

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50470454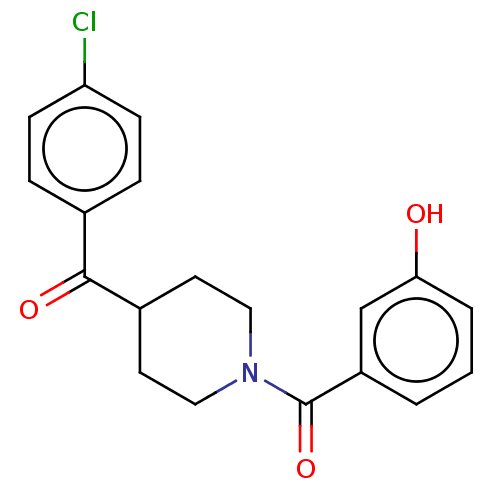 (CHEMBL4282040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human recombinant MAGL using 4-NPA substrate incubated for 30 mins by Michaelis-Menten kinetics analysis | Citation and Details BindingDB Entry DOI: 10.7270/Q20868X2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047103 (2-{3-[4-(1H-Indol-3-yl)-3,6-dihydro-2H-pyridin-1-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047108 (2-{2-[4-(6-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047089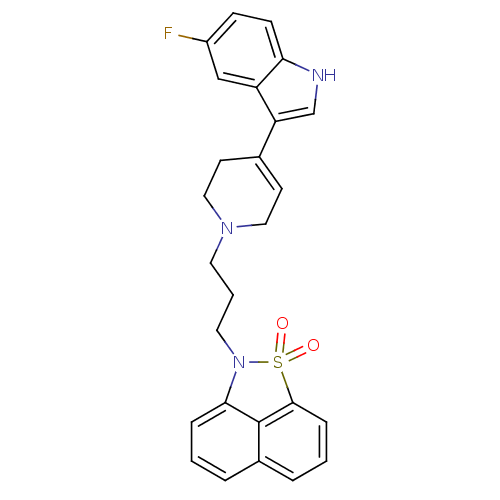 (2-{3-[4-(5-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047092 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047100 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280830 (3-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047086 (2-{3-[4-(1-Methyl-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047097 (2-{2-[4-(1H-Indol-3-yl)-3,6-dihydro-2H-pyridin-1-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047110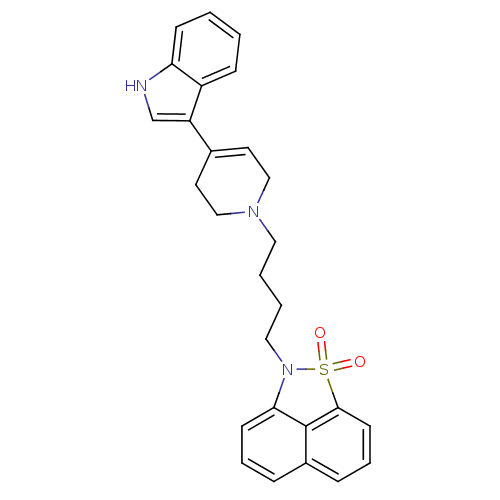 (2-{4-[4-(1H-Indol-3-yl)-3,6-dihydro-2H-pyridin-1-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283917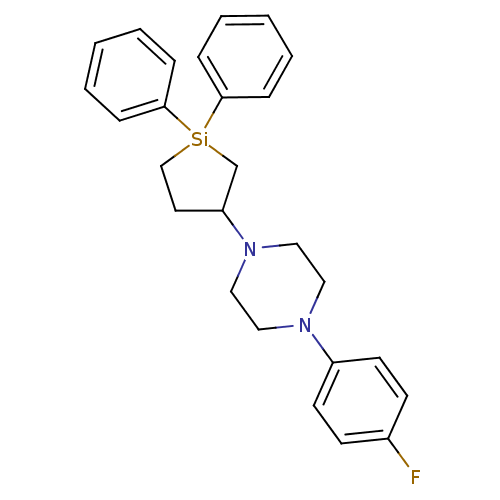 (1-(1,1-Diphenyl-silolan-3-yl)-4-(4-fluoro-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283931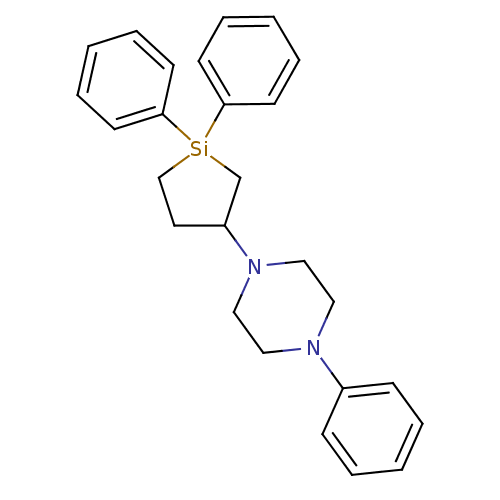 (1-(1,1-Diphenyl-silolan-3-yl)-4-phenyl-piperazine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50250824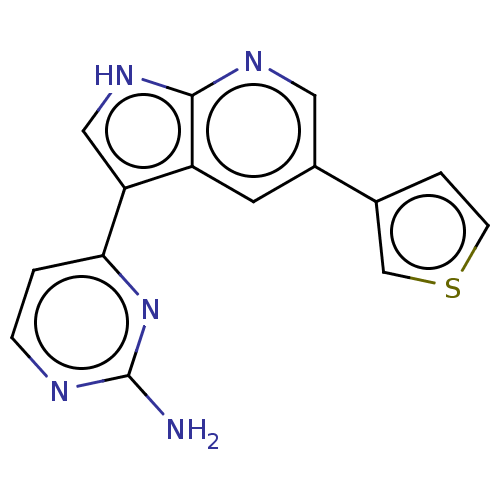 (CHEMBL4101278) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A (unknown origin) using histone H1 as substrate after 30 mins in presence of [33P]-gamma-ATP | J Med Chem 60: 9470-9489 (2017) Article DOI: 10.1021/acs.jmedchem.7b00663 BindingDB Entry DOI: 10.7270/Q2WH2SDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280826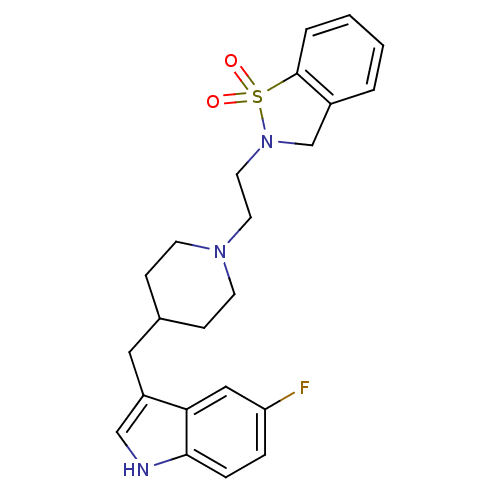 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280822 (4-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047091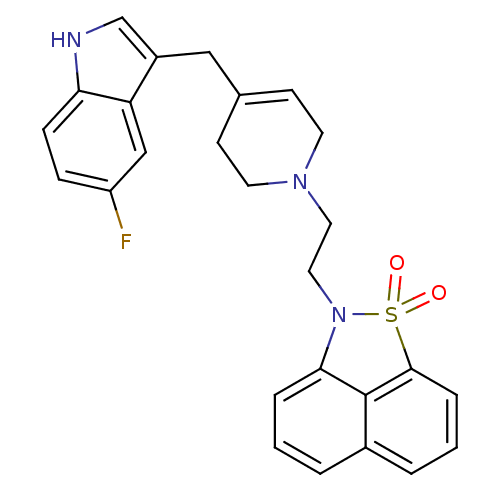 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-3,6-dihydro...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50047098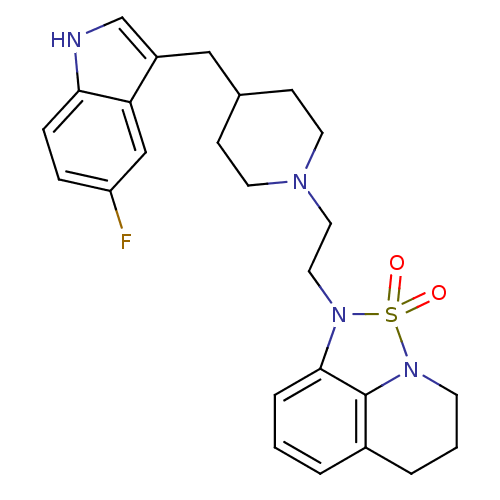 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047098 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047106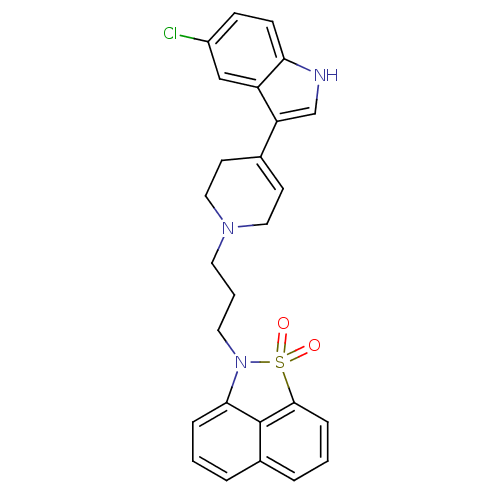 (2-{3-[4-(5-Chloro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047105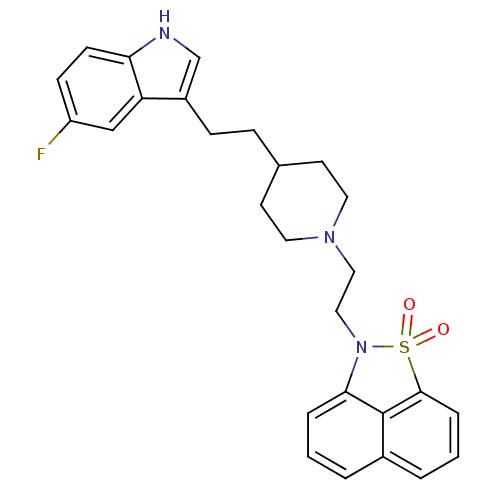 (2-(2-{4-[2-(5-Fluoro-1H-indol-3-yl)-ethyl]-piperid...