 Found 588 hits with Last Name = 'schofield' and Initial = 'p'
Found 588 hits with Last Name = 'schofield' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50366574
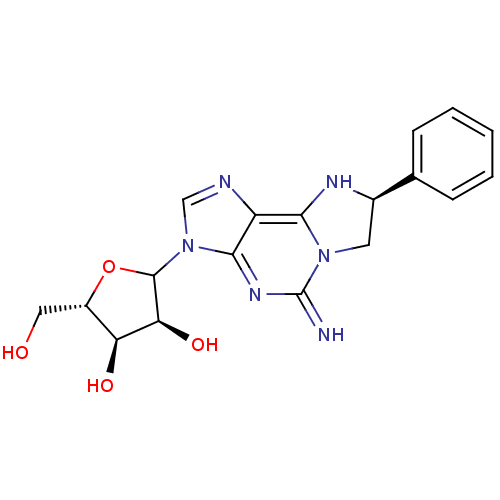
(CHEMBL609646)Show SMILES OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2c3N[C@H](Cn3c(=N)nc12)c1ccccc1 |r| Show InChI InChI=1S/C18H20N6O4/c19-18-22-16-12(15-21-10(6-23(15)18)9-4-2-1-3-5-9)20-8-24(16)17-14(27)13(26)11(7-25)28-17/h1-5,8,10-11,13-14,17,19,21,25-27H,6-7H2/t10-,11+,13+,14+,17?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Binding affinity against adenosine A1 receptor in rat brain using [3H]R-PIA |
Bioorg Med Chem Lett 8: 691-4 (1999)
BindingDB Entry DOI: 10.7270/Q21Z44Z9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50366575
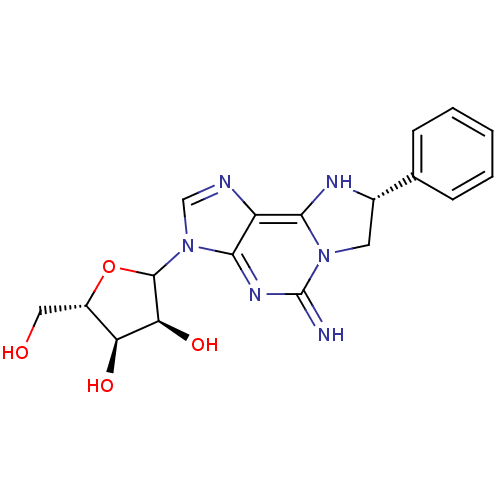
(CHEMBL609645)Show SMILES OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2c3N[C@@H](Cn3c(=N)nc12)c1ccccc1 |r| Show InChI InChI=1S/C18H20N6O4/c19-18-22-16-12(15-21-10(6-23(15)18)9-4-2-1-3-5-9)20-8-24(16)17-14(27)13(26)11(7-25)28-17/h1-5,8,10-11,13-14,17,19,21,25-27H,6-7H2/t10-,11-,13-,14-,17?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Binding affinity against adenosine A1 receptor in rat brain using [3H]R-PIA |
Bioorg Med Chem Lett 8: 691-4 (1999)
BindingDB Entry DOI: 10.7270/Q21Z44Z9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50366573
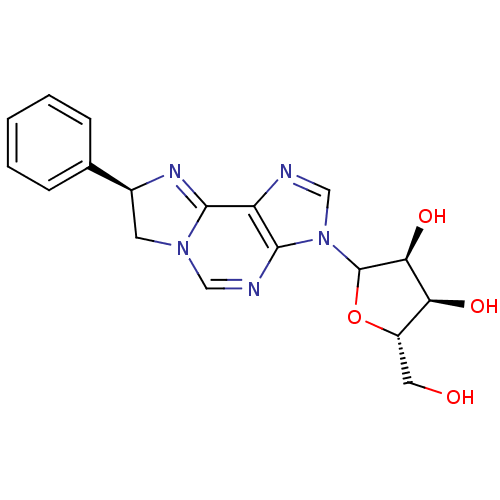
(CHEMBL607912)Show SMILES OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2C3=N[C@@H](CN3C=Nc12)c1ccccc1 |r,c:20,t:14| Show InChI InChI=1S/C18H19N5O4/c24-7-12-14(25)15(26)18(27-12)23-9-19-13-16(23)20-8-22-6-11(21-17(13)22)10-4-2-1-3-5-10/h1-5,8-9,11-12,14-15,18,24-26H,6-7H2/t11-,12-,14-,15-,18?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Binding affinity against adenosine A1 receptor in rat brain using [3H]R-PIA |
Bioorg Med Chem Lett 8: 691-4 (1999)
BindingDB Entry DOI: 10.7270/Q21Z44Z9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50366572
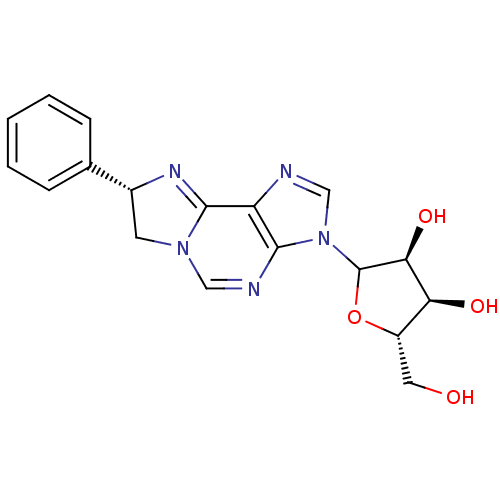
(CHEMBL607911)Show SMILES OC[C@@H]1OC([C@@H](O)[C@H]1O)n1cnc2C3=N[C@H](CN3C=Nc12)c1ccccc1 |r,c:20,t:14| Show InChI InChI=1S/C18H19N5O4/c24-7-12-14(25)15(26)18(27-12)23-9-19-13-16(23)20-8-22-6-11(21-17(13)22)10-4-2-1-3-5-10/h1-5,8-9,11-12,14-15,18,24-26H,6-7H2/t11-,12+,14+,15+,18?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Griffith University
Curated by ChEMBL
| Assay Description
Binding affinity against adenosine A1 receptor in rat brain using [3H]R-PIA |
Bioorg Med Chem Lett 8: 691-4 (1999)
BindingDB Entry DOI: 10.7270/Q21Z44Z9 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Rattus norvegicus (rat)) | BDBM50385398
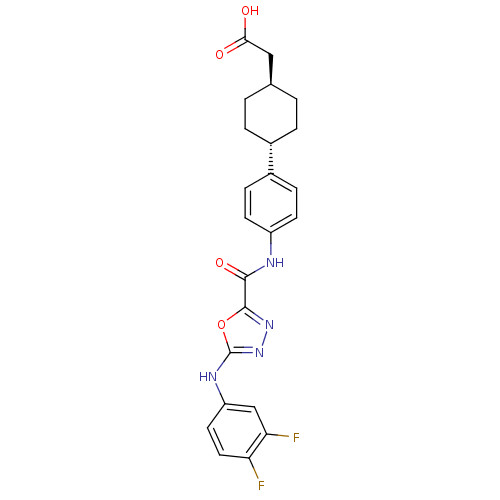
(CHEMBL2036730)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccc(F)c(F)c3)o2)cc1 |r,wU:4.3,wD:7.10,(19.62,-39.47,;18.85,-40.8,;17.31,-40.79,;19.62,-42.14,;21.16,-42.14,;21.93,-40.81,;23.47,-40.82,;24.23,-42.16,;23.46,-43.49,;21.93,-43.48,;25.77,-42.17,;26.55,-40.84,;28.08,-40.85,;28.84,-42.19,;30.38,-42.2,;31.16,-40.87,;30.4,-39.53,;32.71,-40.88,;33.18,-39.41,;34.72,-39.41,;35.19,-40.87,;36.52,-41.64,;37.85,-40.86,;37.84,-39.33,;39.16,-38.55,;40.51,-39.31,;41.84,-38.53,;40.52,-40.85,;41.85,-41.61,;39.19,-41.63,;33.95,-41.78,;28.07,-43.51,;26.54,-43.51,)| Show InChI InChI=1S/C23H22F2N4O4/c24-18-10-9-17(12-19(18)25)27-23-29-28-22(33-23)21(32)26-16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-20(30)31/h5-10,12-14H,1-4,11H2,(H,26,32)(H,27,29)(H,30,31)/t13-,14- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of rat DGAT1 |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385398

