 Found 59 hits with Last Name = 'bryan' and Initial = 'dl'
Found 59 hits with Last Name = 'bryan' and Initial = 'dl' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-10
(Rattus norvegicus) | BDBM50366779
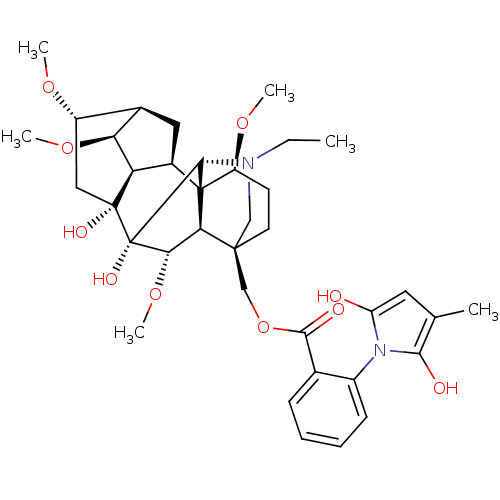
(METHYLLYCACONITINE)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)[C@@]34[C@@H]5C[C@H]6[C@H](OC)[C@@H]5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)[C@H]23)[C@H]14 |r,wU:4.4,48.54,44.48,42.46,36.39,39.43,wD:28.52,35.36,31.32,29.31,47.50,25.27,32.34,TLB:23:4:28:44.42,4:47:36.29.35:48,1:2:28:44.42,45:44:36.29.35:48,44:47:24.23.25:3.48.2,42:36:32:29.30,THB:37:36:32:29.30,40:39:32:29.30,5:4:28:44.42,(5.29,-1.99,;6.48,-1.02,;7.91,-1.57,;7.91,-4.71,;8.6,-3.19,;9.67,-4.27,;9.27,-5.75,;10.35,-6.84,;11.83,-6.45,;9.94,-8.32,;8.46,-8.7,;8.06,-10.18,;9.14,-11.27,;10.63,-10.87,;11.02,-9.39,;12.51,-8.99,;13.7,-9.95,;13.62,-11.48,;14.98,-9.12,;14.58,-7.64,;15.55,-6.45,;13.05,-7.56,;11.52,-7.55,;7.27,-2.43,;7.27,-.88,;8.6,-.11,;8.59,1.42,;7.27,2.19,;9.93,-.88,;11.13,.09,;10.97,1.62,;12.38,2.24,;13.41,1.1,;17.14,1.1,;17.91,-.25,;12.64,-.24,;13.31,-1.62,;14.39,-2.7,;15.18,-1.64,;15.11,2.18,;16.19,3.26,;15.8,4.74,;12.66,-3.02,;13.74,-4.1,;11.15,-3.38,;10.82,-4.87,;12.29,-5.26,;9.94,-2.42,;9.88,1.15,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22-,24+,25+,27-,28+,29-,30+,33-,34+,35-,36+,37+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Binding affinity towards alpha3-beta4 neuronal nicotinic acetylcholine receptor |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193985
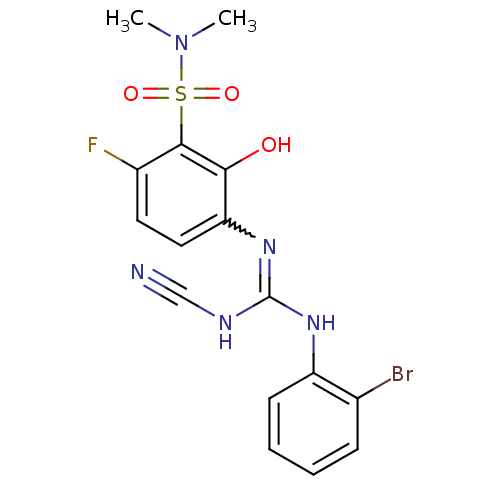
(3-{[[(2-bromophenyl)amino](cyanoimino)methyl]amino...)Show SMILES CN(C)S(=O)(=O)c1c(F)ccc(N=C(NC#N)Nc2ccccc2Br)c1O |w:12.11| Show InChI InChI=1S/C16H15BrFN5O3S/c1-23(2)27(25,26)15-11(18)7-8-13(14(15)24)22-16(20-9-19)21-12-6-4-3-5-10(12)17/h3-8,24H,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193979
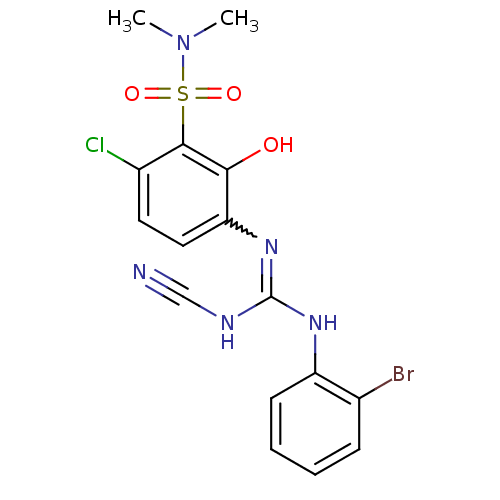
(3-{[[(2-bromophenyl)amino](cyanoimino)methyl]amino...)Show SMILES CN(C)S(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2Br)c1O |w:12.11| Show InChI InChI=1S/C16H15BrClN5O3S/c1-23(2)27(25,26)15-11(18)7-8-13(14(15)24)22-16(20-9-19)21-12-6-4-3-5-10(12)17/h3-8,24H,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193972