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280819 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50029154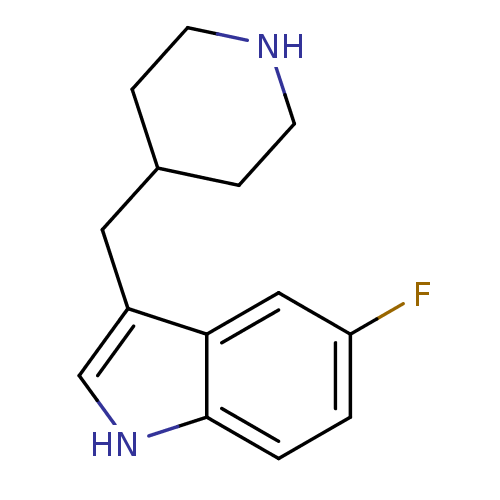 (5-Fluoro-3-piperidin-4-ylmethyl-1H-indole | CHEMBL...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001775 ((ritanserin)6-(2-{4-[Bis-(4-fluoro-phenyl)-methyle...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047102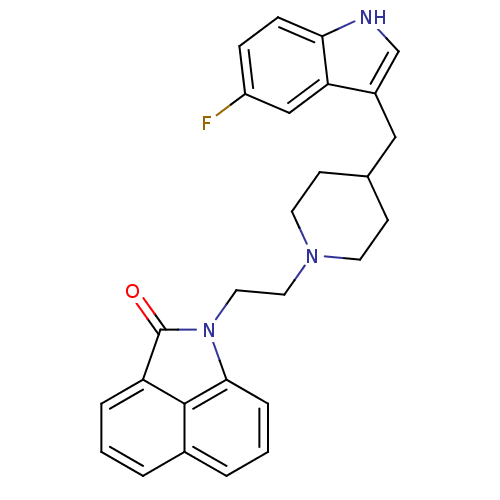 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280831 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047109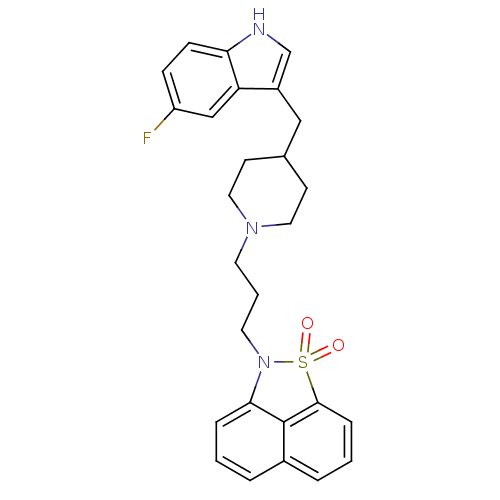 (2-{3-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Paris Curated by ChEMBL | Assay Description Immunosuppressive activity was measured by inhibition of the IL-2 production in Jurkat cells. | Bioorg Med Chem Lett 13: 4415-9 (2003) BindingDB Entry DOI: 10.7270/Q2183715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283920 (1-[1,1-Bis-(4-fluoro-phenyl)-silolan-3-yl]-4-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280817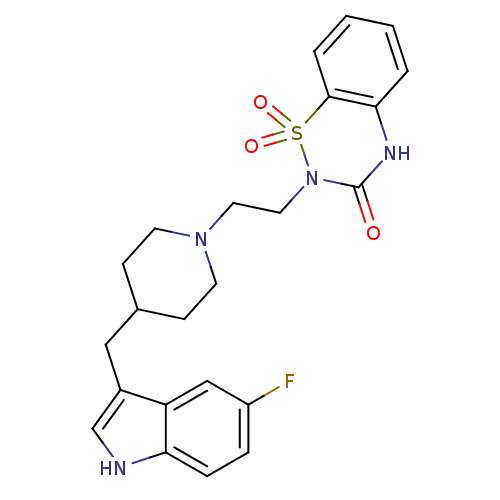 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280828 (3-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50136490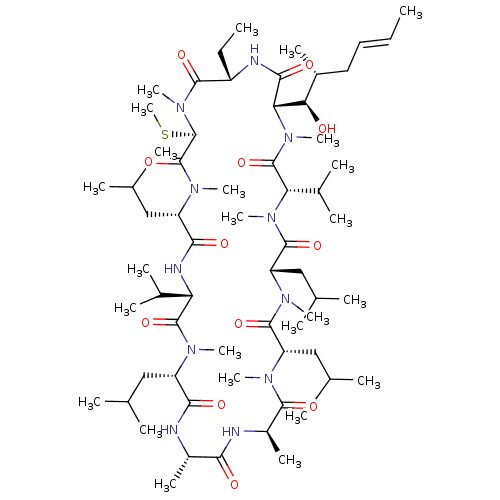 (CHEMBL3038087 | Cyclosporin A analogue) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Paris Curated by ChEMBL | Assay Description Immunosuppressive activity was