-(4-(5-methyl-1H-pyrazol-3-ylamin...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)c2ccccc2Br)[nH]n1 Show InChI InChI=1S/C21H21BrN8O/c1-14-13-18(26-25-14)23-19-17-7-4-8-30(17)27-21(24-19)29-11-9-28(10-12-29)20(31)15-5-2-3-6-16(15)22/h2-8,13H,9-12H2,1H3,(H2,23,24,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50094616
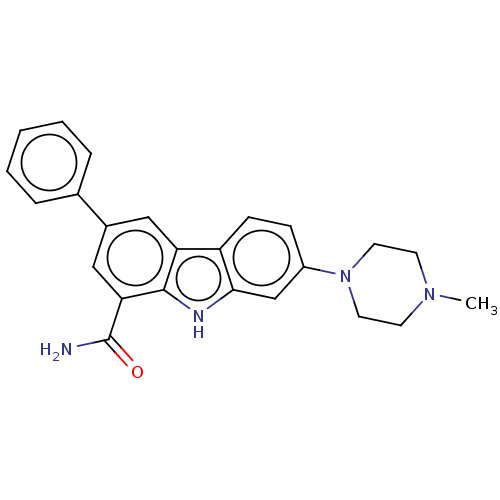
(CHEMBL3589325)Show SMILES CN1CCN(CC1)c1ccc2c(c1)[nH]c1c(cc(cc21)-c1ccccc1)C(N)=O Show InChI InChI=1S/C24H24N4O/c1-27-9-11-28(12-10-27)18-7-8-19-20-13-17(16-5-3-2-4-6-16)14-21(24(25)29)23(20)26-22(19)15-18/h2-8,13-15,26H,9-12H2,1H3,(H2,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) |
Bioorg Med Chem Lett 25: 2809-12 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.101
BindingDB Entry DOI: 10.7270/Q2BC419G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50121377
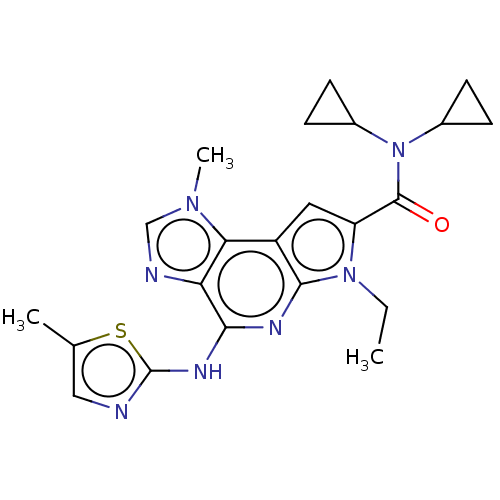
(CHEMBL3622136)Show SMILES CCn1c(cc2c1nc(Nc1ncc(C)s1)c1ncn(C)c21)C(=O)N(C1CC1)C1CC1 Show InChI InChI=1S/C22H25N7OS/c1-4-28-16(21(30)29(13-5-6-13)14-7-8-14)9-15-18-17(24-11-27(18)3)19(25-20(15)28)26-22-23-10-12(2)31-22/h9-11,13-14H,4-8H2,1-3H3,(H,23,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 (unknown origin) using 5-FAMKKKKEEIYFFFG-OH as substrate after 60 mins by HTRF assay |
ACS Med Chem Lett 6: 845-9 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00225
BindingDB Entry DOI: 10.7270/Q2Q2421M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337341
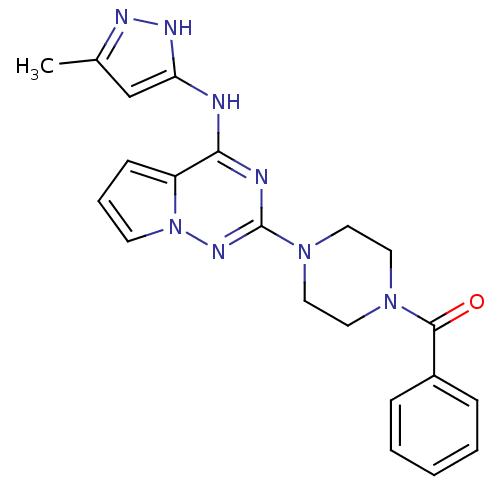
((4-(4-(5-methyl-1H-pyrazol-3-ylamino)pyrrolo[1,2-f...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)c2ccccc2)[nH]n1 Show InChI InChI=1S/C21H22N8O/c1-15-14-18(25-24-15)22-19-17-8-5-9-29(17)26-21(23-19)28-12-10-27(11-13-28)20(30)16-6-3-2-4-7-16/h2-9,14H,10-13H2,1H3,(H2,22,23,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50337342

(2-(2,6-difluorophenyl)-1-(4-(4-(5-methyl-1H-pyrazo...)Show SMILES Cc1cc(Nc2nc(nn3cccc23)N2CCN(CC2)C(=O)Cc2ccc(F)cc2F)[nH]n1 Show InChI InChI=1S/C22H22F2N8O/c1-14-11-19(28-27-14)25-21-18-3-2-6-32(18)29-22(26-21)31-9-7-30(8-10-31)20(33)12-15-4-5-16(23)13-17(15)24/h2-6,11,13H,7-10,12H2,1H3,(H2,25,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co.
Curated by ChEMBL
| Assay Description
Inhibition of JAK2 after 60 mins |
Bioorg Med Chem Lett 21: 1425-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.022
BindingDB Entry DOI: 10.7270/Q2MG7PRT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data