(3-(2-bromophenyl)-2-cyano-1-(2-hydroxy-4-methyl-3-...)Show SMILES CCC(CC)(NS(=O)(=O)c1c(C)ccc(N=C(NC#N)Nc2ccccc2Br)c1O)N1CCOCC1 |w:15.14| Show InChI InChI=1S/C24H31BrN6O4S/c1-4-24(5-2,31-12-14-35-15-13-31)30-36(33,34)22-17(3)10-11-20(21(22)32)29-23(27-16-26)28-19-9-7-6-8-18(19)25/h6-11,30,32H,4-5,12-15H2,1-3H3,(H2,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193971

(3-{[[(2-bromophenyl)amino](cyanoimino)methyl]amino...)Show SMILES CN(C)S(=O)(=O)c1c(O)c(ccc1C(F)(F)F)N=C(NC#N)Nc1ccccc1Br |w:17.17| Show InChI InChI=1S/C17H15BrF3N5O3S/c1-26(2)30(28,29)15-10(17(19,20)21)7-8-13(14(15)27)25-16(23-9-22)24-12-6-4-3-5-11(12)18/h3-8,27H,1-2H3,(H2,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193980
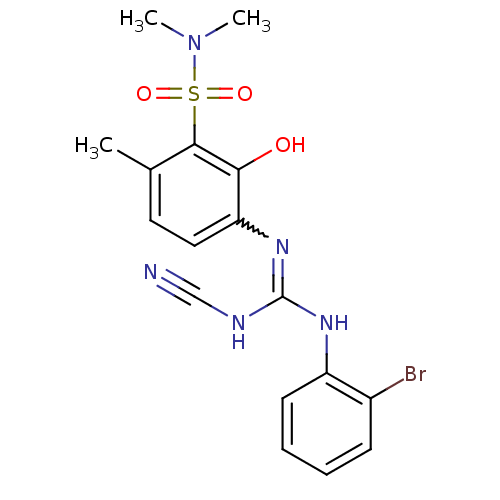
(3-{[[(2-bromophenyl)amino](cyanoimino)methyl]amino...)Show SMILES CN(C)S(=O)(=O)c1c(C)ccc(N=C(NC#N)Nc2ccccc2Br)c1O |w:12.11| Show InChI InChI=1S/C17H18BrN5O3S/c1-11-8-9-14(15(24)16(11)27(25,26)23(2)3)22-17(20-10-19)21-13-7-5-4-6-12(13)18/h4-9,24H,1-3H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193974
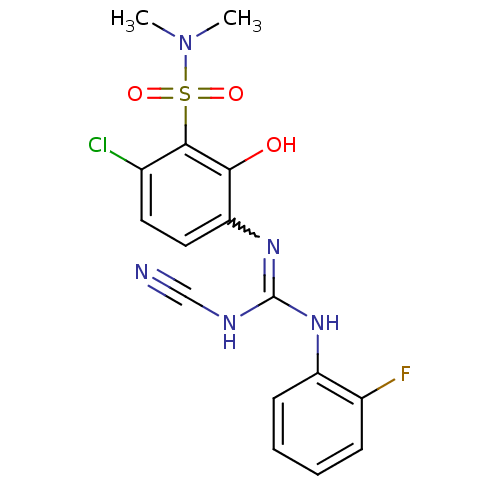
(6-chloro-3-({(cyanoimino)[(2-fluorophenyl)amino]me...)Show SMILES CN(C)S(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2F)c1O |w:12.11| Show InChI InChI=1S/C16H15ClFN5O3S/c1-23(2)27(25,26)15-10(17)7-8-13(14(15)24)22-16(20-9-19)21-12-6-4-3-5-11(12)18/h3-8,24H,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193978