(CHEMBL2036730)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccc(F)c(F)c3)o2)cc1 |r,wU:4.3,wD:7.10,(19.62,-39.47,;18.85,-40.8,;17.31,-40.79,;19.62,-42.14,;21.16,-42.14,;21.93,-40.81,;23.47,-40.82,;24.23,-42.16,;23.46,-43.49,;21.93,-43.48,;25.77,-42.17,;26.55,-40.84,;28.08,-40.85,;28.84,-42.19,;30.38,-42.2,;31.16,-40.87,;30.4,-39.53,;32.71,-40.88,;33.18,-39.41,;34.72,-39.41,;35.19,-40.87,;36.52,-41.64,;37.85,-40.86,;37.84,-39.33,;39.16,-38.55,;40.51,-39.31,;41.84,-38.53,;40.52,-40.85,;41.85,-41.61,;39.19,-41.63,;33.95,-41.78,;28.07,-43.51,;26.54,-43.51,)| Show InChI InChI=1S/C23H22F2N4O4/c24-18-10-9-17(12-19(18)25)27-23-29-28-22(33-23)21(32)26-16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-20(30)31/h5-10,12-14H,1-4,11H2,(H,26,32)(H,27,29)(H,30,31)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1-mediated triacylglycerol synthesis in human HuTu80 cells |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385398

(CHEMBL2036730)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccc(F)c(F)c3)o2)cc1 |r,wU:4.3,wD:7.10,(19.62,-39.47,;18.85,-40.8,;17.31,-40.79,;19.62,-42.14,;21.16,-42.14,;21.93,-40.81,;23.47,-40.82,;24.23,-42.16,;23.46,-43.49,;21.93,-43.48,;25.77,-42.17,;26.55,-40.84,;28.08,-40.85,;28.84,-42.19,;30.38,-42.2,;31.16,-40.87,;30.4,-39.53,;32.71,-40.88,;33.18,-39.41,;34.72,-39.41,;35.19,-40.87,;36.52,-41.64,;37.85,-40.86,;37.84,-39.33,;39.16,-38.55,;40.51,-39.31,;41.84,-38.53,;40.52,-40.85,;41.85,-41.61,;39.19,-41.63,;33.95,-41.78,;28.07,-43.51,;26.54,-43.51,)| Show InChI InChI=1S/C23H22F2N4O4/c24-18-10-9-17(12-19(18)25)27-23-29-28-22(33-23)21(32)26-16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-20(30)31/h5-10,12-14H,1-4,11H2,(H,26,32)(H,27,29)(H,30,31)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DGAT1 expressed in baculovirus infected insect sf9 cells using [14C] oleoyl coenzyme A after 30 mins by scintillation... |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116590
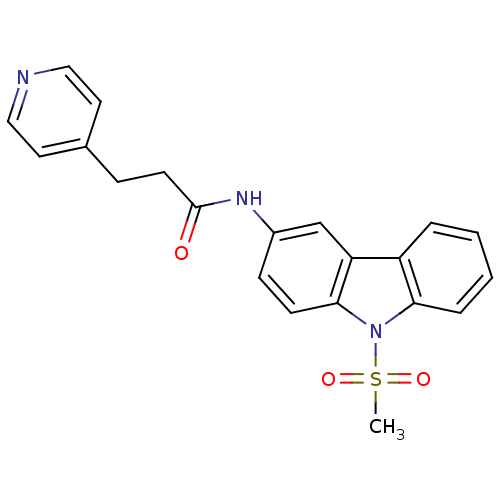
(CHEMBL325486 | N-(9-Methanesulfonyl-9H-carbazol-3-...)Show SMILES CS(=O)(=O)n1c2ccccc2c2cc(NC(=O)CCc3ccncc3)ccc12 Show InChI InChI=1S/C21H19N3O3S/c1-28(26,27)24-19-5-3-2-4-17(19)18-14-16(7-8-20(18)24)23-21(25)9-6-15-10-12-22-13-11-15/h2-5,7-8,10-14H,6,9H2,1H3,(H,23,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116610
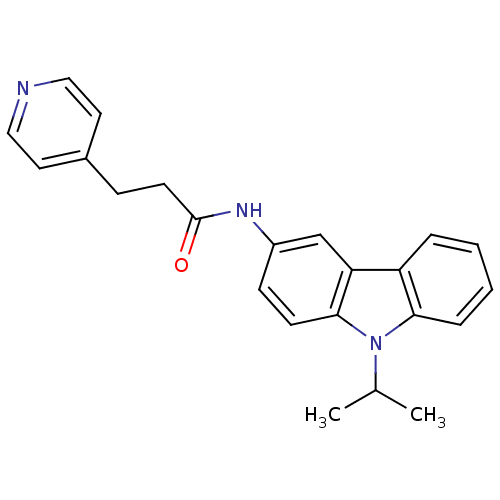
(CHEMBL119743 | N-(9-Isopropyl-9H-carbazol-3-yl)-3-...)Show InChI InChI=1S/C23H23N3O/c1-16(2)26-21-6-4-3-5-19(21)20-15-18(8-9-22(20)26)25-23(27)10-7-17-11-13-24-14-12-17/h3-6,8-9,11-16H,7,10H2,1-2H3,(H,25,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433856
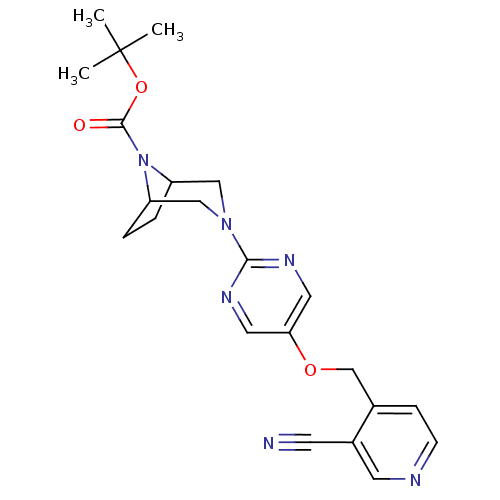
(CHEMBL2382410)Show SMILES CC(C)(C)OC(=O)N1C2CCC1CN(C2)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C22H26N6O3/c1-22(2,3)31-21(29)28-17-4-5-18(28)13-27(12-17)20-25-10-19(11-26-20)30-14-15-6-7-24-9-16(15)8-23/h6-7,9-11,17-18H,4-5,12-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50420842

(CHEMBL2086684)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccncc2C#N)cn1)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C21H26N6O3/c1-15-13-26(20(28)30-21(2,3)4)7-8-27(15)19-24-11-18(12-25-19)29-14-16-5-6-23-10-17(16)9-22/h5-6,10-12,15H,7-8,13-14H2,1-4H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Mus musculus (mouse)) | BDBM50385398

