

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50176062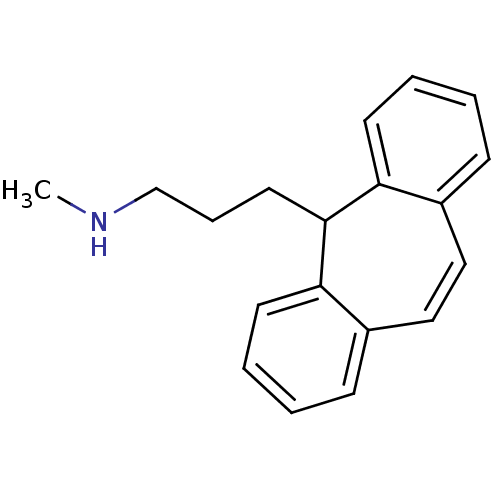 (3-(5H-dibenzo[a,d][7]annulen-5-yl)-N-methylpropan-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 55: 3488-501 (2012) Article DOI: 10.1021/jm300138g BindingDB Entry DOI: 10.7270/Q2KW5H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50255365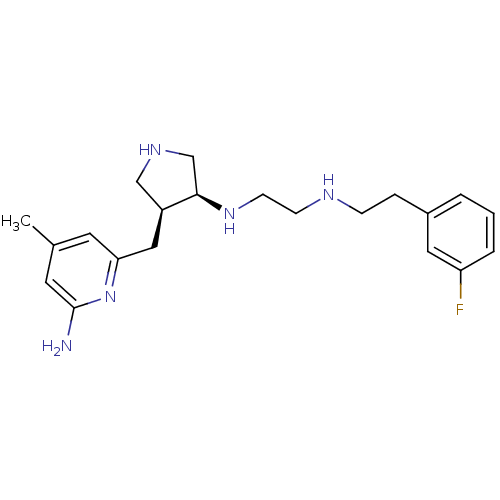 ((+/-)-cis-N1-((3S,4S)-4-((6-amino-4-methylpyridin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vibrant Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat nNOS | J Med Chem 58: 1064-6 (2015) Article DOI: 10.1021/acs.jmedchem.5b00057 BindingDB Entry DOI: 10.7270/Q2348N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50278675 ((+/-)-cis-6-((4-(2-(3-Fluorophenethylamino)-ethoxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vibrant Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat nNOS | J Med Chem 58: 1064-6 (2015) Article DOI: 10.1021/acs.jmedchem.5b00057 BindingDB Entry DOI: 10.7270/Q2348N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50072408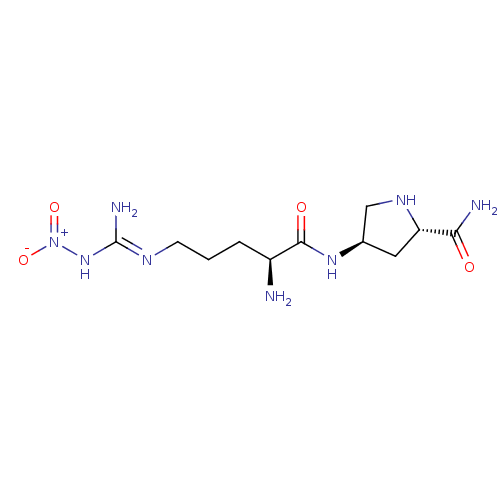 (CHEMBL228077) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vibrant Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat nNOS | J Med Chem 58: 1064-6 (2015) Article DOI: 10.1021/acs.jmedchem.5b00057 BindingDB Entry DOI: 10.7270/Q2348N2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50072407 (CHEMBL44833) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vibrant Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of rat nNOS | J Med Chem 58: 1064-6 (2015) Article DOI: 10.1021/acs.jmedchem.5b00057 BindingDB Entry DOI: 10.7270/Q2348N2Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM50176062 (3-(5H-dibenzo[a,d][7]annulen-5-yl)-N-methylpropan-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Displacement of [3H]nisoxetine from human NET expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 55: 3488-501 (2012) Article DOI: 10.1021/jm300138g BindingDB Entry DOI: 10.7270/Q2KW5H2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM111496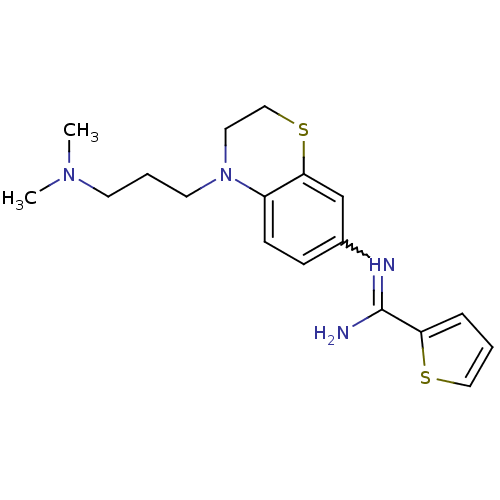 (US8618286, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... | US Patent US8618286 (2013) BindingDB Entry DOI: 10.7270/Q2C53JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50352593 (CHEMBL1825174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as conversion of [3H]-L-arginine to [3H]-L-citrulline by radiometric method | Bioorg Med Chem Lett 21: 5301-4 (2011) Article DOI: 10.1016/j.bmcl.2011.07.022 BindingDB Entry DOI: 10.7270/Q2FN16KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM111497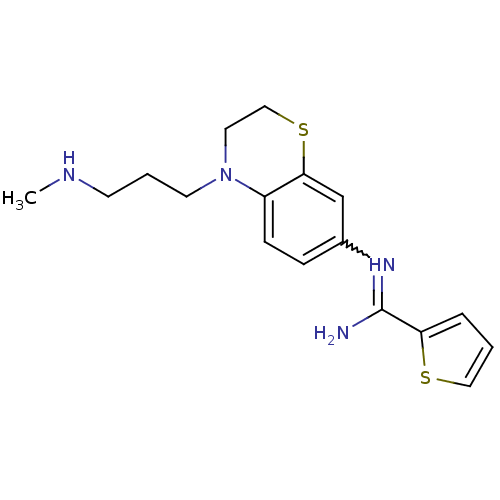 (US8618286, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... | US Patent US8618286 (2013) BindingDB Entry DOI: 10.7270/Q2C53JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206074 ((+/-)-N-{2-[2-(1-methyl-pyrrolidin-2-yl)-ethylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM111490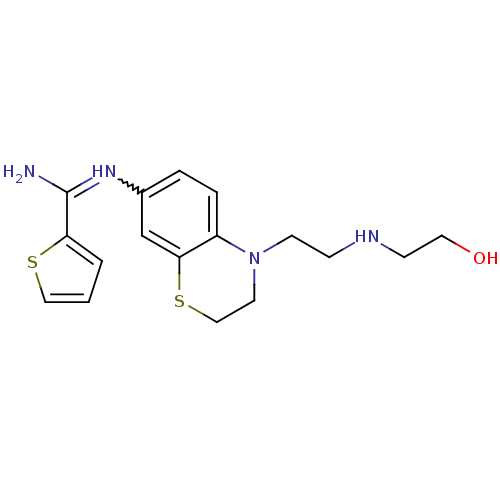 (US8618286, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... | US Patent US8618286 (2013) BindingDB Entry DOI: 10.7270/Q2C53JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50401280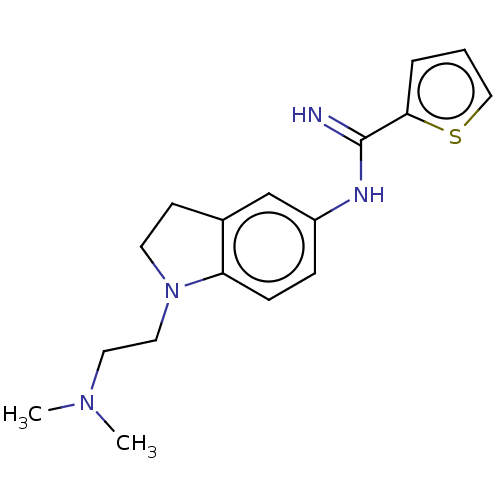 (CHEMBL3216124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in baculovirus-infected Sf9 cells assessed as conversion of [3H]-larginine to [3H]-L-citrulline preinc... | Eur J Med Chem 55: 94-107 (2012) Article DOI: 10.1016/j.ejmech.2012.07.006 BindingDB