(3-(2-bromophenyl)-1-[4-chloro-3-({3-[(2R,6S)-2,6-d...)Show SMILES CCC(CC)(NS(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2Br)c1O)N1C[C@H](C)O[C@H](C)C1 |w:15.14| Show InChI InChI=1S/C25H32BrClN6O4S/c1-5-25(6-2,33-13-16(3)37-17(4)14-33)32-38(35,36)23-19(27)11-12-21(22(23)34)31-24(29-15-28)30-20-10-8-7-9-18(20)26/h7-12,16-17,32,34H,5-6,13-14H2,1-4H3,(H2,29,30,31)/t16-,17+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193970
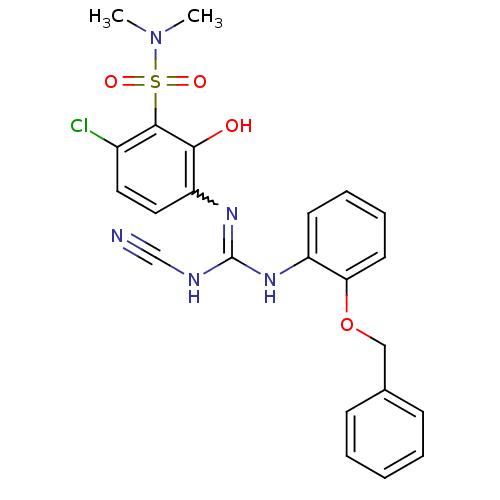
(3-[2-(benzyloxy)phenyl]-1-[4-chloro-3-(dimethylsul...)Show SMILES CN(C)S(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2OCc2ccccc2)c1O |w:12.11| Show InChI InChI=1S/C23H22ClN5O4S/c1-29(2)34(31,32)22-17(24)12-13-19(21(22)30)28-23(26-15-25)27-18-10-6-7-11-20(18)33-14-16-8-4-3-5-9-16/h3-13,30H,14H2,1-2H3,(H2,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193981
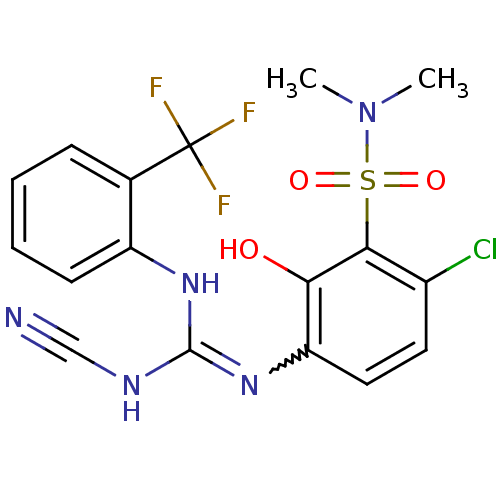
(6-chloro-3-[((cyanoimino){[2-(trifluoromethyl)phen...)Show SMILES CN(C)S(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2C(F)(F)F)c1O |w:12.11| Show InChI InChI=1S/C17H15ClF3N5O3S/c1-26(2)30(28,29)15-11(18)7-8-13(14(15)27)25-16(23-9-22)24-12-6-4-3-5-10(12)17(19,20)21/h3-8,27H,1-2H3,(H2,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193982
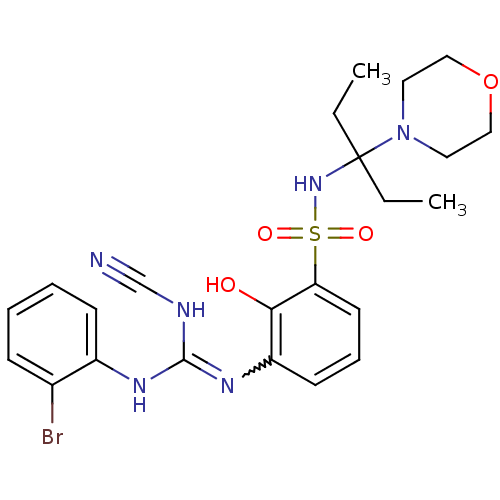
(3-(2-bromophenyl)-2-cyano-1-(2-hydroxy-3-{[3-(morp...)Show SMILES CCC(CC)(NS(=O)(=O)c1cccc(N=C(NC#N)Nc2ccccc2Br)c1O)N1CCOCC1 |w:14.13| Show InChI InChI=1S/C23H29BrN6O4S/c1-3-23(4-2,30-12-14-34-15-13-30)29-35(32,33)20-11-7-10-19(21(20)31)28-22(26-16-25)27-18-9-6-5-8-17(18)24/h5-11,29,31H,3-4,12-15H2,1-2H3,(H2,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193977
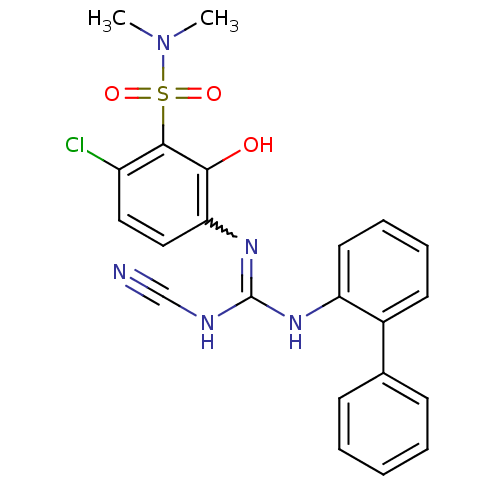
(1-[4-chloro-3-(dimethylsulfamoyl)-2-hydroxyphenyl]...)Show SMILES CN(C)S(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2-c2ccccc2)c1O |w:12.11| Show InChI InChI=1S/C22H20ClN5O3S/c1-28(2)32(30,31)21-17(23)12-13-19(20(21)29)27-22(25-14-24)26-18-11-7-6-10-16(18)15-8-4-3-5-9-15/h3-13,29H,1-2H3,(H2,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193984

