 Found 571 hits with Last Name = 'bae' and Initial = 'k'
Found 571 hits with Last Name = 'bae' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50135168
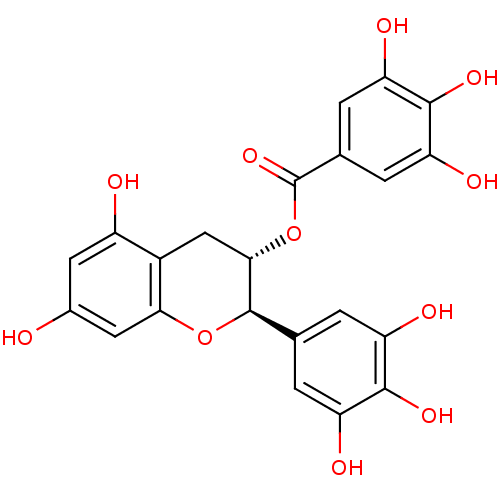
(3,4,5-Trihydroxy-benzoic acid (2R,3S)-5,7-dihydrox...)Show SMILES Oc1cc(O)c2C[C@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University
Curated by ChEMBL
| Assay Description
Inhibition of human Beta-secretase 1 |
Bioorg Med Chem Lett 13: 3905-8 (2003)
BindingDB Entry DOI: 10.7270/Q28K78HN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50135163
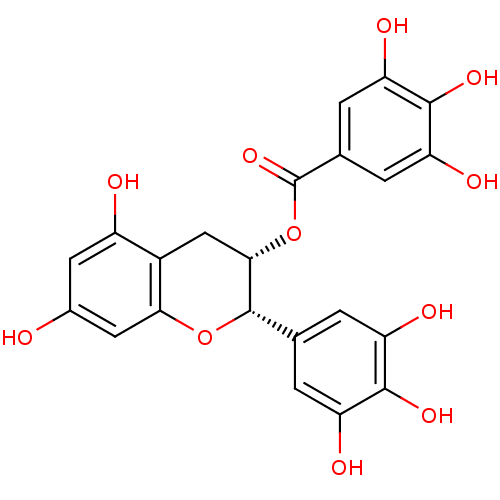
(3,4,5-Trihydroxy-benzoic acid (2S,3S)-5,7-dihydrox...)Show SMILES Oc1cc(O)c2C[C@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1cc(O)c(O)c(O)c1 Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University
Curated by ChEMBL
| Assay Description
Inhibition of human Beta-secretase 1 |
Bioorg Med Chem Lett 13: 3905-8 (2003)
BindingDB Entry DOI: 10.7270/Q28K78HN |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50135164
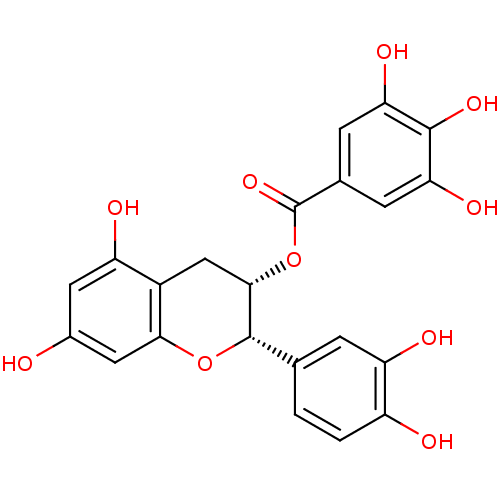
(3,4,5-Trihydroxy-benzoic acid (2S,3S)-2-(3,4-dihyd...)Show SMILES Oc1cc(O)c2C[C@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@@H](Oc2c1)c1ccc(O)c(O)c1 Show InChI InChI=1S/C22H18O10/c23-11-6-14(25)12-8-19(32-22(30)10-4-16(27)20(29)17(28)5-10)21(31-18(12)7-11)9-1-2-13(24)15(26)3-9/h1-7,19,21,23-29H,8H2/t19-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyungpook National University
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against recombinant human Beta-secretase 1 |
Bioorg Med Chem Lett 13: 3905-8 (2003)
BindingDB Entry DOI: 10.7270/Q28K78HN |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50228403

((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...)Show SMILES CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1 Show InChI InChI=1S/C25H28N8O2/c1-4-5-13-32-21-22(29-24(32)31-12-8-9-17(26)14-31)30(3)25(35)33(23(21)34)15-20-27-16(2)18-10-6-7-11-19(18)28-20/h6-7,10-11,17H,8-9,12-15,26H2,1-3H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364171
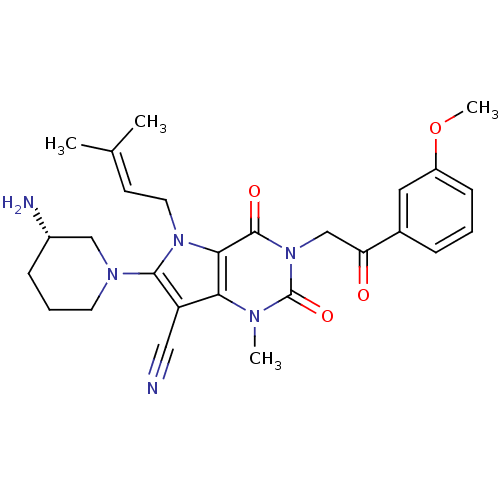
(CHEMBL1951598)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1c(=O)n(-[#6])c2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O |r| Show InChI InChI=1S/C27H32N6O4/c1-17(2)10-12-32-24-23(21(14-28)25(32)31-11-6-8-19(29)15-31)30(3)27(36)33(26(24)35)16-22(34)18-7-5-9-20(13-18)37-4/h5,7,9-10,13,19H,6,8,11-12,15-16,29H2,1-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364156