Entry DOI: 10.7270/Q2GQ6ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106707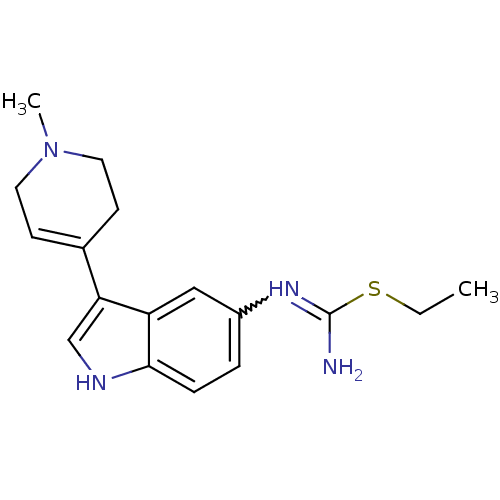 (CHEMBL1957358 | US8586620, 67) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50206073 (CHEMBL233652 | N-[2-(2-pyridin-2-yl-ethylamino)-be...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM111498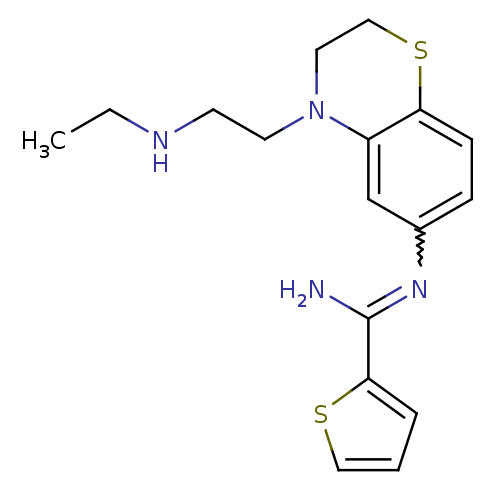 (US8618286, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... | US Patent US8618286 (2013) BindingDB Entry DOI: 10.7270/Q2C53JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106699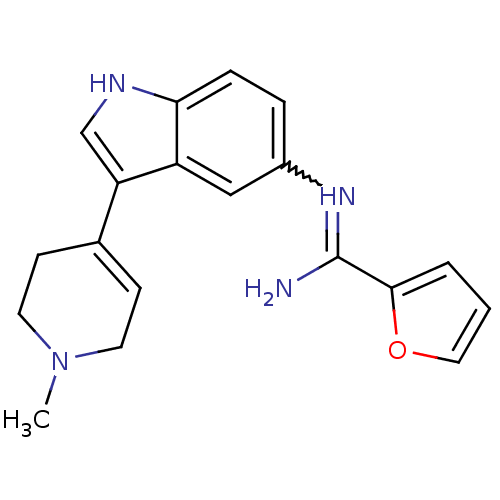 (CHEMBL1957350 | US8586620, 46) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106698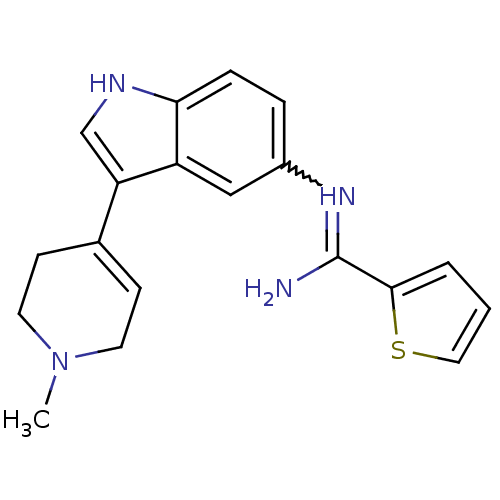 (CHEMBL1957349 | US8586620, 42) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106706 (CHEMBL1957356 | US8586620, 64) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM111482 (US8618286, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... | US Patent US8618286 (2013) BindingDB Entry DOI: 10.7270/Q2C53JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50352592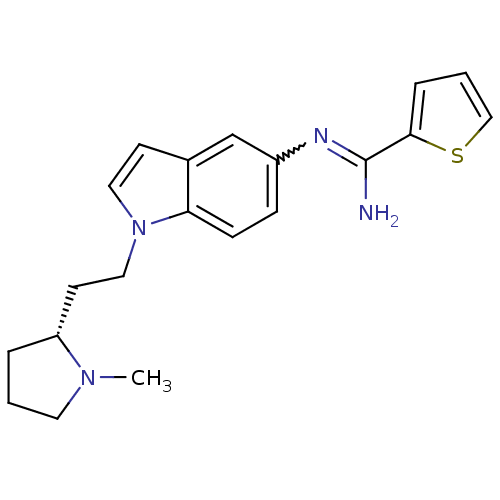 (CHEMBL1825173) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as conversion of [3H]-L-arginine to [3H]-L-citrulline by radiometric method | Bioorg Med Chem Lett 21: 5301-4 (2011) Article DOI: 10.1016/j.bmcl.2011.07.022 BindingDB Entry DOI: 10.7270/Q2FN16KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50365335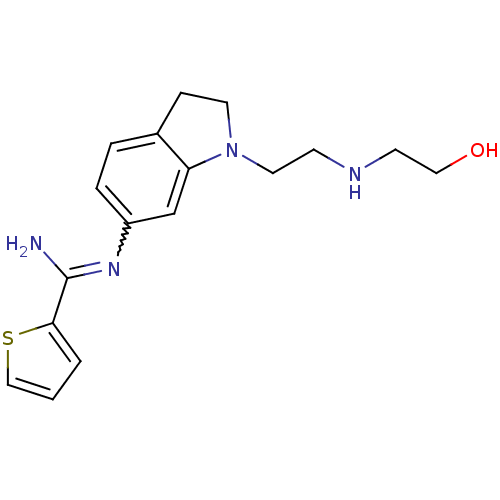 (CHEMBL1955937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in baculovirus infected insect Sf9 cells assessed as conversion of [3H]L-arginine to [3H]L-citrulline ... | J Med Chem 55: 943-55 (2012) Article DOI: 10.1021/jm201564u