(6-chloro-3-({(cyanoimino)[(2-ethylphenyl)amino]met...)Show SMILES CCc1ccccc1NC(NC#N)=Nc1ccc(Cl)c(c1O)S(=O)(=O)N(C)C |w:13.14| Show InChI InChI=1S/C18H20ClN5O3S/c1-4-12-7-5-6-8-14(12)22-18(21-11-20)23-15-10-9-13(19)17(16(15)25)28(26,27)24(2)3/h5-10,25H,4H2,1-3H3,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193973
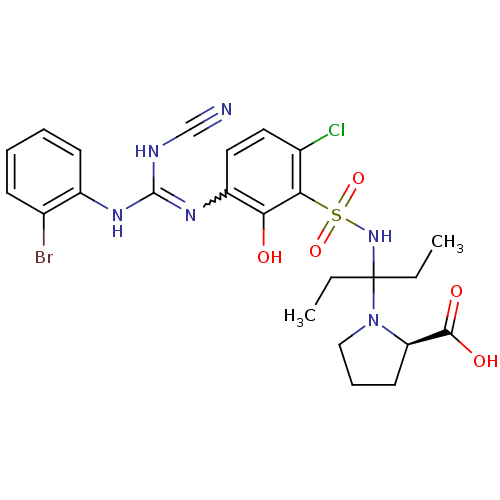
((R)-1-(3-(3-(3-(2-bromophenyl)-2-cyanoguanidino)-6...)Show SMILES CCC(CC)(NS(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2Br)c1O)N1CCC[C@@H]1C(O)=O |w:15.14| Show InChI InChI=1S/C24H28BrClN6O5S/c1-3-24(4-2,32-13-7-10-19(32)22(34)35)31-38(36,37)21-16(26)11-12-18(20(21)33)30-23(28-14-27)29-17-9-6-5-8-15(17)25/h5-6,8-9,11-12,19,31,33H,3-4,7,10,13H2,1-2H3,(H,34,35)(H2,28,29,30)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193976
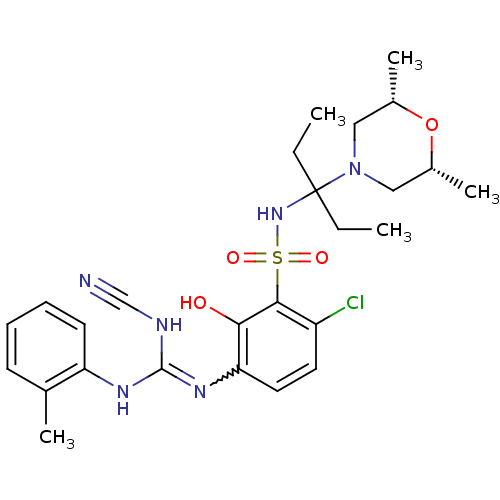
(1-[4-chloro-3-({3-[(2R,6S)-2,6-dimethylmorpholin-4...)Show SMILES CCC(CC)(NS(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2C)c1O)N1C[C@H](C)O[C@H](C)C1 |w:15.14| Show InChI InChI=1S/C26H35ClN6O4S/c1-6-26(7-2,33-14-18(4)37-19(5)15-33)32-38(35,36)24-20(27)12-13-22(23(24)34)31-25(29-16-28)30-21-11-9-8-10-17(21)3/h8-13,18-19,32,34H,6-7,14-15H2,1-5H3,(H2,29,30,31)/t18-,19+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193986