(CHEMBL2036730)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccc(F)c(F)c3)o2)cc1 |r,wU:4.3,wD:7.10,(19.62,-39.47,;18.85,-40.8,;17.31,-40.79,;19.62,-42.14,;21.16,-42.14,;21.93,-40.81,;23.47,-40.82,;24.23,-42.16,;23.46,-43.49,;21.93,-43.48,;25.77,-42.17,;26.55,-40.84,;28.08,-40.85,;28.84,-42.19,;30.38,-42.2,;31.16,-40.87,;30.4,-39.53,;32.71,-40.88,;33.18,-39.41,;34.72,-39.41,;35.19,-40.87,;36.52,-41.64,;37.85,-40.86,;37.84,-39.33,;39.16,-38.55,;40.51,-39.31,;41.84,-38.53,;40.52,-40.85,;41.85,-41.61,;39.19,-41.63,;33.95,-41.78,;28.07,-43.51,;26.54,-43.51,)| Show InChI InChI=1S/C23H22F2N4O4/c24-18-10-9-17(12-19(18)25)27-23-29-28-22(33-23)21(32)26-16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-20(30)31/h5-10,12-14H,1-4,11H2,(H,26,32)(H,27,29)(H,30,31)/t13-,14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mouse DGAT1 |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50392216
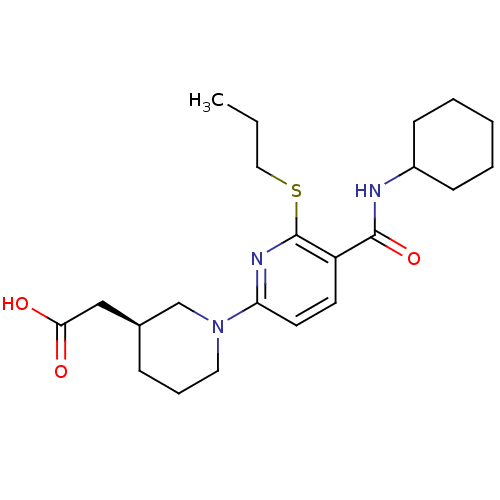
(CHEMBL2153191 | US8673938, 7)Show SMILES CCCSc1nc(ccc1C(=O)NC1CCCCC1)N1CCC[C@@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C22H33N3O3S/c1-2-13-29-22-18(21(28)23-17-8-4-3-5-9-17)10-11-19(24-22)25-12-6-7-16(15-25)14-20(26)27/h10-11,16-17H,2-9,12-15H2,1H3,(H,23,28)(H,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human isolated adipocytes using [3H]cortisone as substrate after 6 hrs by flow scintillation analysis |
J Med Chem 55: 5951-64 (2012)
Article DOI: 10.1021/jm300592r
BindingDB Entry DOI: 10.7270/Q24F1RTK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM24393
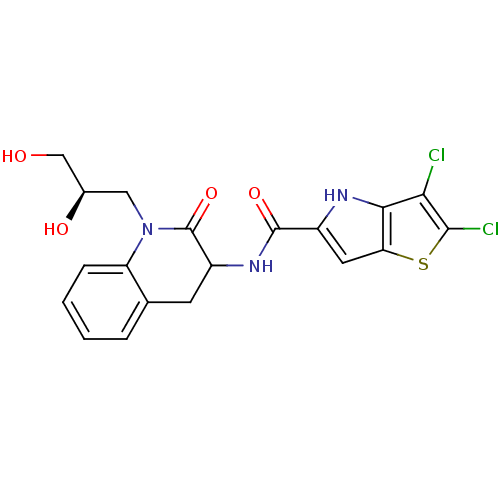
(2,3-dichloro-N-{1-[(2R)-2,3-dihydroxypropyl]-2-oxo...)Show SMILES OC[C@H](O)CN1C(=O)C(Cc2ccccc12)NC(=O)c1cc2sc(Cl)c(Cl)c2[nH]1 |r| Show InChI InChI=1S/C19H17Cl2N3O4S/c20-15-16-14(29-17(15)21)6-11(22-16)18(27)23-12-5-9-3-1-2-4-13(9)24(19(12)28)7-10(26)8-25/h1-4,6,10,12,22,25-26H,5,7-8H2,(H,23,27)/t10-,12?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
| Assay Description
The inhibitory activity of the test compounds against human recombinant glycogen phosphorylase a (GPa) was monitored using 96-well microplate format.... |
Bioorg Med Chem Lett 17: 394-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.037
BindingDB Entry DOI: 10.7270/Q2TM78DM |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116619
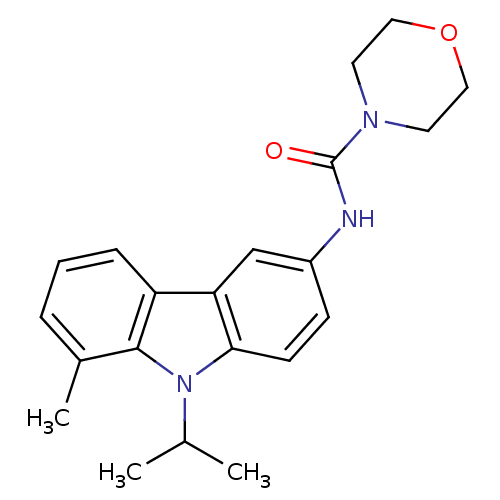
(CHEMBL117563 | Morpholine-4-carboxylic acid (9-iso...)Show SMILES CC(C)n1c2ccc(NC(=O)N3CCOCC3)cc2c2cccc(C)c12 Show InChI InChI=1S/C21H25N3O2/c1-14(2)24-19-8-7-16(22-21(25)23-9-11-26-12-10-23)13-18(19)17-6-4-5-15(3)20(17)24/h4-8,13-14H,9-12H2,1-3H3,(H,22,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116602
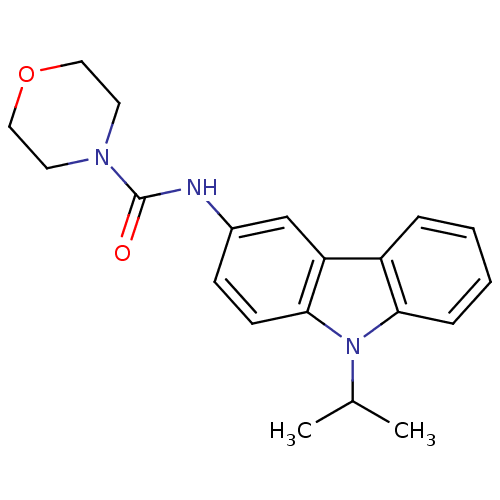
(CHEMBL419951 | Morpholine-4-carboxylic acid (9-iso...)Show InChI InChI=1S/C20H23N3O2/c1-14(2)23-18-6-4-3-5-16(18)17-13-15(7-8-19(17)23)21-20(24)22-9-11-25-12-10-22/h3-8,13-14H,9-12H2,1-2H3,(H,21,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394015
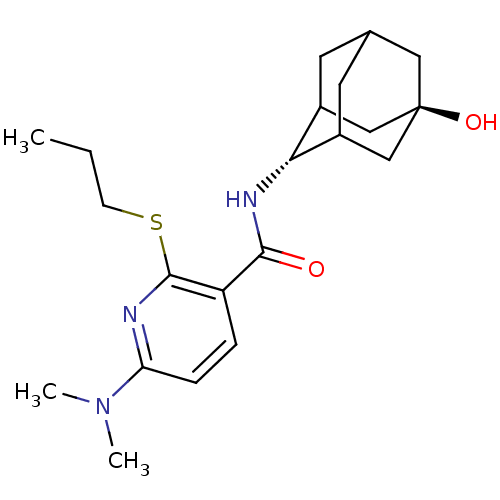
(CHEMBL2158468)Show SMILES CCCSc1nc(ccc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2)N(C)C |r,wU:13.13,wD:20.22,TLB:17:18:23:15.16.22,17:16:13.18.19:23,21:20:13:15.17.16,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(1.9,-44.89,;.56,-45.66,;-.77,-44.89,;-.77,-43.35,;-2.11,-42.59,;-3.45,-43.36,;-4.78,-42.59,;-4.78,-41.04,;-3.45,-40.27,;-2.11,-41.04,;-.78,-40.26,;-.79,-38.72,;.55,-41.03,;1.88,-40.25,;3.05,-38.95,;4.39,-39.41,;5.78,-39.04,;4.79,-40.33,;3.37,-39.8,;3.34,-38.2,;4.35,-36.96,;4.34,-35.41,;5.76,-37.51,;3.01,-37.46,;-6.11,-43.36,;-7.45,-42.59,;-6.12,-44.9,)| Show InChI InChI=1S/C21H31N3O2S/c1-4-7-27-20-16(5-6-17(22-20)24(2)3)19(25)23-18-14-8-13-9-15(18)12-21(26,10-13)11-14/h5-6,13-15,18,26H,4,7-12H2,1-3H3,(H,23,25)/t13?,14?,15?,18-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116600
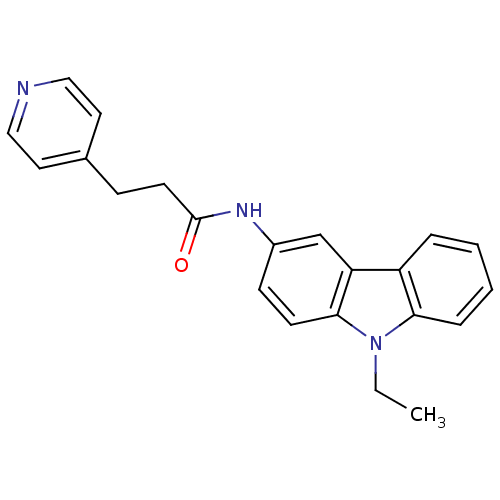
(CHEMBL325475 | N-(9-Ethyl-9H-carbazol-3-yl)-3-pyri...)Show InChI InChI=1S/C22H21N3O/c1-2-25-20-6-4-3-5-18(20)19-15-17(8-9-21(19)25)24-22(26)10-7-16-11-13-23-14-12-16/h3-6,8-9,11-15H,2,7,10H2,1H3,(H,24,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116592