BindingDB Entry DOI: 10.7270/Q20P10HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50352591 (CHEMBL1825172) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as conversion of [3H]-L-arginine to [3H]-L-citrulline by radiometric method | Bioorg Med Chem Lett 21: 5301-4 (2011) Article DOI: 10.1016/j.bmcl.2011.07.022 BindingDB Entry DOI: 10.7270/Q2FN16KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50401282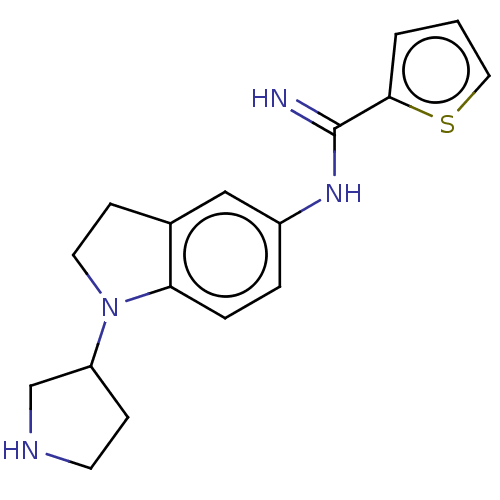 (CHEMBL3216566) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in baculovirus-infected Sf9 cells assessed as conversion of [3H]-larginine to [3H]-L-citrulline preinc... | Eur J Med Chem 55: 94-107 (2012) Article DOI: 10.1016/j.ejmech.2012.07.006 BindingDB Entry DOI: 10.7270/Q2GQ6ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50379744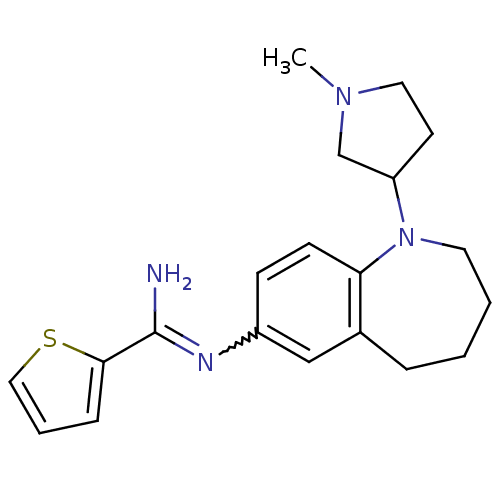 (CHEMBL2011136) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in baculovirus infected insect sf9 cells assessed as conversion of [3H]-L-arginine into [3H]-L-citrull... | Bioorg Med Chem Lett 22: 2510-3 (2012) Article DOI: 10.1016/j.bmcl.2012.02.004 BindingDB Entry DOI: 10.7270/Q2639QRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM111484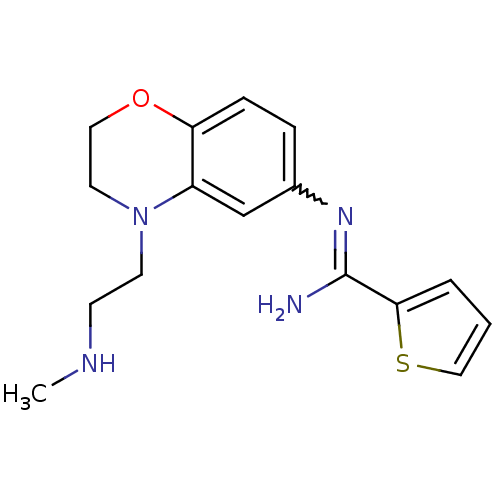 (US8618286, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... | US Patent US8618286 (2013) BindingDB Entry DOI: 10.7270/Q2C53JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM111502 (US8618286, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... | US Patent US8618286 (2013) BindingDB Entry DOI: 10.7270/Q2C53JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50357692 (CHEMBL1915288) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in baculovirus infected Sf9 cells assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincu... | J Med Chem 54: 5562-75 (2011) Article DOI: 10.1021/jm200648s BindingDB Entry DOI: 10.7270/Q2222V58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50206064 (CHEMBL233857 | N-[2-(1-benzyl-piperidin-4-ylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of rat neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50352590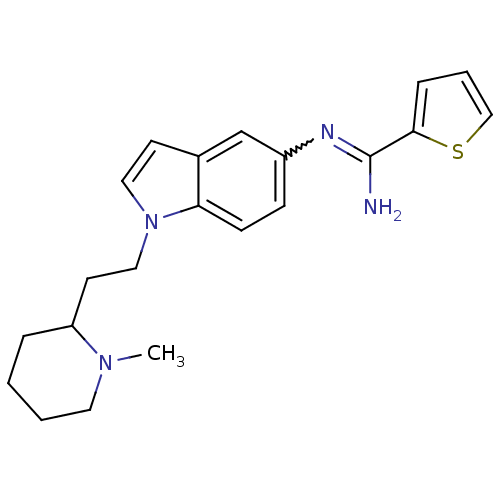 (CHEMBL1825171) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as conversion of [3H]-L-arginine to [3H]-L-citrulline by radiometric method | Bioorg Med Chem Lett 21: 5301-4 (2011) Article DOI: 10.1016/j.bmcl.2011.07.022 BindingDB Entry DOI: 10.7270/Q2FN16KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50357690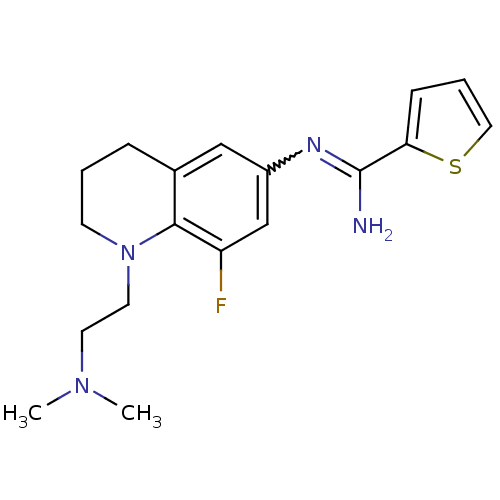 (CHEMBL1915286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in baculovirus infected Sf9 cells assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincu... | J Med Chem 54: 5562-75 (2011) Article DOI: 10.1021/jm200648s BindingDB Entry DOI: 10.7270/Q2222V58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50394745 (CHEMBL2165821) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human nNOS expressed in Sf9 cells assessed as reduction in conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins by... | J Med Chem 55: 2882-93 (2012) Article DOI: 10.1021/jm3000449 BindingDB Entry DOI: 10.7270/Q24F1RV1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50357687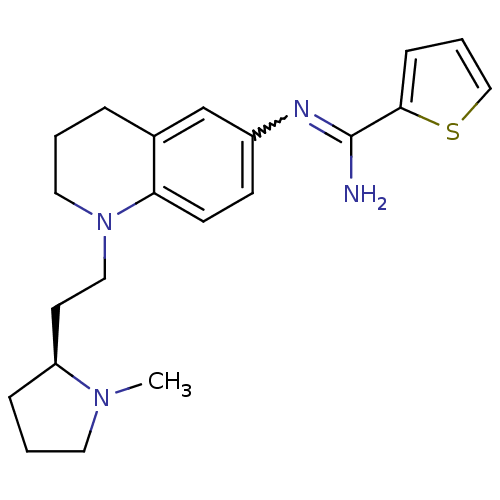 (CHEMBL1956112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in baculovirus infected Sf9 cells assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincu... | J Med Chem 54: 5562-75 (2011) Article DOI: 10.1021/jm200648s BindingDB Entry DOI: 