(6-chloro-3-({(cyanoimino)[(2-methylphenyl)amino]me...)Show SMILES CN(C)S(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2C)c1O |w:12.11| Show InChI InChI=1S/C17H18ClN5O3S/c1-11-6-4-5-7-13(11)21-17(20-10-19)22-14-9-8-12(18)16(15(14)24)27(25,26)23(2)3/h4-9,24H,1-3H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193973

((R)-1-(3-(3-(3-(2-bromophenyl)-2-cyanoguanidino)-6...)Show SMILES CCC(CC)(NS(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2Br)c1O)N1CCC[C@@H]1C(O)=O |w:15.14| Show InChI InChI=1S/C24H28BrClN6O5S/c1-3-24(4-2,32-13-7-10-19(32)22(34)35)31-38(36,37)21-16(26)11-12-18(20(21)33)30-23(28-14-27)29-17-9-6-5-8-15(17)25/h5-6,8-9,11-12,19,31,33H,3-4,7,10,13H2,1-2H3,(H,34,35)(H2,28,29,30)/t19-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193969
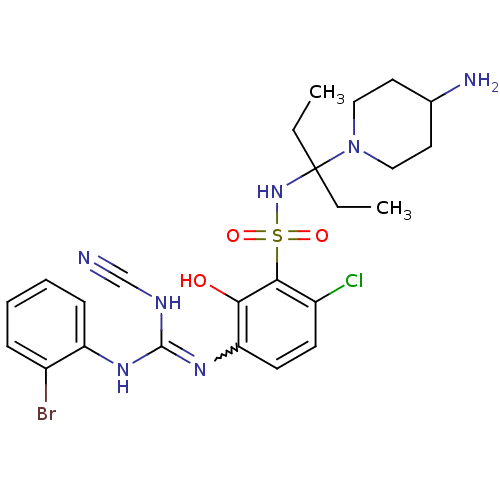
(1-(3-{[3-(4-aminopiperidin-1-yl)pentan-3-yl]sulfam...)Show SMILES CCC(CC)(NS(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2Br)c1O)N1CCC(N)CC1 |w:15.14| Show InChI InChI=1S/C24H31BrClN7O3S/c1-3-24(4-2,33-13-11-16(28)12-14-33)32-37(35,36)22-18(26)9-10-20(21(22)34)31-23(29-15-27)30-19-8-6-5-7-17(19)25/h5-10,16,32,34H,3-4,11-14,28H2,1-2H3,(H2,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193975