measured by inhibition of the IL-2 production in Jurkat cells. | Bioorg Med Chem Lett 13: 4415-9 (2003) BindingDB Entry DOI: 10.7270/Q2183715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50250823 (CHEMBL4065940) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A (unknown origin) using histone H1 as substrate after 30 mins in presence of [33P]-gamma-ATP | J Med Chem 60: 9470-9489 (2017) Article DOI: 10.1021/acs.jmedchem.7b00663 BindingDB Entry DOI: 10.7270/Q2WH2SDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50136490 (CHEMBL3038087 | Cyclosporin A analogue) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Paris Curated by ChEMBL | Assay Description In vitro inhibitory activity against HIV-1 RT in CEM4 cell line | Bioorg Med Chem Lett 13: 4415-9 (2003) BindingDB Entry DOI: 10.7270/Q2183715 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283920 (1-[1,1-Bis-(4-fluoro-phenyl)-silolan-3-yl]-4-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280824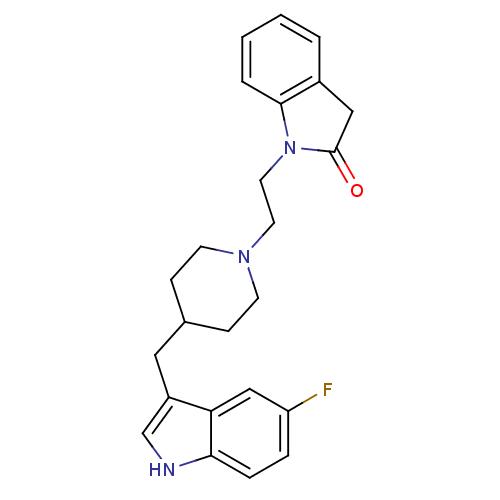 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50283928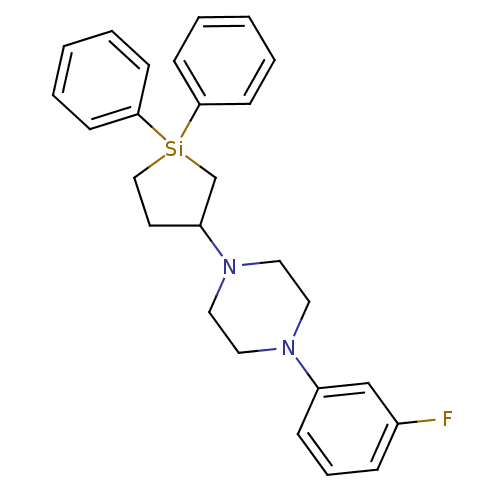 (1-(1,1-Diphenyl-silolan-3-yl)-4-(3-fluoro-phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin 5-HT2A receptor in rat cortical membranes using [3H]ketanserin as radioligand | Bioorg Med Chem Lett 4: 