(CHEMBL1951432)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H29N7O/c1-18(2)10-13-34-25-24(22(14-28)26(34)32-12-5-7-20(29)15-32)31-17-33(27(25)35)16-23-21-8-4-3-6-19(21)9-11-30-23/h3-4,6,8-11,17,20H,5,7,12-13,15-16,29H2,1-2H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM347359
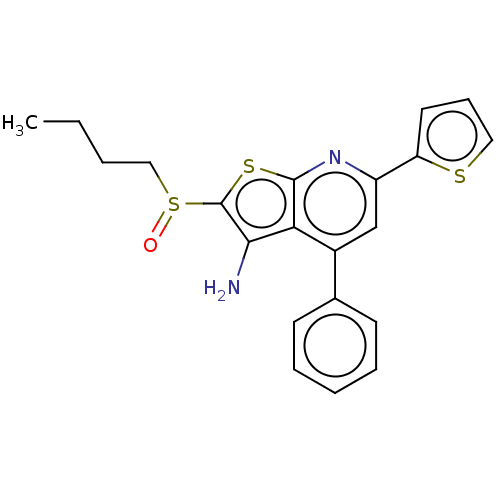
(US10301320, ID SW033291 | US10869871, ID # SW03329...)Show SMILES CCCCS(=O)c1sc2nc(cc(-c3ccccc3)c2c1N)-c1cccs1 Show InChI InChI=1S/C21H20N2OS3/c1-2-3-12-27(24)21-19(22)18-15(14-8-5-4-6-9-14)13-16(23-20(18)26-21)17-10-7-11-25-17/h4-11,13H,2-3,12,22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University; Board of Regents of the University of Texas System; University of Kentucky Research Foundation
US Patent
| Assay Description
In vivo assays for 15-PGDH expression or 15-PGDH activity, e.g., ligands, agonists, antagonists, and their homologs and mimetics. The term modulator ... |
US Patent US9790233 (2017)
BindingDB Entry DOI: 10.7270/Q2SQ92HZ |
More data for this
Ligand-Target Pair | |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM347365

(US10301320, ID SW206992 | US10869871, ID # SW20699...)Show InChI InChI=1S/C14H15N3OS3/c1-2-3-8-21(18)14-11(15)9-4-5-10(17-12(9)20-14)13-16-6-7-19-13/h4-7H,2-3,8,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University; Board of Regents of the University of Texas System; University of Kentucky Research Foundation
US Patent
| Assay Description
In vivo assays for 15-PGDH expression or 15-PGDH activity, e.g., ligands, agonists, antagonists, and their homologs and mimetics. The term modulator ... |
US Patent US9790233 (2017)
BindingDB Entry DOI: 10.7270/Q2SQ92HZ |
More data for this
Ligand-Target Pair | |
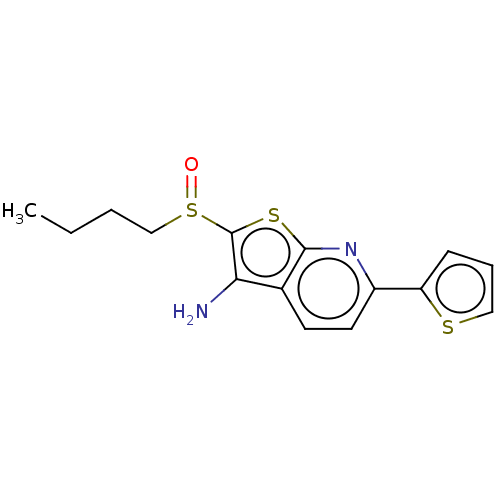
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM347364
(US10301320, ID SW206980 | US10869871, ID # SW20698...)Show InChI InChI=1S/C15H16N2OS3/c1-2-3-9-21(18)15-13(16)10-6-7-11(17-14(10)20-15)12-5-4-8-19-12/h4-8H,2-3,9,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
This Example provides data on a group of structural analogues of SW033291. Data provided includes level of induction of a 15-PGDH-luciferase fusion g... |
J Med Chem 50: 685-95 (2007)
BindingDB Entry DOI: 10.7270/Q2BV7JZ5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364187

(CHEMBL1951416)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3ncc4ccccc4c3C#N)c(=O)c12 Show InChI InChI=1S/C28H26N8O2/c1-3-4-13-35-25-24(22(16-30)26(35)34-12-7-10-31-11-14-34)33(2)28(38)36(27(25)37)18-23-21(15-29)20-9-6-5-8-19(20)17-32-23/h5-6,8-9,17,31H,7,10-14,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
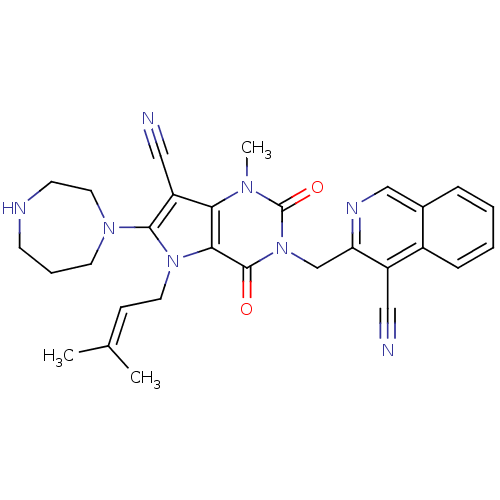
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364184
(CHEMBL1951611)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3ncc4ccccc4c3C#N)c(=O)c12 Show InChI InChI=1S/C29H30N8O2/c1-19(2)9-13-36-26-25(23(16-31)27(36)35-12-6-10-32-11-14-35)34(3)29(39)37(28(26)38)18-24-22(15-30)21-8-5-4-7-20(21)17-33-24/h4-5,7-9,17,32H,6,10-14,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364158