10.7270/Q2222V58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50379740 (CHEMBL2011132) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in baculovirus infected insect sf9 cells assessed as conversion of [3H]-L-arginine into [3H]-L-citrull... | Bioorg Med Chem Lett 22: 2510-3 (2012) Article DOI: 10.1016/j.bmcl.2012.02.004 BindingDB Entry DOI: 10.7270/Q2639QRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50206065 (CHEMBL233655 | N-{2-[1-(3-methoxy-benzyl)-piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human neuronal NOS activity | Bioorg Med Chem Lett 17: 2540-4 (2007) Article DOI: 10.1016/j.bmcl.2007.02.011 BindingDB Entry DOI: 10.7270/Q2WM1F6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM111503 (US8618286, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... | US Patent US8618286 (2013) BindingDB Entry DOI: 10.7270/Q2C53JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50365334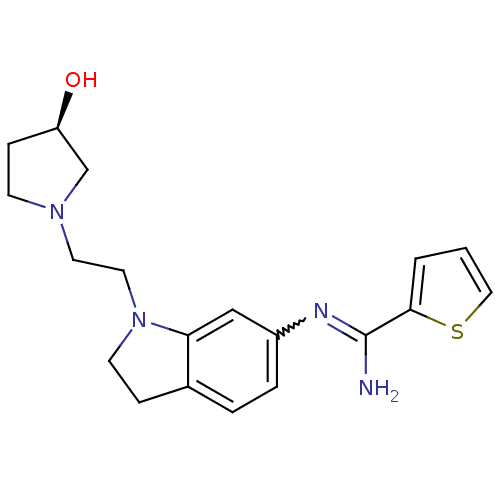 (CHEMBL1955936) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in baculovirus infected insect Sf9 cells assessed as conversion of [3H]L-arginine to [3H]L-citrulline ... | J Med Chem 55: 943-55 (2012) Article DOI: 10.1021/jm201564u BindingDB Entry DOI: 10.7270/Q20P10HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50401271 (CHEMBL2203713 | CHEMBL3216328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in baculovirus-infected Sf9 cells assessed as conversion of [3H]-larginine to [3H]-L-citrulline preinc... | Eur J Med Chem 55: 94-107 (2012) Article DOI: 10.1016/j.ejmech.2012.07.006 BindingDB Entry DOI: 10.7270/Q2GQ6ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50394739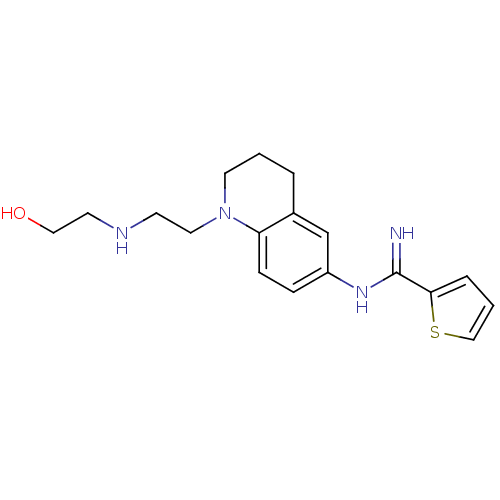 (CHEMBL2165827) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human