415-420 (1994) Article DOI: 10.1016/0960-894X(94)80007-3 BindingDB Entry DOI: 10.7270/Q2HX1D6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM5655 (2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of CDK9/cyclin T (unknown origin) using YSPTSPSYSPTSPSYSPTSPKKK as substrate after 30 mins in presence of [33P]-gamma-ATP | J Med Chem 60: 9470-9489 (2017) Article DOI: 10.1021/acs.jmedchem.7b00663 BindingDB Entry DOI: 10.7270/Q2WH2SDH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280823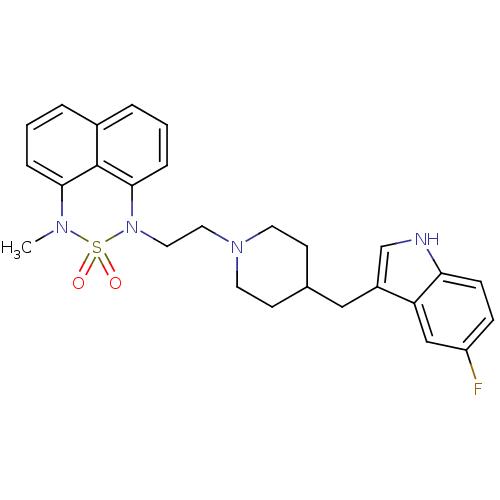 (1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280832 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047107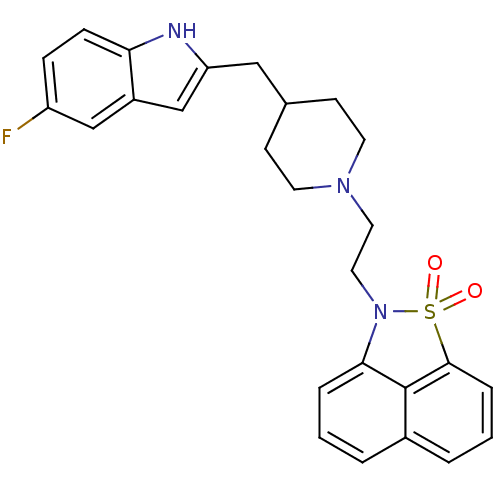 (2-{2-[4-(5-Fluoro-1H-indol-2-ylmethyl)-piperidin-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50250837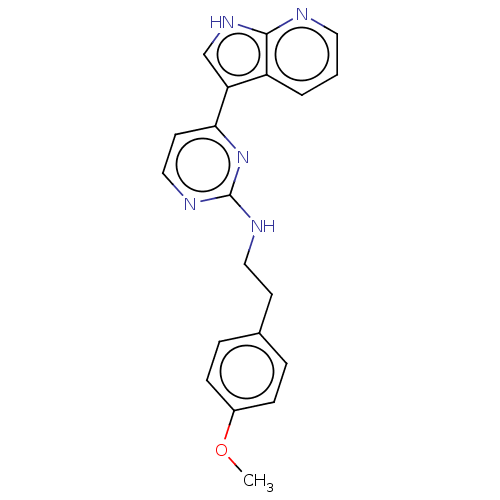 (CHEMBL4063017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A (unknown origin) using histone H1 as substrate after 30 mins in presence of [33P]-gamma-ATP | J Med Chem 60: 9470-9489 (2017) Article DOI: 10.1021/acs.jmedchem.7b00663 BindingDB Entry DOI: 10.7270/Q2WH2SDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280827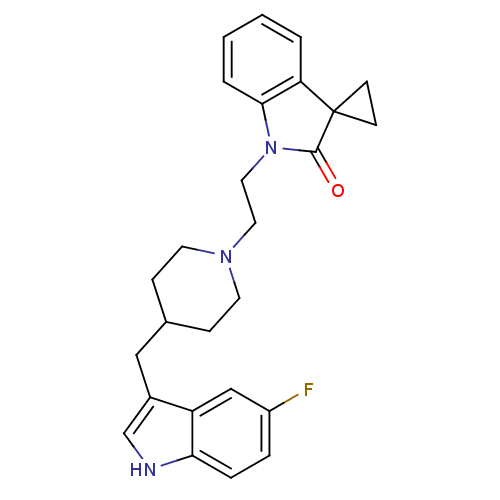 (1'-{2-[4-(5-fluoro-1H-3-indolylmethyl)hexahydro-1-...