(CHEMBL1951430)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1cnc2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O |r| Show InChI InChI=1S/C26H30N6O3/c1-17(2)9-11-32-24-23(21(13-27)25(32)30-10-5-7-19(28)14-30)29-16-31(26(24)34)15-22(33)18-6-4-8-20(12-18)35-3/h4,6,8-9,12,16,19H,5,7,10-11,14-15,28H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364173
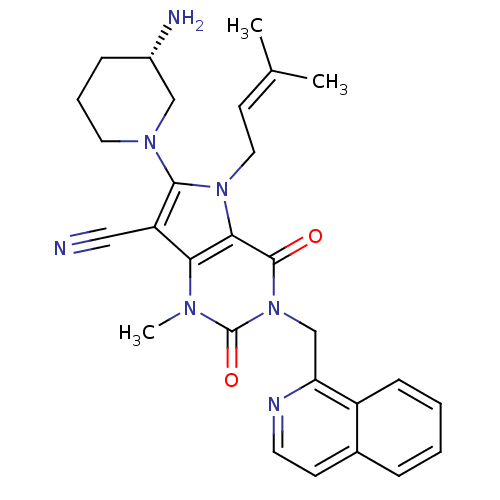
(CHEMBL1951599)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C28H31N7O2/c1-18(2)11-14-34-25-24(22(15-29)26(34)33-13-6-8-20(30)16-33)32(3)28(37)35(27(25)36)17-23-21-9-5-4-7-19(21)10-12-31-23/h4-5,7,9-12,20H,6,8,13-14,16-17,30H2,1-3H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM347359

(US10301320, ID SW033291 | US10869871, ID # SW03329...)Show SMILES CCCCS(=O)c1sc2nc(cc(-c3ccccc3)c2c1N)-c1cccs1 Show InChI InChI=1S/C21H20N2OS3/c1-2-3-12-27(24)21-19(22)18-15(14-8-5-4-6-9-14)13-16(23-20(18)26-21)17-10-7-11-25-17/h4-11,13H,2-3,12,22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University; Board of Regents of the University of Texas System; University of Kentucky Research Foundation
US Patent
| Assay Description
In vivo assays for 15-PGDH expression or 15-PGDH activity, e.g., ligands, agonists, antagonists, and their homologs and mimetics. The term modulator ... |
US Patent US9790233 (2017)
BindingDB Entry DOI: 10.7270/Q2SQ92HZ |
More data for this
Ligand-Target Pair | |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM347359

(US10301320, ID SW033291 | US10869871, ID # SW03329...)Show SMILES CCCCS(=O)c1sc2nc(cc(-c3ccccc3)c2c1N)-c1cccs1 Show InChI InChI=1S/C21H20N2OS3/c1-2-3-12-27(24)21-19(22)18-15(14-8-5-4-6-9-14)13-16(23-20(18)26-21)17-10-7-11-25-17/h4-11,13H,2-3,12,22H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.23 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
This Example provides data on a group of structural analogues of SW033291. Data provided includes level of induction of a 15-PGDH-luciferase fusion g... |
J Med Chem 50: 685-95 (2007)
BindingDB Entry DOI: 10.7270/Q2BV7JZ5 |
More data for this
Ligand-Target Pair | |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM347368

(US10301320, ID SW208066 | US10869871, ID # SW20806...)Show SMILES CCCCS(=O)c1sc2nc(cc(-c3ccccc3)c2c1N)-c1nccs1 Show InChI InChI=1S/C20H19N3OS3/c1-2-3-11-27(24)20-17(21)16-14(13-7-5-4-6-8-13)12-15(23-19(16)26-20)18-22-9-10-25-18/h4-10,12H,2-3,11,21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
This Example provides data on a group of structural analogues of SW033291. Data provided includes level of induction of a 15-PGDH-luciferase fusion g... |
J Med Chem 50: 685-95 (2007)
BindingDB Entry DOI: 10.7270/Q2BV7JZ5 |
More data for this
Ligand-Target Pair | |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM347368

(US10301320, ID SW208066 | US10869871, ID # SW20806...)Show SMILES CCCCS(=O)c1sc2nc(cc(-c3ccccc3)c2c1N)-c1nccs1 Show InChI InChI=1S/C20H19N3OS3/c1-2-3-11-27(24)20-17(21)16-14(13-7-5-4-6-8-13)12-15(23-19(16)26-20)18-22-9-10-25-18/h4-10,12H,2-3,11,21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University; Board of Regents of the University of Texas System; University of Kentucky Research Foundation
US Patent
| Assay Description
In vivo assays for 15-PGDH expression or 15-PGDH activity, e.g., ligands, agonists, antagonists, and their homologs and mimetics. The term modulator ... |
US Patent US9790233 (2017)
BindingDB Entry DOI: 10.7270/Q2SQ92HZ |
More data for this
Ligand-Target Pair | |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM348758

(US9790233, ID SW206992)Show InChI InChI=1S/C15H15N3OS2/c1-2-3-4-11(19)13-12(16)9-5-6-10(18-14(9)21-13)15-17-7-8-20-15/h5-8H,2-4,16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University; Board of Regents of the University of Texas System; University of Kentucky Research Foundation
US Patent
| Assay Description
In vivo assays for 15-PGDH expression or 15-PGDH activity, e.g., ligands, agonists, antagonists, and their homologs and mimetics. The term modulator ... |
US Patent US9790233 (2017)
BindingDB Entry DOI: 10.7270/Q2SQ92HZ |
More data for this
Ligand-Target Pair | |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM347365

(US10301320, ID SW206992 | US10869871, ID # SW20699...)Show InChI InChI=1S/C14H15N3OS3/c1-2-3-8-21(18)14-11(15)9-4-5-10(17-12(9)20-14)13-16-6-7-19-13/h4-7H,2-3,8,15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.41 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
This Example provides data on a group of structural analogues of SW033291. Data provided includes level of induction of a 15-PGDH-luciferase fusion g... |
J Med Chem 50: 685-95 (2007)
BindingDB Entry DOI: 10.7270/Q2BV7JZ5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364147