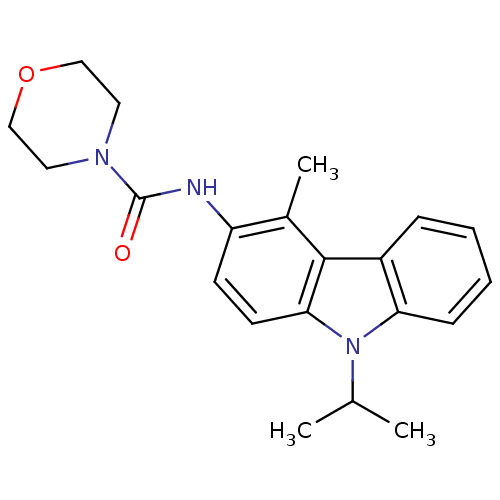
(CHEMBL325226 | Morpholine-4-carboxylic acid (9-iso...)Show SMILES CC(C)n1c2ccccc2c2c(C)c(NC(=O)N3CCOCC3)ccc12 Show InChI InChI=1S/C21H25N3O2/c1-14(2)24-18-7-5-4-6-16(18)20-15(3)17(8-9-19(20)24)22-21(25)23-10-12-26-13-11-23/h4-9,14H,10-13H2,1-3H3,(H,22,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116605
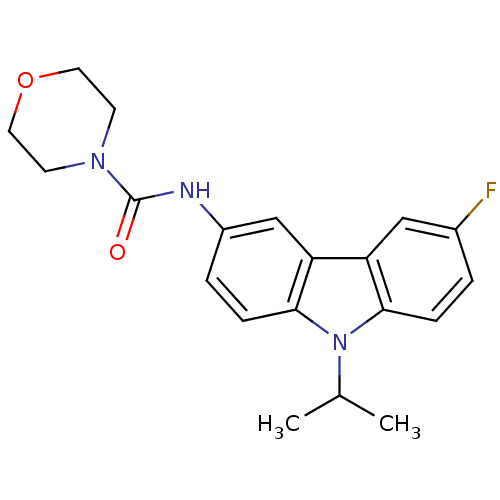
(CHEMBL432628 | Morpholine-4-carboxylic acid (6-flu...)Show SMILES CC(C)n1c2ccc(F)cc2c2cc(NC(=O)N3CCOCC3)ccc12 Show InChI InChI=1S/C20H22FN3O2/c1-13(2)24-18-5-3-14(21)11-16(18)17-12-15(4-6-19(17)24)22-20(25)23-7-9-26-10-8-23/h3-6,11-13H,7-10H2,1-2H3,(H,22,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116617
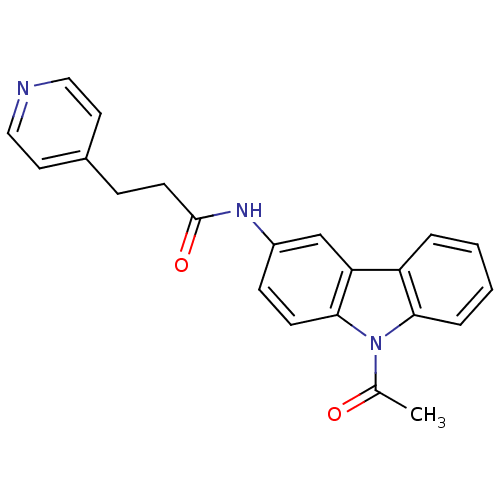
(CHEMBL116210 | N-(9-Acetyl-9H-carbazol-3-yl)-3-pyr...)Show SMILES CC(=O)n1c2ccccc2c2cc(NC(=O)CCc3ccncc3)ccc12 Show InChI InChI=1S/C22H19N3O2/c1-15(26)25-20-5-3-2-4-18(20)19-14-17(7-8-21(19)25)24-22(27)9-6-16-10-12-23-13-11-16/h2-5,7-8,10-14H,6,9H2,1H3,(H,24,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394013
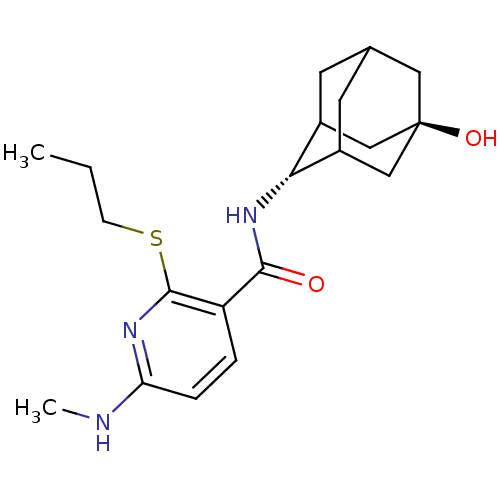
(CHEMBL2158466)Show SMILES CCCSc1nc(NC)ccc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:15.15,wD:22.24,TLB:19:20:25:17.18.24,19:18:15.20.21:25,23:22:15:17.19.18,THB:14:15:25:17.18.24,24:18:15:21.22.25,24:22:15:17.19.18,(1.31,-29.92,;-.02,-30.69,;-1.35,-29.92,;-1.36,-28.38,;-2.69,-27.61,;-4.03,-28.39,;-5.36,-27.62,;-6.7,-28.38,;-8.03,-27.61,;-5.36,-26.07,;-4.03,-25.3,;-2.7,-26.06,;-1.37,-25.29,;-1.37,-23.75,;-.03,-26.05,;1.3,-25.28,;2.47,-23.97,;3.81,-24.44,;5.19,-24.06,;4.2,-25.36,;2.79,-24.82,;2.75,-23.23,;3.77,-21.98,;3.76,-20.44,;5.18,-22.54,;2.43,-22.49,)| Show InChI InChI=1S/C20H29N3O2S/c1-3-6-26-19-15(4-5-16(21-2)22-19)18(24)23-17-13-7-12-8-14(17)11-20(25,9-12)10-13/h4-5,12-14,17,25H,3,6-11H2,1-2H3,(H,21,22)(H,23,24)/t12?,13?,14?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50420836
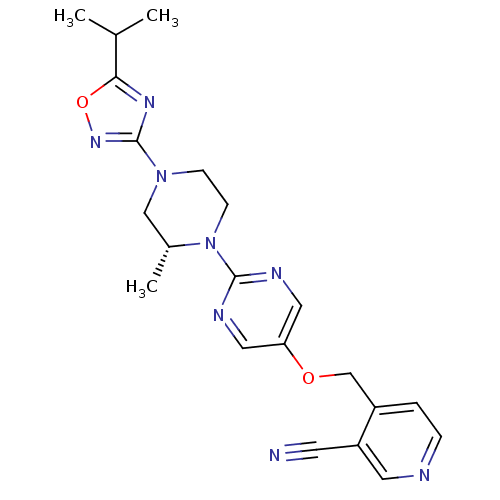
(CHEMBL2086690)Show SMILES CC(C)c1nc(no1)N1CCN([C@H](C)C1)c1ncc(OCc2ccncc2C#N)cn1 |r| Show InChI InChI=1S/C21H24N8O2/c1-14(2)19-26-21(27-31-19)28-6-7-29(15(3)12-28)20-24-10-18(11-25-20)30-13-16-4-5-23-9-17(16)8-22/h4-5,9-11,14-15H,6-7,12-13H2,1-3H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433861
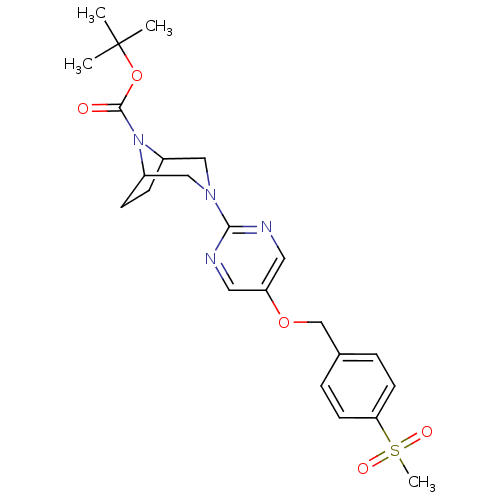
(CHEMBL2382409)Show SMILES CC(C)(C)OC(=O)N1C2CCC1CN(C2)c1ncc(OCc2ccc(cc2)S(C)(=O)=O)cn1 Show InChI InChI=1S/C23H30N4O5S/c1-23(2,3)32-22(28)27-17-7-8-18(27)14-26(13-17)21-24-11-19(12-25-21)31-15-16-5-9-20(10-6-16)33(4,29)30/h5-6,9-12,17-18H,7-8,13-15H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394014
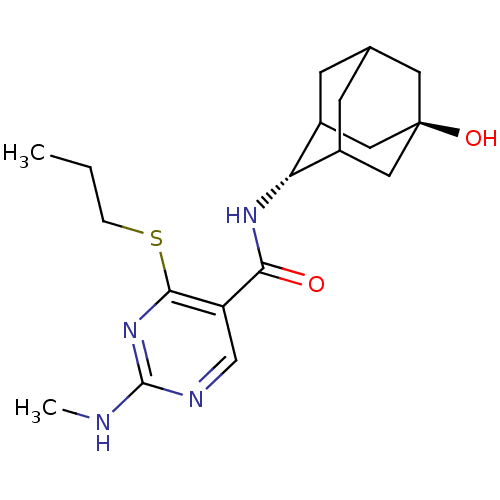
(CHEMBL2158467)Show SMILES CCCSc1nc(NC)ncc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:15.15,wD:22.24,TLB:19:20:25:17.18.24,19:18:15.20.21:25,23:22:15:17.19.18,THB:14:15:25:17.18.24,24:18:15:21.22.25,24:22:15:17.19.18,(21.02,-29.8,;19.69,-30.57,;18.35,-29.81,;18.35,-28.27,;17.02,-27.5,;15.68,-28.27,;14.35,-27.5,;13.01,-28.27,;11.68,-27.5,;14.35,-25.95,;15.68,-25.18,;17.01,-25.95,;18.34,-25.17,;18.34,-23.63,;19.68,-25.94,;21.01,-25.16,;22.18,-23.86,;23.51,-24.32,;24.9,-23.95,;23.91,-25.24,;22.5,-24.71,;22.46,-23.12,;23.48,-21.87,;23.47,-20.32,;24.88,-22.42,;22.14,-22.37,)| Show InChI InChI=1S/C19H28N4O2S/c1-3-4-26-17-14(10-21-18(20-2)23-17)16(24)22-15-12-5-11-6-13(15)9-19(25,7-11)8-12/h10-13,15,25H,3-9H2,1-2H3,(H,22,24)(H,20,21,23)/t11?,12?,13?,15-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394016
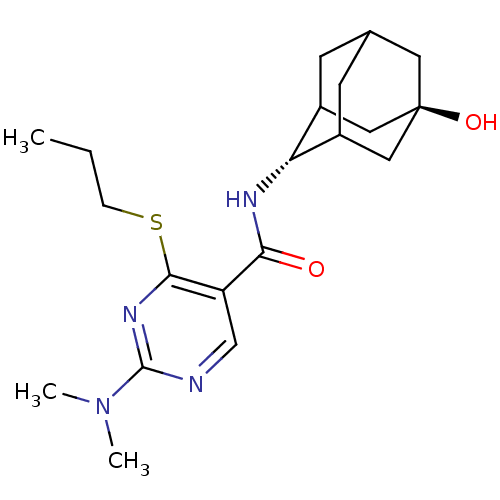