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50250815 (CHEMBL4089950) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A (unknown origin) using histone H1 as substrate after 30 mins in presence of [33P]-gamma-ATP | J Med Chem 60: 9470-9489 (2017) Article DOI: 10.1021/acs.jmedchem.7b00663 BindingDB Entry DOI: 10.7270/Q2WH2SDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50047095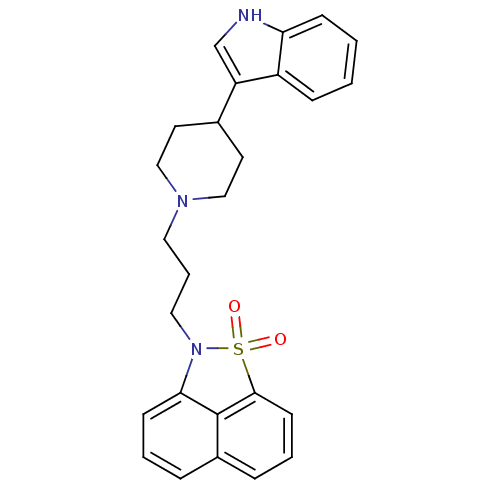 (2-{3-[4-(1H-Indol-3-yl)-piperidin-1-yl]-propyl}-2H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Binding affinity at dopamine receptor D2 by the inhibition of binding to [3H]-spiperone in rat striatal membranes | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047085 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperazin-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50250825 (CHEMBL4074567) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A (unknown origin) using histone H1 as substrate after 30 mins in presence of [33P]-gamma-ATP | J Med Chem 60: 9470-9489 (2017) Article DOI: 10.1021/acs.jmedchem.7b00663 BindingDB Entry DOI: 10.7270/Q2WH2SDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50250814 (CHEMBL4068858) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A (unknown origin) using histone H1 as substrate after 30 mins in presence of [33P]-gamma-ATP | J Med Chem 60: 9470-9489 (2017) Article