(CHEMBL1951595)Show SMILES COc1cccc(c1)C(=O)Cn1c(=O)n(C)c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c1=O |r| Show InChI InChI=1S/C29H30N6O4/c1-32-25-23(15-30)27(33-13-7-11-21(31)17-33)34(16-19-8-4-3-5-9-19)26(25)28(37)35(29(32)38)18-24(36)20-10-6-12-22(14-20)39-2/h3-6,8-10,12,14,21H,7,11,13,16-18,31H2,1-2H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364186
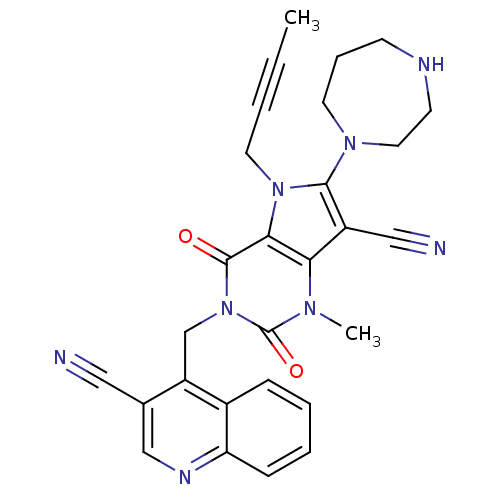
(CHEMBL1951614)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3c(cnc4ccccc34)C#N)c(=O)c12 Show InChI InChI=1S/C28H26N8O2/c1-3-4-13-35-25-24(21(16-30)26(35)34-12-7-10-31-11-14-34)33(2)28(38)36(27(25)37)18-22-19(15-29)17-32-23-9-6-5-8-20(22)23/h5-6,8-9,17,31H,7,10-14,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364179

(CHEMBL1951607)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1c(=O)n(-[#6])c2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O Show InChI InChI=1S/C27H32N6O4/c1-18(2)9-13-32-24-23(21(16-28)25(32)31-12-6-10-29-11-14-31)30(3)27(36)33(26(24)35)17-22(34)19-7-5-8-20(15-19)37-4/h5,7-9,15,29H,6,10-14,17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364167

(CHEMBL1951600)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C28H32N8O2/c1-17(2)11-13-35-25-24(21(14-29)26(35)34-12-7-8-19(30)15-34)33(4)28(38)36(27(25)37)16-23-31-18(3)20-9-5-6-10-22(20)32-23/h5-6,9-11,19H,7-8,12-13,15-16,30H2,1-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364177
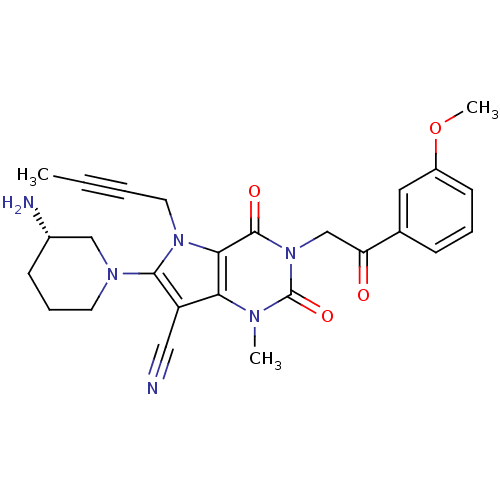
(CHEMBL1951603)Show SMILES COc1cccc(c1)C(=O)Cn1c(=O)n(C)c2c(C#N)c(N3CCC[C@H](N)C3)n(CC#CC)c2c1=O |r| Show InChI InChI=1S/C26H28N6O4/c1-4-5-12-31-23-22(20(14-27)24(31)30-11-7-9-18(28)15-30)29(2)26(35)32(25(23)34)16-21(33)17-8-6-10-19(13-17)36-3/h6,8,10,13,18H,7,9,11-12,15-16,28H2,1-3H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364183
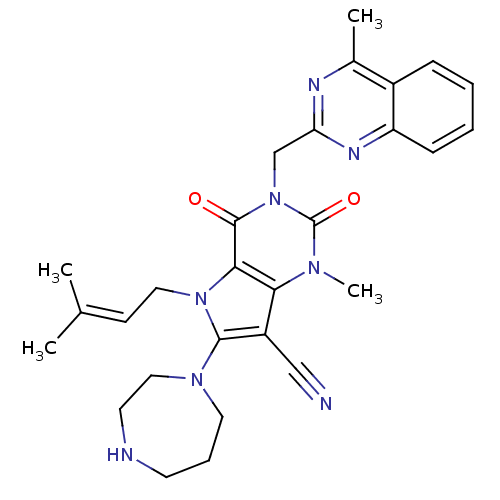
(CHEMBL1951609)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C28H32N8O2/c1-18(2)10-14-35-25-24(21(16-29)26(35)34-13-7-11-30-12-15-34)33(4)28(38)36(27(25)37)17-23-31-19(3)20-8-5-6-9-22(20)32-23/h5-6,8-10,30H,7,11-15,17H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM347369
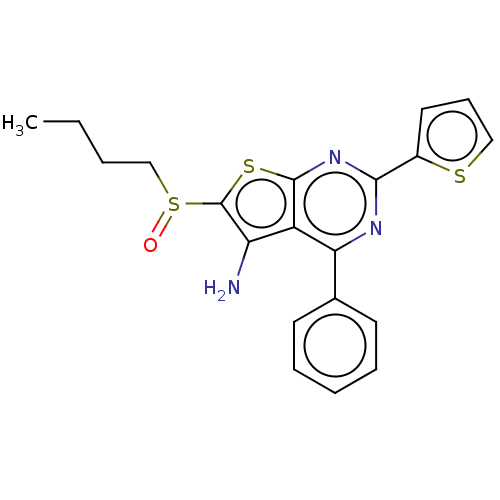
(US10869871, ID # SW208067 | US20230285402, ID # SW...)Show SMILES CCCCS(=O)c1sc2nc(nc(-c3ccccc3)c2c1N)-c1cccs1 Show InChI InChI=1S/C20H19N3OS3/c1-2-3-12-27(24)20-16(21)15-17(13-8-5-4-6-9-13)22-18(23-19(15)26-20)14-10-7-11-25-14/h4-11H,2-3,12,21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University; Board of Regents of the University of Texas System; University of Kentucky Research Foundation
US Patent
| Assay Description
In vivo assays for 15-PGDH expression or 15-PGDH activity, e.g., ligands, agonists, antagonists, and their homologs and mimetics. The term modulator ... |
US Patent US9790233 (2017)
BindingDB Entry DOI: 10.7270/Q2SQ92HZ |
More data for this
Ligand-Target Pair | |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM393003