(CHEMBL2158469)Show SMILES CCCSc1nc(ncc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2)N(C)C |r,wU:13.13,wD:20.22,TLB:17:18:23:15.16.22,17:16:13.18.19:23,21:20:13:15.17.16,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(23.18,-44.42,;21.85,-45.2,;20.52,-44.43,;20.51,-42.89,;19.18,-42.12,;17.84,-42.89,;16.51,-42.12,;16.51,-40.58,;17.84,-39.81,;19.17,-40.57,;20.5,-39.79,;20.5,-38.25,;21.84,-40.56,;23.17,-39.79,;24.34,-38.48,;25.68,-38.94,;27.07,-38.57,;26.08,-39.87,;24.66,-39.33,;24.62,-37.74,;25.64,-36.49,;25.63,-34.95,;27.05,-37.04,;24.3,-36.99,;15.17,-42.89,;13.84,-42.12,;15.17,-44.43,)| Show InChI InChI=1S/C20H30N4O2S/c1-4-5-27-18-15(11-21-19(23-18)24(2)3)17(25)22-16-13-6-12-7-14(16)10-20(26,8-12)9-13/h11-14,16,26H,4-10H2,1-3H3,(H,22,25)/t12?,13?,14?,16-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394017
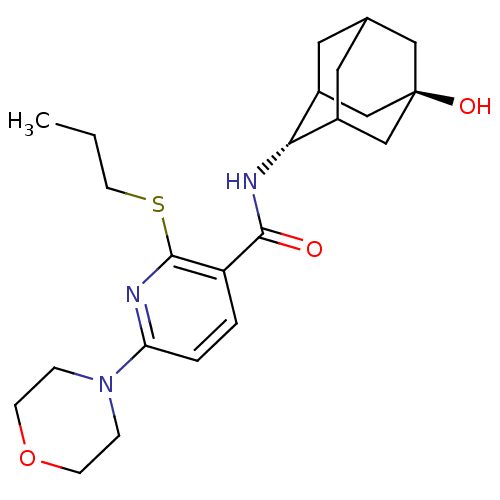
(CHEMBL2158470)Show SMILES CCCSc1nc(ccc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2)N1CCOCC1 |r,wU:13.13,wD:20.22,TLB:17:18:23:15.16.22,17:16:13.18.19:23,21:20:13:15.17.16,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(2.84,-3.33,;1.5,-4.1,;.17,-3.33,;.17,-1.79,;-1.17,-1.02,;-2.5,-1.79,;-3.84,-1.02,;-3.84,.52,;-2.51,1.29,;-1.17,.53,;.16,1.3,;.15,2.84,;1.49,.54,;2.82,1.31,;3.99,2.62,;5.33,2.15,;6.72,2.53,;5.73,1.23,;4.31,1.77,;4.28,3.36,;5.29,4.61,;5.28,6.15,;6.7,4.06,;3.96,4.1,;-5.17,-1.8,;-6.5,-1.02,;-7.83,-1.78,;-7.84,-3.32,;-6.51,-4.1,;-5.17,-3.33,)| Show InChI InChI=1S/C23H33N3O3S/c1-2-9-30-22-18(3-4-19(24-22)26-5-7-29-8-6-26)21(27)25-20-16-10-15-11-17(20)14-23(28,12-15)13-16/h3-4,15-17,20,28H,2,5-14H2,1H3,(H,25,27)/t15?,16?,17?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433857
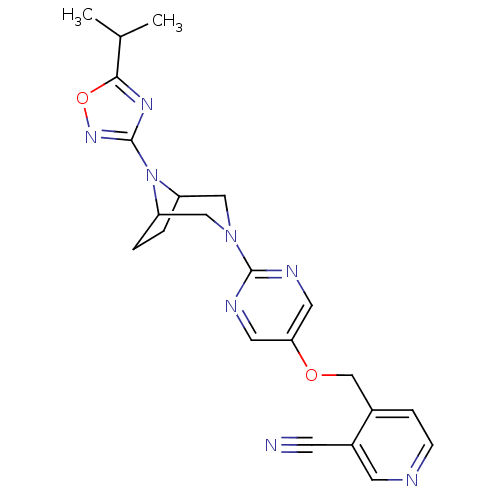
(CHEMBL2382417)Show SMILES CC(C)c1nc(no1)N1C2CCC1CN(C2)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C22H24N8O2/c1-14(2)20-27-22(28-32-20)30-17-3-4-18(30)12-29(11-17)21-25-9-19(10-26-21)31-13-15-5-6-24-8-16(15)7-23/h5-6,8-10,14,17-18H,3-4,11-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385439
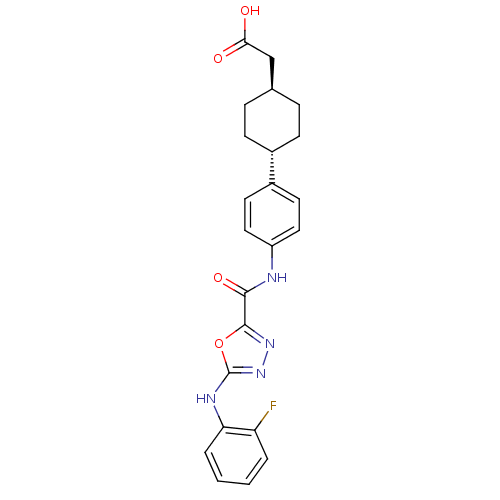
(CHEMBL2036745)Show SMILES OC(=O)C[C@H]1CC[C@@H](CC1)c1ccc(NC(=O)c2nnc(Nc3ccccc3F)o2)cc1 |r,wU:7.10,wD:4.3,(45.29,-27.64,;44.52,-28.98,;42.98,-28.97,;45.28,-30.31,;46.82,-30.32,;47.6,-31.66,;49.13,-31.66,;49.9,-30.34,;49.14,-29,;47.6,-28.99,;51.43,-30.34,;52.21,-29.01,;53.75,-29.02,;54.51,-30.36,;56.05,-30.37,;56.83,-29.04,;56.07,-27.71,;58.38,-29.05,;58.85,-27.59,;60.38,-27.59,;60.86,-29.05,;62.19,-29.81,;63.52,-29.04,;63.51,-27.5,;64.83,-26.72,;66.18,-27.49,;66.19,-29.03,;64.86,-29.81,;64.86,-31.35,;59.62,-29.96,;53.74,-31.69,;52.2,-31.69,)| Show InChI InChI=1S/C23H23FN4O4/c24-18-3-1-2-4-19(18)26-23-28-27-22(32-23)21(31)25-17-11-9-16(10-12-17)15-7-5-14(6-8-15)13-20(29)30/h1-4,9-12,14-15H,5-8,13H2,(H,25,31)(H,26,28)(H,29,30)/t14-,15- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DGAT1 expressed in baculovirus infected insect sf9 cells using [14C] oleoyl coenzyme A after 30 mins by scintillation... |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194411
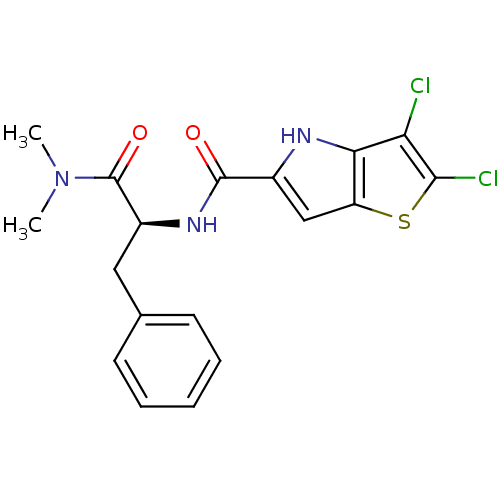
((S)-2,3-dichloro-N-(1-(dimethylamino)-1-oxo-3-phen...)Show SMILES CN(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc2sc(Cl)c(Cl)c2[nH]1 Show InChI InChI=1S/C18H17Cl2N3O2S/c1-23(2)18(25)12(8-10-6-4-3-5-7-10)22-17(24)11-9-13-15(21-11)14(19)16(20)26-13/h3-7,9,12,21H,8H2,1-2H3,(H,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human liver GPa by multienzyme coupled assay |
Bioorg Med Chem Lett 16: 5567-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.047
BindingDB Entry DOI: 10.7270/Q2KW5FP3 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50433861

(CHEMBL2382409)Show SMILES CC(C)(C)OC(=O)N1C2CCC1CN(C2)c1ncc(OCc2ccc(cc2)S(C)(=O)=O)cn1 Show InChI InChI=1S/C23H30N4O5S/c1-23(2,3)32-22(28)27-17-7-8-18(27)14-26(13-17)21-24-11-19(12-25-21)31-15-16-5-9-20(10-6-16)33(4,29)30/h5-6,9-12,17-18H,7-8,13-15H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385429