nNOS expressed in Sf9 cells assessed as reduction in conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins by... | J Med Chem 55: 2882-93 (2012) Article DOI: 10.1021/jm3000449 BindingDB Entry DOI: 10.7270/Q24F1RV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50379737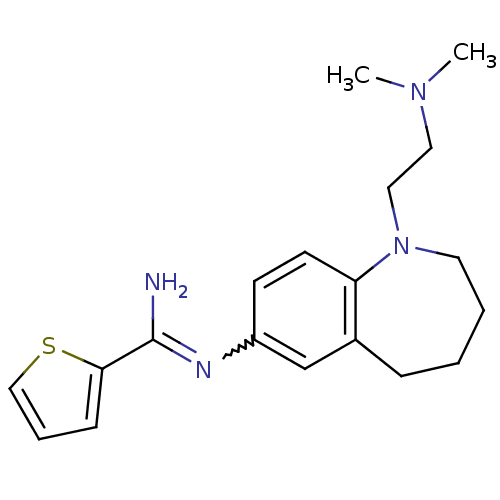 (CHEMBL2011128) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of rat nNOS | Bioorg Med Chem Lett 22: 2510-3 (2012) Article DOI: 10.1016/j.bmcl.2012.02.004 BindingDB Entry DOI: 10.7270/Q2639QRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50355311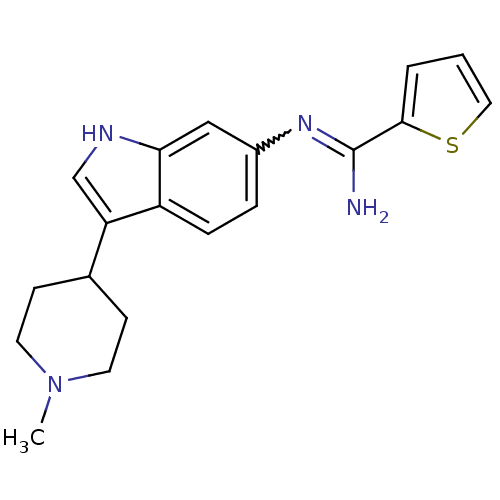 (CHEMBL1835114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM111486 (US8618286, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... | US Patent US8618286 (2013) BindingDB Entry DOI: 10.7270/Q2C53JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50352595 (CHEMBL1825176) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as conversion of [3H]-L-arginine to [3H]-L-citrulline by radiometric method | Bioorg Med Chem Lett 21: 5301-4 (2011) Article DOI: 10.1016/j.bmcl.2011.07.022 BindingDB Entry DOI: 10.7270/Q2FN16KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50401279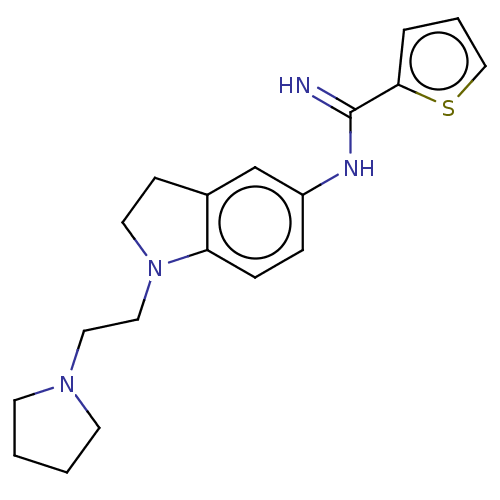 (CHEMBL3215893) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in baculovirus-infected Sf9 cells assessed as conversion of [3H]-larginine to [3H]-L-citrulline preinc... | Eur J Med Chem 55: 94-107 (2012) Article DOI: 10.1016/j.ejmech.2012.07.006 BindingDB Entry DOI: 10.7270/Q2GQ6ZWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine 1D receptor (Bos taurus (Bovine)) | BDBM106715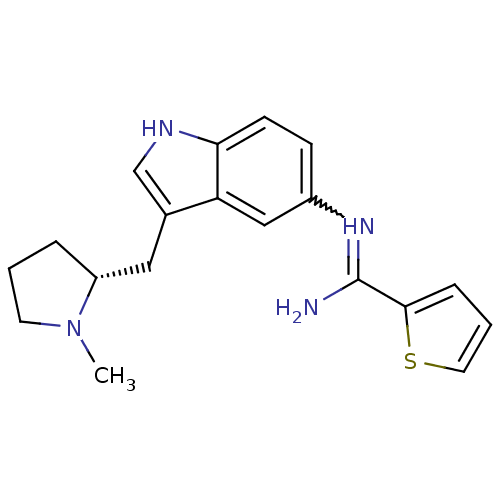 (US8586620, 97) | PDB Reactome pathway UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description 