(US10301320, ID SW208067)Show InChI InChI=1S/C20H19N3S3/c1-2-3-11-25-20-16(21)15-17(13-8-5-4-6-9-13)22-18(23-19(15)26-20)14-10-7-12-24-14/h4-10,12H,2-3,11,21H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
This Example provides data on a group of structural analogues of SW033291. Data provided includes level of induction of a 15-PGDH-luciferase fusion g... |
J Med Chem 50: 685-95 (2007)
BindingDB Entry DOI: 10.7270/Q2BV7JZ5 |
More data for this
Ligand-Target Pair | |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM347375
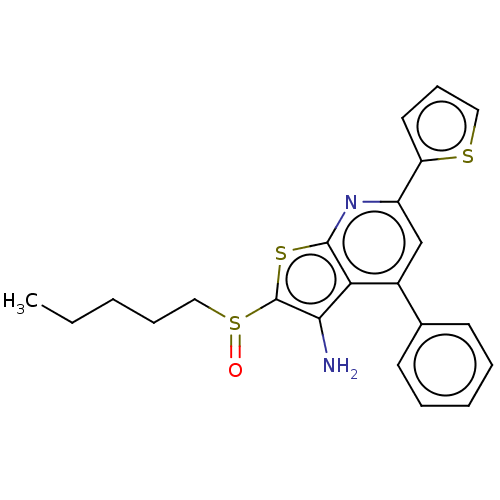
(US10301320, ID SW208080 | US10869871, ID # SW20808...)Show SMILES CCCCCS(=O)c1sc2nc(cc(-c3ccccc3)c2c1N)-c1cccs1 Show InChI InChI=1S/C22H22N2OS3/c1-2-3-7-13-28(25)22-20(23)19-16(15-9-5-4-6-10-15)14-17(24-21(19)27-22)18-11-8-12-26-18/h4-6,8-12,14H,2-3,7,13,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.64 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
This Example provides data on a group of structural analogues of SW033291. Data provided includes level of induction of a 15-PGDH-luciferase fusion g... |
J Med Chem 50: 685-95 (2007)
BindingDB Entry DOI: 10.7270/Q2BV7JZ5 |
More data for this
Ligand-Target Pair | |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM347375

(US10301320, ID SW208080 | US10869871, ID # SW20808...)Show SMILES CCCCCS(=O)c1sc2nc(cc(-c3ccccc3)c2c1N)-c1cccs1 Show InChI InChI=1S/C22H22N2OS3/c1-2-3-7-13-28(25)22-20(23)19-16(15-9-5-4-6-10-15)14-17(24-21(19)27-22)18-11-8-12-26-18/h4-6,8-12,14H,2-3,7,13,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University; Board of Regents of the University of Texas System; University of Kentucky Research Foundation
US Patent
| Assay Description
In vivo assays for 15-PGDH expression or 15-PGDH activity, e.g., ligands, agonists, antagonists, and their homologs and mimetics. The term modulator ... |
US Patent US9790233 (2017)
BindingDB Entry DOI: 10.7270/Q2SQ92HZ |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364181
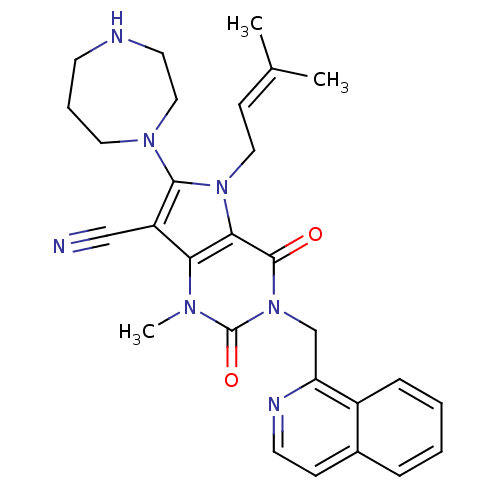
(CHEMBL1951608)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C28H31N7O2/c1-19(2)10-15-34-25-24(22(17-29)26(34)33-14-6-11-30-13-16-33)32(3)28(37)35(27(25)36)18-23-21-8-5-4-7-20(21)9-12-31-23/h4-5,7-10,12,30H,6,11,13-16,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364155