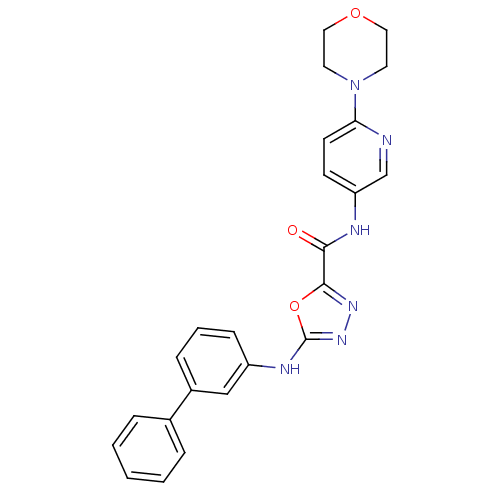
(CHEMBL2036734)Show SMILES O=C(Nc1ccc(nc1)N1CCOCC1)c1nnc(Nc2cccc(c2)-c2ccccc2)o1 Show InChI InChI=1S/C24H22N6O3/c31-22(26-20-9-10-21(25-16-20)30-11-13-32-14-12-30)23-28-29-24(33-23)27-19-8-4-7-18(15-19)17-5-2-1-3-6-17/h1-10,15-16H,11-14H2,(H,26,31)(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DGAT1 expressed in baculovirus infected insect sf9 cells using [14C] oleoyl coenzyme A after 30 mins by scintillation... |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116592

(CHEMBL325226 | Morpholine-4-carboxylic acid (9-iso...)Show SMILES CC(C)n1c2ccccc2c2c(C)c(NC(=O)N3CCOCC3)ccc12 Show InChI InChI=1S/C21H25N3O2/c1-14(2)24-18-7-5-4-6-16(18)20-15(3)17(8-9-19(20)24)22-21(25)23-10-12-26-13-11-23/h4-9,14H,10-13H2,1-3H3,(H,22,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Compound was evaluated for functional antagonism of Neuropeptide Y receptor Y5 activity in cellular Ca flux |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50394000
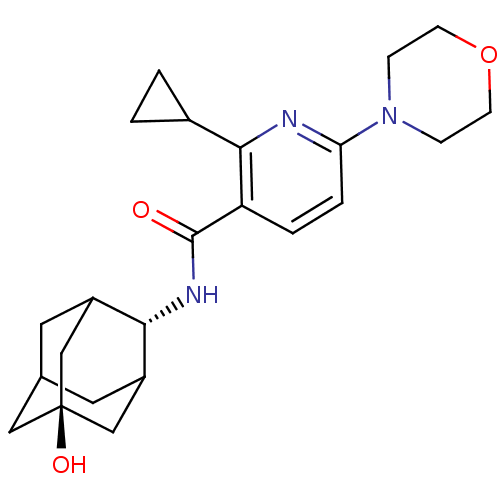
(CHEMBL2158481)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1ccc(nc1C1CC1)N1CCOCC1)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:0:1:7:4.27.3,27:26:6:4.3.2,THB:8:7:6:4.3.2,27:3:7.26.28:6,2:3:7:28.1.6,2:1:7:4.27.3,(5.69,-7.49,;5.71,-9.03,;7.11,-9.59,;7.13,-11.11,;5.74,-11.49,;4.4,-11.02,;4.37,-9.54,;3.23,-12.33,;1.91,-13.1,;.57,-12.34,;.56,-10.8,;-.76,-13.11,;-2.1,-12.35,;-3.42,-13.12,;-3.43,-14.67,;-2.09,-15.44,;-.76,-14.66,;.58,-15.43,;1.35,-16.76,;2.12,-15.43,;-4.76,-15.44,;-6.09,-14.66,;-7.42,-15.42,;-7.43,-16.97,;-6.1,-17.74,;-4.76,-16.98,;4.73,-11.87,;6.14,-12.41,;4.69,-10.28,)| Show InChI InChI=1S/C23H31N3O3/c27-22(25-20-16-9-14-10-17(20)13-23(28,11-14)12-16)18-3-4-19(24-21(18)15-1-2-15)26-5-7-29-8-6-26/h3-4,14-17,20,28H,1-2,5-13H2,(H,25,27)/t14?,16?,17?,20-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433854
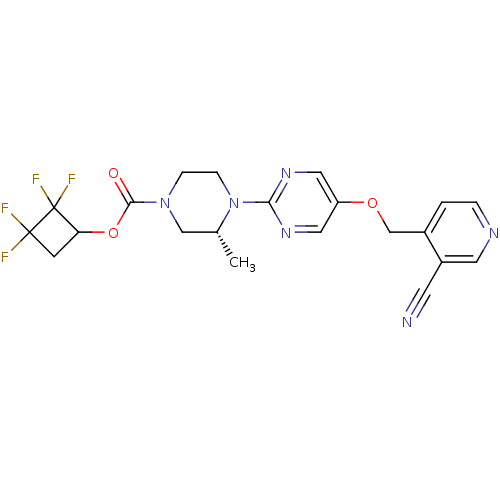
(CHEMBL2382412)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccncc2C#N)cn1)C(=O)OC1CC(F)(F)C1(F)F |r| Show InChI InChI=1S/C21H20F4N6O3/c1-13-11-30(19(32)34-17-6-20(22,23)21(17,24)25)4-5-31(13)18-28-9-16(10-29-18)33-12-14-2-3-27-8-15(14)7-26/h2-3,8-10,13,17H,4-6,11-12H2,1H3/t13-,17?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385424
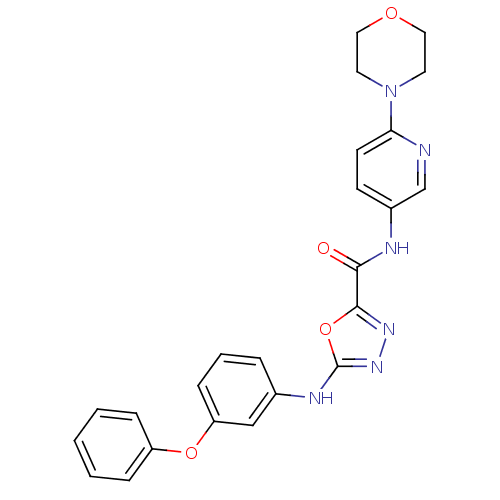
(CHEMBL2036596)Show SMILES O=C(Nc1ccc(nc1)N1CCOCC1)c1nnc(Nc2cccc(Oc3ccccc3)c2)o1 Show InChI InChI=1S/C24H22N6O4/c31-22(26-18-9-10-21(25-16-18)30-11-13-32-14-12-30)23-28-29-24(34-23)27-17-5-4-8-20(15-17)33-19-6-2-1-3-7-19/h1-10,15-16H,11-14H2,(H,26,31)(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DGAT1 expressed in baculovirus infected insect sf9 cells using [14C] oleoyl coenzyme A after 30 mins by scintillation... |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50385412
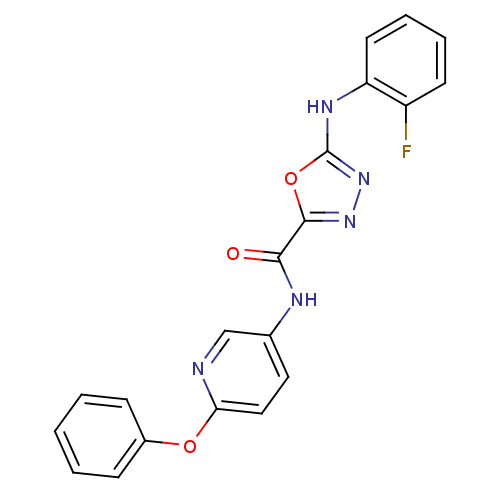
(CHEMBL2036583)Show SMILES Fc1ccccc1Nc1nnc(o1)C(=O)Nc1ccc(Oc2ccccc2)nc1 Show InChI InChI=1S/C20H14FN5O3/c21-15-8-4-5-9-16(15)24-20-26-25-19(29-20)18(27)23-13-10-11-17(22-12-13)28-14-6-2-1-3-7-14/h1-12H,(H,23,27)(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DGAT1 expressed in baculovirus infected insect sf9 cells using [14C] oleoyl coenzyme A after 30 mins by scintillation... |
Bioorg Med Chem Lett 22: 3873-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.117
BindingDB Entry DOI: 10.7270/Q2T72JG4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50392222
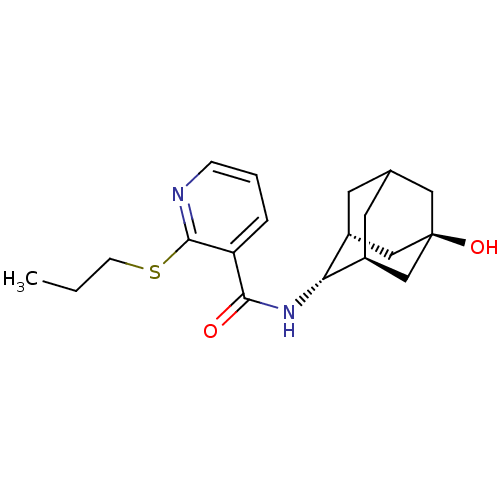