5-HT1D binding assays (agonist radioligand)were performed using bovine caudate membranes according to the methods of Heuring and Peroutka (J. Neurosc... | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM111501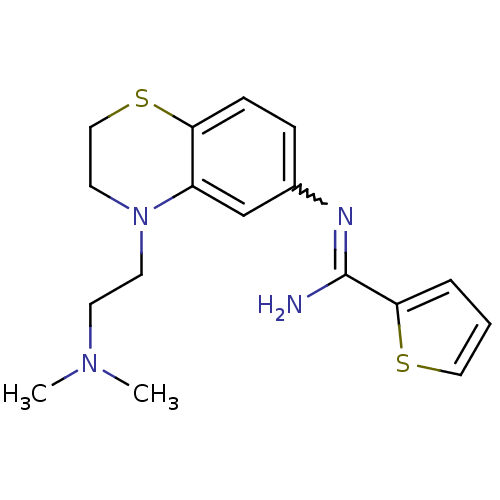 (US8618286, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Primary stock solutions of test compounds at a concentration of 6 mM were prepared from the 2 to 5 mg powder. The primary stock solutions of each tes... | US Patent US8618286 (2013) BindingDB Entry DOI: 10.7270/Q2C53JH7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50357688 (CHEMBL1915284) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in baculovirus infected Sf9 cells assessed as conversion of [3H]L-arginine to [3H]L-citrulline preincu... | J Med Chem 54: 5562-75 (2011) Article DOI: 10.1021/jm200648s BindingDB Entry DOI: 10.7270/Q2222V58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50352587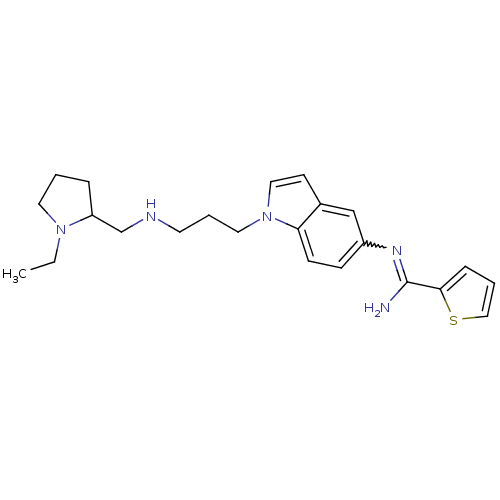 (CHEMBL1825167) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as conversion of [3H]-L-arginine to [3H]-L-citrulline by radiometric method | Bioorg Med Chem Lett 21: 5301-4 (2011) Article DOI: 10.1016/j.bmcl.2011.07.022 BindingDB Entry DOI: 10.7270/Q2FN16KP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50394744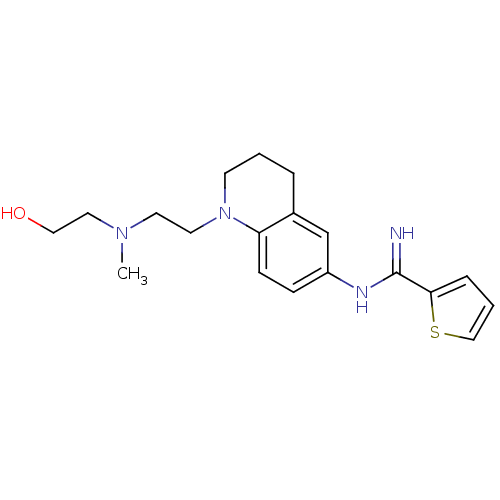 (CHEMBL2165822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human nNOS expressed in Sf9 cells assessed as reduction in conversion of [3H]L-arginine to [3H]L-citrulline preincubated for 15 mins by... | J Med Chem 55: 2882-93 (2012) Article DOI: 10.1021/jm3000449 BindingDB Entry DOI: 10.7270/Q24F1RV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 811 total ) | Next | Last >> |