(CHEMBL1951433)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3nc(-[#6])c4ccccc4n3)c(=O)c12 |r| Show InChI InChI=1S/C27H30N8O/c1-17(2)10-12-35-25-24(21(13-28)26(35)33-11-6-7-19(29)14-33)30-16-34(27(25)36)15-23-31-18(3)20-8-4-5-9-22(20)32-23/h4-5,8-10,16,19H,6-7,11-12,14-15,29H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM23866
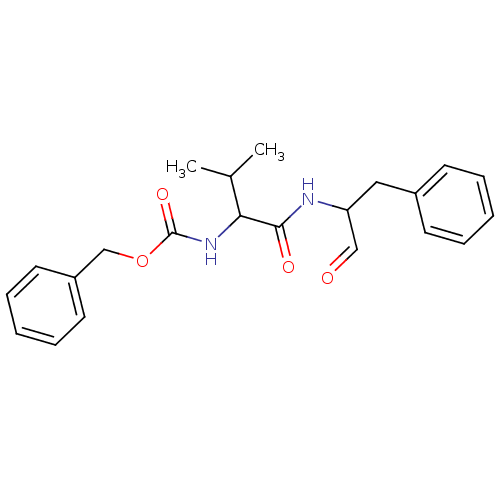
(MDL28170 | Z-Val-Phe-CHO | benzyl N-[(1S)-2-methyl...)Show SMILES CC(C)C(NC(=O)OCc1ccccc1)C(=O)NC(Cc1ccccc1)C=O Show InChI InChI=1S/C22H26N2O4/c1-16(2)20(24-22(27)28-15-18-11-7-4-8-12-18)21(26)23-19(14-25)13-17-9-5-3-6-10-17/h3-12,14,16,19-20H,13,15H2,1-2H3,(H,23,26)(H,24,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Ewha Womans University, Seoul 120-750, Republic of Korea.
| Assay Description
Inhibition of cathepsin L was assayed in reaction buffer (0.1M NaOAc-HCl, 1 mM EDTA, 0.1% β-mercaptoethanol, pH 5.5) containing 20 µM subst... |
Bioorg Chem 51: 24-30 (2013)
Article DOI: 10.1016/j.bioorg.2013.09.002
BindingDB Entry DOI: 10.7270/Q2NP233R |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluorogenic HDAC substrate 3 pre-incubated for 30 mins followed by HDAC developer addition and measured a... |
Bioorg Med Chem 27: 3408-3420 (2019)
Article DOI: 10.1016/j.bmc.2019.06.036
BindingDB Entry DOI: 10.7270/Q2CR5XRJ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50427040

(CHEMBL2322207)Show SMILES COCCCCC1(CNC(=O)[C@@H]2CNC[C@@H](C2)NS(=O)(=O)c2ccc(C)cc2)c2ccccc2Oc2ccccc12 |r| Show InChI InChI=1S/C32H39N3O5S/c1-23-13-15-26(16-14-23)41(37,38)35-25-19-24(20-33-21-25)31(36)34-22-32(17-7-8-18-39-2)27-9-3-5-11-29(27)40-30-12-6-4-10-28(30)32/h3-6,9-16,24-25,33,35H,7-8,17-22H2,1-2H3,(H,34,36)/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human plasma renin |
J Med Chem 56: 2196-206 (2013)
Article DOI: 10.1021/jm301706j
BindingDB Entry DOI: 10.7270/Q25X2B8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50427040

(CHEMBL2322207)Show SMILES COCCCCC1(CNC(=O)[C@@H]2CNC[C@@H](C2)NS(=O)(=O)c2ccc(C)cc2)c2ccccc2Oc2ccccc12 |r| Show InChI InChI=1S/C32H39N3O5S/c1-23-13-15-26(16-14-23)41(37,38)35-25-19-24(20-33-21-25)31(36)34-22-32(17-7-8-18-39-2)27-9-3-5-11-29(27)40-30-12-6-4-10-28(30)32/h3-6,9-16,24-25,33,35H,7-8,17-22H2,1-2H3,(H,34,36)/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant renin using fluorescence-quenched (RE(EDANS)IHPFHLVIHTK(Dabcyl)R as substrate incubated for 1 hr prior to substrate a... |
J Med Chem 56: 2196-206 (2013)
Article DOI: 10.1021/jm301706j
BindingDB Entry DOI: 10.7270/Q25X2B8Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364176

(CHEMBL1951604)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2n(C)c(=O)n(Cc3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H27N7O2/c1-3-4-14-33-24-23(21(15-28)25(33)32-13-7-9-19(29)16-32)31(2)27(36)34(26(24)35)17-22-20-10-6-5-8-18(20)11-12-30-22/h5-6,8,10-12,19H,7,9,13-14,16-17,29H2,1-2H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364170

(CHEMBL1951596)Show SMILES Cn1c2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c(=O)n(Cc2nccc3ccccc23)c1=O |r| Show InChI InChI=1S/C30H29N7O2/c1-34-26-24(16-31)28(35-15-7-11-22(32)18-35)36(17-20-8-3-2-4-9-20)27(26)29(38)37(30(34)39)19-25-23-12-6-5-10-21(23)13-14-33-25/h2-6,8-10,12-14,22H,7,11,15,17-19,32H2,1H3/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364157