DOI: 10.1021/acs.jmedchem.7b00663 BindingDB Entry DOI: 10.7270/Q2WH2SDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50047088 (2-{2-[4-(3H-Inden-1-ylmethyl)-piperidin-1-yl]-ethy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-peroxitine binding to rat cortical membranes as measure of inhibitory activity towards 5-HT uptake | J Med Chem 36: 1194-202 (1993) BindingDB Entry DOI: 10.7270/Q2MC8Z3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50280829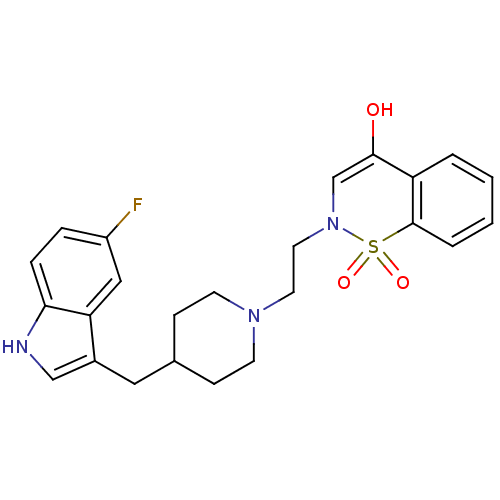 (2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of 5-HT uptake by measuring its ability to inhibit [3H]paroxetine binding to rat cortical membranes | Bioorg Med Chem Lett 3: 1913-1918 (1993) Article DOI: 10.1016/S0960-894X(01)80986-0 BindingDB Entry DOI: 10.7270/Q27944M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50250813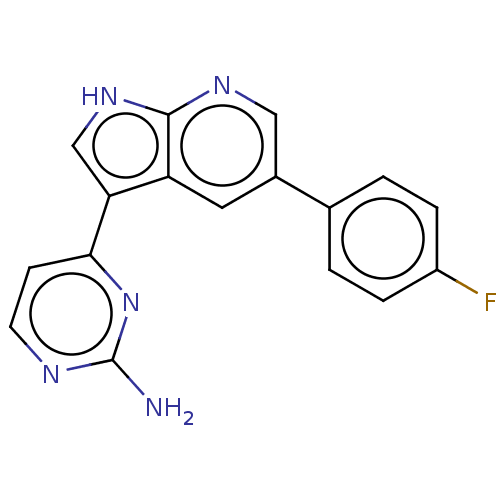 (CHEMBL4095226) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Integrative Medicine Curated by ChEMBL | Assay Description Inhibition of CDK2/cyclin A (unknown origin) using histone H1 as substrate after 30 mins in presence of [33P]-gamma-ATP | J Med Chem 60: 9470-9489 (2017) Article DOI: 10.1021/acs.jmedchem.7b00663 BindingDB Entry DOI: 10.7270/Q2WH2SDH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 303 total ) | Next | Last >> |