(CHEMBL2153178)Show SMILES CCCSc1ncccc1C(=O)N[C@H]1[C@H]2CC3C[C@@H]1C[C@](O)(C3)C2 |r,TLB:23:20:14.13.15:17,21:20:14.13.15:17| Show InChI InChI=1S/C19H26N2O2S/c1-2-6-24-18-15(4-3-5-20-18)17(22)21-16-13-7-12-8-14(16)11-19(23,9-12)10-13/h3-5,12-14,16,23H,2,6-11H2,1H3,(H,21,22)/t12?,13-,14+,16-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged full length human 11betaHSD1 expressed in baculovirus infected cells using cortisone as substrate after 25 mins ... |
J Med Chem 55: 5951-64 (2012)
Article DOI: 10.1021/jm300592r
BindingDB Entry DOI: 10.7270/Q24F1RTK |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM24385
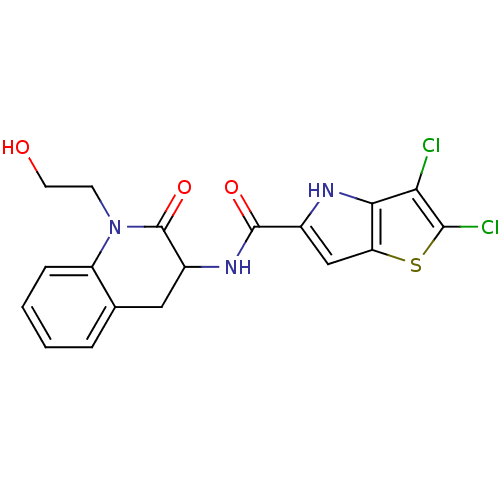
(2,3-dichloro-N-[1-(2-hydroxyethyl)-2-oxo-1,2,3,4-t...)Show SMILES OCCN1C(=O)C(Cc2ccccc12)NC(=O)c1cc2sc(Cl)c(Cl)c2[nH]1 Show InChI InChI=1S/C18H15Cl2N3O3S/c19-14-15-13(27-16(14)20)8-10(21-15)17(25)22-11-7-9-3-1-2-4-12(9)23(5-6-24)18(11)26/h1-4,8,11,21,24H,5-7H2,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.2 | 22 |
AstraZeneca
| Assay Description
The inhibitory activity of the test compounds against human recombinant glycogen phosphorylase a (GPa) was monitored using 96-well microplate format.... |
Bioorg Med Chem Lett 17: 394-9 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.037
BindingDB Entry DOI: 10.7270/Q2TM78DM |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50392216

(CHEMBL2153191 | US8673938, 7)Show SMILES CCCSc1nc(ccc1C(=O)NC1CCCCC1)N1CCC[C@@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C22H33N3O3S/c1-2-13-29-22-18(21(28)23-17-8-4-3-5-9-17)10-11-19(24-22)25-12-6-7-16(15-25)14-20(26)27/h10-11,16-17H,2-9,12-15H2,1H3,(H,23,28)(H,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged full length human 11betaHSD1 expressed in baculovirus infected cells using cortisone as substrate after 25 mins ... |
J Med Chem 55: 5951-64 (2012)
Article DOI: 10.1021/jm300592r
BindingDB Entry DOI: 10.7270/Q24F1RTK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuropeptide Y receptor type 5
(Rat 6B) | BDBM50116600

(CHEMBL325475 | N-(9-Ethyl-9H-carbazol-3-yl)-3-pyri...)Show InChI InChI=1S/C22H21N3O/c1-2-25-20-6-4-3-5-18(20)19-15-17(8-9-21(19)25)24-22(26)10-7-16-11-13-23-14-12-16/h3-6,8-9,11-15H,2,7,10H2,1H3,(H,24,26) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity against rat Neuropeptide Y receptor Y5 |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394011
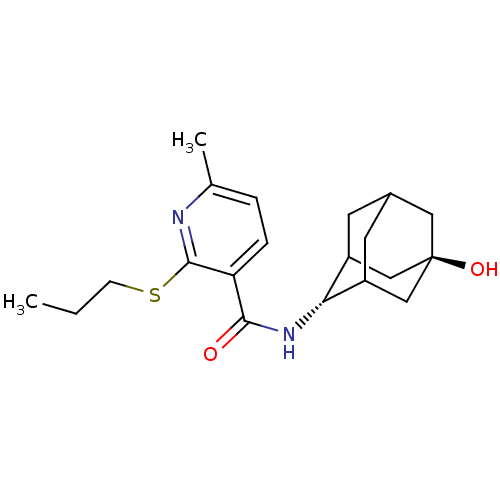
(CHEMBL2158464)Show SMILES CCCSc1nc(C)ccc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:14.14,wD:21.23,TLB:18:19:24:16.17.23,18:17:14.19.20:24,22:21:14:16.18.17,THB:13:14:24:16.17.23,23:17:14:20.21.24,23:21:14:16.18.17,(1.43,-17.15,;.1,-17.92,;-1.24,-17.15,;-1.24,-15.61,;-2.58,-14.84,;-3.91,-15.62,;-5.25,-14.84,;-6.58,-15.61,;-5.24,-13.3,;-3.92,-12.53,;-2.58,-13.29,;-1.25,-12.52,;-1.26,-10.98,;.09,-13.28,;1.41,-12.51,;2.58,-11.2,;3.92,-11.67,;5.31,-11.29,;4.32,-12.59,;2.91,-12.05,;2.87,-10.46,;3.89,-9.21,;3.87,-7.67,;5.29,-9.77,;2.55,-9.72,)| Show InChI InChI=1S/C20H28N2O2S/c1-3-6-25-19-16(5-4-12(2)21-19)18(23)22-17-14-7-13-8-15(17)11-20(24,9-13)10-14/h4-5,13-15,17,24H,3,6-11H2,1-2H3,(H,22,23)/t13?,14?,15?,17-,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116597
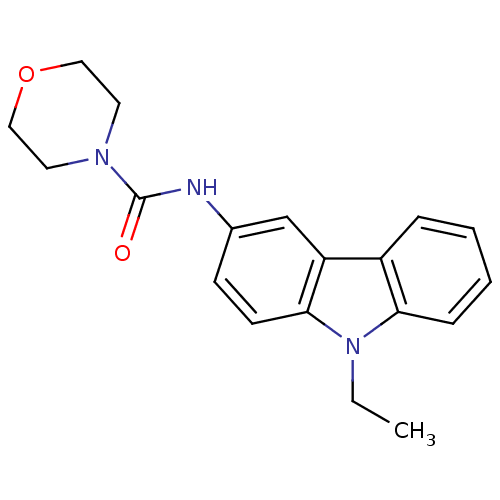
(CHEMBL119247 | Morpholine-4-carboxylic acid (9-eth...)Show InChI InChI=1S/C19H21N3O2/c1-2-22-17-6-4-3-5-15(17)16-13-14(7-8-18(16)22)20-19(23)21-9-11-24-12-10-21/h3-8,13H,2,9-12H2,1H3,(H,20,23) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433859
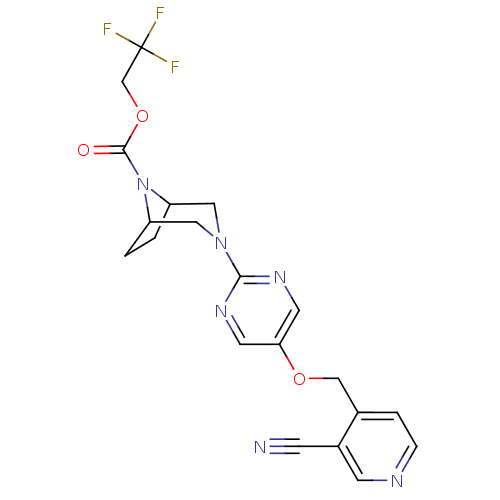
(CHEMBL2382415)Show SMILES FC(F)(F)COC(=O)N1C2CCC1CN(C2)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C20H19F3N6O3/c21-20(22,23)12-32-19(30)29-15-1-2-16(29)10-28(9-15)18-26-7-17(8-27-18)31-11-13-3-4-25-6-14(13)5-24/h3-4,6-8,15-16H,1-2,9-12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50116608