(CHEMBL1951431)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#6@H](-[#7])-[#6]-2)c(C#N)c2ncn(-[#6]-c3ccnc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C27H29N7O/c1-18(2)10-13-34-25-24(22(14-28)26(34)32-12-5-6-20(29)16-32)31-17-33(27(25)35)15-19-9-11-30-23-8-4-3-7-21(19)23/h3-4,7-11,17,20H,5-6,12-13,15-16,29H2,1-2H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364159
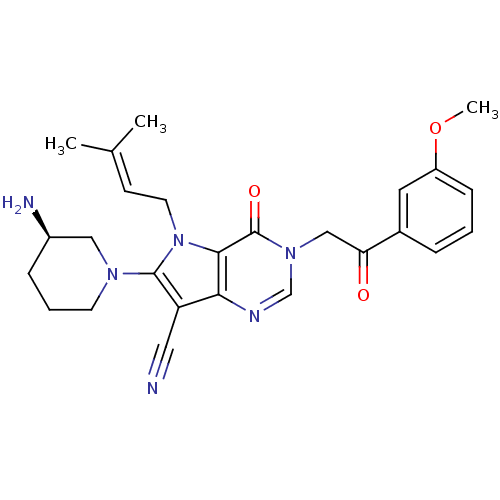
(CHEMBL1951429)Show SMILES [#6]-[#8]-c1cccc(c1)-[#6](=O)-[#6]-n1cnc2c(C#N)c(-[#7]-3-[#6]-[#6]-[#6]-[#6@@H](-[#7])-[#6]-3)n(-[#6]\[#6]=[#6](\[#6])-[#6])c2c1=O |r| Show InChI InChI=1S/C26H30N6O3/c1-17(2)9-11-32-24-23(21(13-27)25(32)30-10-5-7-19(28)14-30)29-16-31(26(24)34)15-22(33)18-6-4-8-20(12-18)35-3/h4,6,8-9,12,16,19H,5,7,10-11,14-15,28H2,1-3H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364165

(CHEMBL1951423)Show SMILES COc1cccc(c1)C(=O)Cn1cnc2c(C#N)c(N3CCC[C@H](N)C3)n(Cc3ccccc3)c2c1=O |r| Show InChI InChI=1S/C28H28N6O3/c1-37-22-11-5-9-20(13-22)24(35)17-33-18-31-25-23(14-29)27(32-12-6-10-21(30)16-32)34(26(25)28(33)36)15-19-7-3-2-4-8-19/h2-5,7-9,11,13,18,21H,6,10,12,15-17,30H2,1H3/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM16509
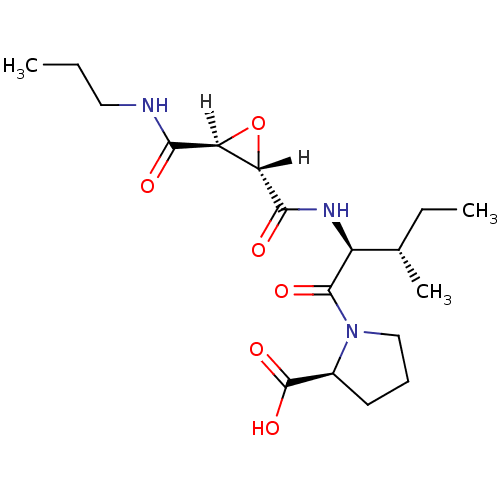
((2S)-1-[(2S,3S)-3-methyl-2-{[(2S,3S)-3-(propylcarb...)Show SMILES [H][C@@]1(O[C@]1([H])C(=O)N[C@@H]([C@@H](C)CC)C(=O)N1CCC[C@H]1C(O)=O)C(=O)NCCC |r| Show InChI InChI=1S/C18H29N3O6/c1-4-8-19-15(22)13-14(27-13)16(23)20-12(10(3)5-2)17(24)21-9-6-7-11(21)18(25)26/h10-14H,4-9H2,1-3H3,(H,19,22)(H,20,23)(H,25,26)/t10-,11-,12-,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Ewha Womans University, Seoul 120-750, Republic of Korea.
| Assay Description
Inhibition of cathepsin B was assayed in reaction buffer (50 mM NaOAc-HCl, 2 mM dithiothreitol, 2 mM EDTA, pH 5.5) containing 20 µM substrate an... |
Bioorg Chem 51: 24-30 (2013)
Article DOI: 10.1016/j.bioorg.2013.09.002
BindingDB Entry DOI: 10.7270/Q2NP233R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM347367
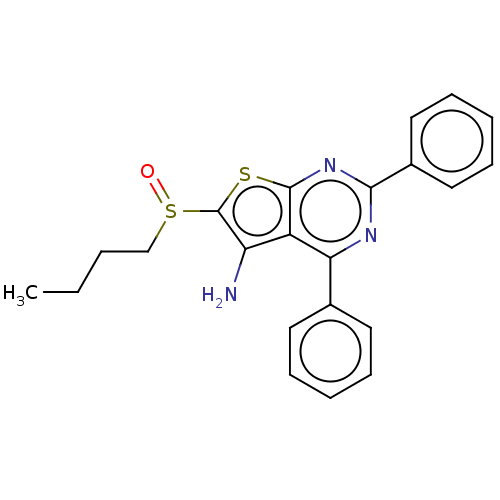
(US10301320, ID SW208065 | US10869871, ID # SW20806...)Show SMILES CCCCS(=O)c1sc2nc(nc(-c3ccccc3)c2c1N)-c1ccccc1 Show InChI InChI=1S/C22H21N3OS2/c1-2-3-14-28(26)22-18(23)17-19(15-10-6-4-7-11-15)24-20(25-21(17)27-22)16-12-8-5-9-13-16/h4-13H,2-3,14,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.87 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University; Board of Regents of the University of Texas System; University of Kentucky Research Foundation
US Patent
| Assay Description
In vivo assays for 15-PGDH expression or 15-PGDH activity, e.g., ligands, agonists, antagonists, and their homologs and mimetics. The term modulator ... |
US Patent US9790233 (2017)
BindingDB Entry DOI: 10.7270/Q2SQ92HZ |
More data for this
Ligand-Target Pair | |
15-hydroxyprostaglandin dehydrogenase [NAD(+)]
(Homo sapiens (Human)) | BDBM347367

(US10301320, ID SW208065 | US10869871, ID # SW20806...)Show SMILES CCCCS(=O)c1sc2nc(nc(-c3ccccc3)c2c1N)-c1ccccc1 Show InChI InChI=1S/C22H21N3OS2/c1-2-3-14-28(26)22-18(23)17-19(15-10-6-4-7-11-15)24-20(25-21(17)27-22)16-12-8-5-9-13-16/h4-13H,2-3,14,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.87 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
| Assay Description
This Example provides data on a group of structural analogues of SW033291. Data provided includes level of induction of a 15-PGDH-luciferase fusion g... |
J Med Chem 50: 685-95 (2007)
BindingDB Entry DOI: 10.7270/Q2BV7JZ5 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364169

(CHEMBL1951612)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C27H27N7O2/c1-3-4-15-33-24-23(21(17-28)25(33)32-14-7-11-29-13-16-32)31(2)27(36)34(26(24)35)18-22-20-9-6-5-8-19(20)10-12-30-22/h5-6,8-10,12,29H,7,11,13-16,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364160

(CHEMBL1951428)Show SMILES CC#CCn1c(N2CCC[C@H](N)C2)c(C#N)c2ncn(Cc3nccc4ccccc34)c(=O)c12 |r| Show InChI InChI=1S/C26H25N7O/c1-2-3-13-33-24-23(21(14-27)25(33)31-12-6-8-19(28)15-31)30-17-32(26(24)34)16-22-20-9-5-4-7-18(20)10-11-29-22/h4-5,7,9-11,17,19H,6,8,12-13,15-16,28H2,1H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364185

(CHEMBL1949693)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2n(-[#6])c(=O)n(-[#6]-c3c(cnc4ccccc34)C#N)c(=O)c12 Show InChI InChI=1S/C29H30N8O2/c1-19(2)9-13-36-26-25(22(16-31)27(36)35-12-6-10-32-11-14-35)34(3)29(39)37(28(26)38)18-23-20(15-30)17-33-24-8-5-4-7-21(23)24/h4-5,7-9,17,32H,6,10-14,18H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM110185
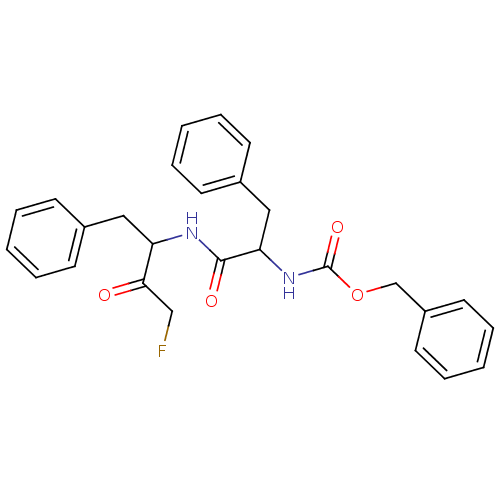
(Z-FF-FMK | benzyl N-[1-[(4-fluoro-3-oxo-1-phenylbu...)Show SMILES FCC(=O)C(Cc1ccccc1)NC(=O)C(Cc1ccccc1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C27H27FN2O4/c28-18-25(31)23(16-20-10-4-1-5-11-20)29-26(32)24(17-21-12-6-2-7-13-21)30-27(33)34-19-22-14-8-3-9-15-22/h1-15,23-24H,16-19H2,(H,29,32)(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Ewha Womans University, Seoul 120-750, Republic of Korea.
| Assay Description
Inhibition of cathepsin L was assayed in reaction buffer (0.1M NaOAc-HCl, 1 mM EDTA, 0.1% β-mercaptoethanol, pH 5.5) containing 20 µM subst... |
Bioorg Chem 51: 24-30 (2013)
Article DOI: 10.1016/j.bioorg.2013.09.002
BindingDB Entry DOI: 10.7270/Q2NP233R |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364150

(CHEMBL1951438)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2ncn(Cc3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C26H25N7O/c1-2-3-14-33-24-23(21(16-27)25(33)31-13-6-10-28-12-15-31)30-18-32(26(24)34)17-22-20-8-5-4-7-19(20)9-11-29-22/h4-5,7-9,11,18,28H,6,10,12-15,17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364152

(CHEMBL1951437)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-n1c(-[#7]-2-[#6]-[#6]-[#6]-[#7]-[#6]-[#6]-2)c(C#N)c2ncn(-[#6]-c3nccc4ccccc34)c(=O)c12 Show InChI InChI=1S/C27H29N7O/c1-19(2)9-14-34-25-24(22(16-28)26(34)32-13-5-10-29-12-15-32)31-18-33(27(25)35)17-23-21-7-4-3-6-20(21)8-11-30-23/h3-4,6-9,11,18,29H,5,10,12-15,17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50364168
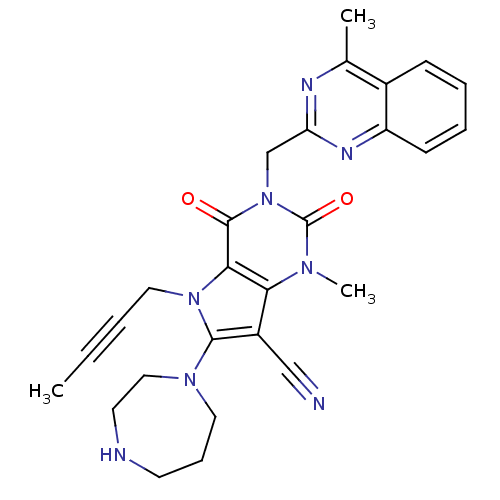
(CHEMBL1951613)Show SMILES CC#CCn1c(N2CCCNCC2)c(C#N)c2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12 Show InChI InChI=1S/C27H28N8O2/c1-4-5-14-34-24-23(20(16-28)25(34)33-13-8-11-29-12-15-33)32(3)27(37)35(26(24)36)17-22-30-18(2)19-9-6-7-10-21(19)31-22/h6-7,9-10,29H,8,11-15,17H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system |
Bioorg Med Chem Lett 22: 1464-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.11.054
BindingDB Entry DOI: 10.7270/Q2SF2WN8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data