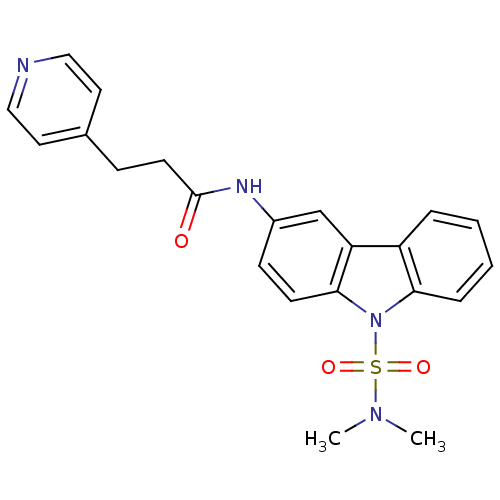
(CHEMBL117922 | N-(9-Dimethylsulfamoyl-9H-carbazol-...)Show SMILES CN(C)S(=O)(=O)n1c2ccccc2c2cc(NC(=O)CCc3ccncc3)ccc12 Show InChI InChI=1S/C22H22N4O3S/c1-25(2)30(28,29)26-20-6-4-3-5-18(20)19-15-17(8-9-21(19)26)24-22(27)10-7-16-11-13-23-14-12-16/h3-6,8-9,11-15H,7,10H2,1-2H3,(H,24,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to the human Neuropeptide Y receptor Y5 (NPY5) |
J Med Chem 45: 3509-23 (2002)
BindingDB Entry DOI: 10.7270/Q27S7N3K |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50194415
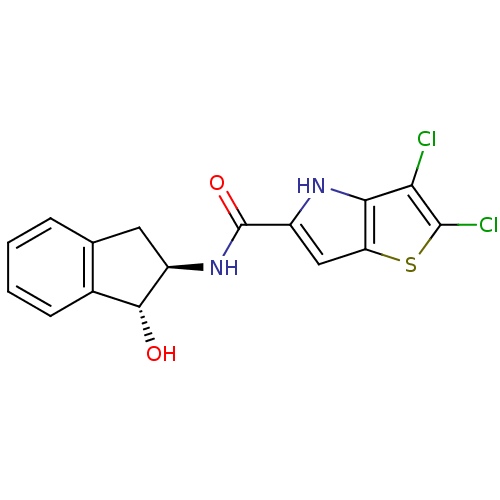
(2,3-dichloro-N-((1R,2R)-1-hydroxy-2,3-dihydro-1H-i...)Show SMILES O[C@H]1[C@@H](Cc2ccccc12)NC(=O)c1cc2sc(Cl)c(Cl)c2[nH]1 Show InChI InChI=1S/C16H12Cl2N2O2S/c17-12-13-11(23-15(12)18)6-10(19-13)16(22)20-9-5-7-3-1-2-4-8(7)14(9)21/h1-4,6,9,14,19,21H,5H2,(H,20,22)/t9-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human liver GPa by multienzyme coupled assay |
Bioorg Med Chem Lett 16: 5567-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.047
BindingDB Entry DOI: 10.7270/Q2KW5FP3 |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433853
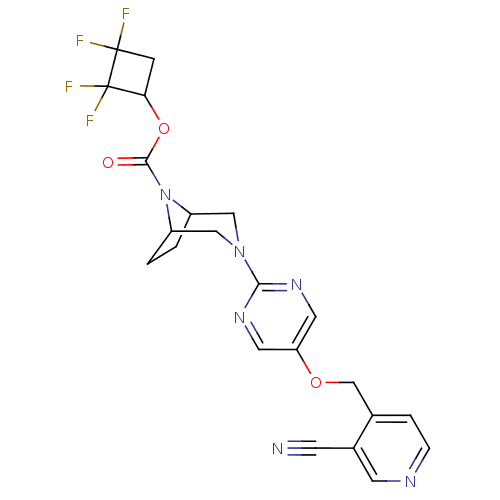
(CHEMBL2382413)Show SMILES FC1(F)CC(OC(=O)N2C3CCC2CN(C3)c2ncc(OCc3ccncc3C#N)cn2)C1(F)F Show InChI InChI=1S/C22H20F4N6O3/c23-21(24)5-18(22(21,25)26)35-20(33)32-15-1-2-16(32)11-31(10-15)19-29-8-17(9-30-19)34-12-13-3-4-28-7-14(13)6-27/h3-4,7-9,15-16,18H,1-2,5,10-12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50433856

(CHEMBL2382410)Show SMILES CC(C)(C)OC(=O)N1C2CCC1CN(C2)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C22H26N6O3/c1-22(2,3)31-21(29)28-17-4-5-18(28)13-27(12-17)20-25-10-19(11-26-20)30-14-15-6-7-24-9-16(15)8-23/h6-7,9-11,17-18H,4-5,12-14H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394010
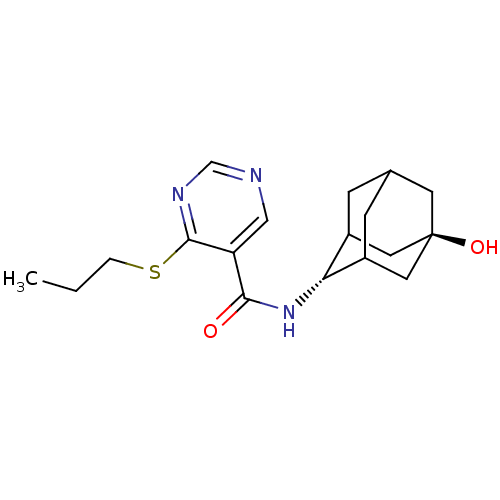
(CHEMBL2158007)Show SMILES CCCSc1ncncc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:13.13,wD:20.22,TLB:17:18:23:15.16.22,17:16:13.18.19:23,21:20:13:15.17.16,THB:12:13:23:15.16.22,22:16:13:19.20.23,22:20:13:15.17.16,(16.53,-3.98,;15.2,-4.75,;13.87,-3.98,;13.86,-2.44,;12.53,-1.68,;11.19,-2.45,;9.86,-1.68,;9.86,-.13,;11.19,.64,;12.52,-.13,;13.85,.65,;13.85,2.19,;15.19,-.12,;16.52,.66,;17.69,1.97,;19.03,1.5,;20.42,1.87,;19.43,.58,;18.01,1.12,;17.97,2.71,;18.99,3.95,;18.98,5.5,;20.4,3.4,;17.65,3.45,)| Show InChI InChI=1S/C18H25N3O2S/c1-2-3-24-17-14(9-19-10-20-17)16(22)21-15-12-4-11-5-13(15)8-18(23,6-11)7-12/h9-13,15,23H,2-8H2,1H3,(H,21,22)/t11?,12?,13?,15-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50394012
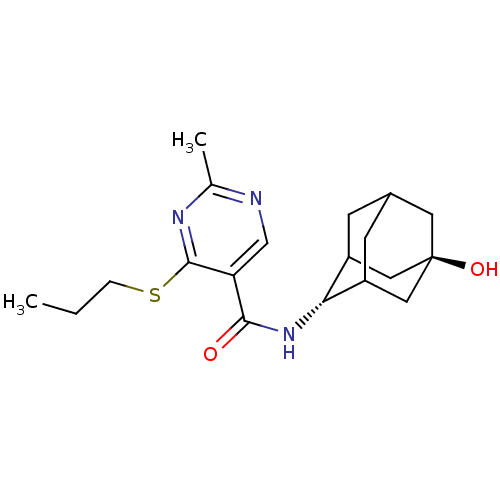
(CHEMBL2158465)Show SMILES CCCSc1nc(C)ncc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:14.14,wD:21.23,TLB:18:19:24:16.17.23,18:17:14.19.20:24,22:21:14:16.18.17,THB:13:14:24:16.17.23,23:17:14:20.21.24,23:21:14:16.18.17,(18.17,-16.91,;16.83,-17.69,;15.5,-16.92,;15.5,-15.38,;14.16,-14.61,;12.83,-15.38,;11.49,-14.61,;10.16,-15.38,;11.49,-13.07,;12.82,-12.3,;14.16,-13.06,;15.49,-12.28,;15.48,-10.74,;16.82,-13.05,;18.15,-12.28,;19.32,-10.97,;20.66,-11.43,;22.05,-11.06,;21.06,-12.36,;19.64,-11.82,;19.61,-10.23,;20.62,-8.98,;20.61,-7.44,;22.03,-9.53,;19.29,-9.48,)| Show InChI InChI=1S/C19H27N3O2S/c1-3-4-25-18-15(10-20-11(2)21-18)17(23)22-16-13-5-12-6-14(16)9-19(24,7-12)8-13/h10,12-14,16,24H,3-9H2,1-2H3,(H,22,23)/t12?,13?,14?,16-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11betaHSD1 by HTRF assay |
Bioorg Med Chem Lett 22: 6756-61 (2012)
Article DOI: 10.1016/j.bmcl.2012.08.070
BindingDB Entry DOI: 10.7270/Q23R0V0G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data