(6-chloro-3-{[[(3-chloro-2-fluorophenyl)amino](cyan...)Show SMILES CN(C)S(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2cccc(Cl)c2F)c1O |w:12.11| Show InChI InChI=1S/C16H14Cl2FN5O3S/c1-24(2)28(26,27)15-10(18)6-7-12(14(15)25)23-16(21-8-20)22-11-5-3-4-9(17)13(11)19/h3-7,25H,1-2H3,(H2,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193979

(3-{[[(2-bromophenyl)amino](cyanoimino)methyl]amino...)Show SMILES CN(C)S(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2Br)c1O |w:12.11| Show InChI InChI=1S/C16H15BrClN5O3S/c1-23(2)27(25,26)15-11(18)7-8-13(14(15)24)22-16(20-9-19)21-12-6-4-3-5-10(12)17/h3-8,24H,1-2H3,(H2,20,21,22) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50369323
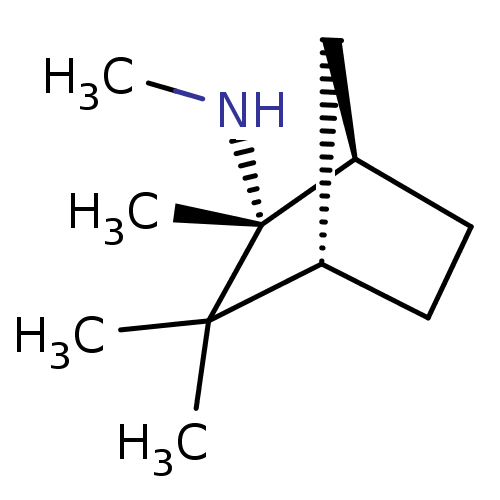
(MECAMYLAMINE)Show InChI InChI=1S/C11H21N/c1-10(2)8-5-6-9(7-8)11(10,3)12-4/h8-9,12H,5-7H2,1-4H3/t8-,9+,11+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Inhibitory activity against nicotinic acetylcholine receptor alpha3-beta4 |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193971

(3-{[[(2-bromophenyl)amino](cyanoimino)methyl]amino...)Show SMILES CN(C)S(=O)(=O)c1c(O)c(ccc1C(F)(F)F)N=C(NC#N)Nc1ccccc1Br |w:17.17| Show InChI InChI=1S/C17H15BrF3N5O3S/c1-26(2)30(28,29)15-10(17(19,20)21)7-8-13(14(15)27)25-16(23-9-22)24-12-6-4-3-5-11(12)18/h3-8,27H,1-2H3,(H2,23,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193972

(3-(2-bromophenyl)-2-cyano-1-(2-hydroxy-4-methyl-3-...)Show SMILES CCC(CC)(NS(=O)(=O)c1c(C)ccc(N=C(NC#N)Nc2ccccc2Br)c1O)N1CCOCC1 |w:15.14| Show InChI InChI=1S/C24H31BrN6O4S/c1-4-24(5-2,31-12-14-35-15-13-31)30-36(33,34)22-17(3)10-11-20(21(22)32)29-23(27-16-26)28-19-9-7-6-8-18(19)25/h6-11,30,32H,4-5,12-15H2,1-3H3,(H2,27,28,29) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50193983

(1-[4-chloro-3-(dimethylsulfamoyl)-2-hydroxyphenyl]...)Show SMILES CC(C)c1ccccc1NC(NC#N)=Nc1ccc(Cl)c(c1O)S(=O)(=O)N(C)C |w:14.15| Show InChI InChI=1S/C19H22ClN5O3S/c1-12(2)13-7-5-6-8-15(13)23-19(22-11-21)24-16-10-9-14(20)18(17(16)26)29(27,28)25(3)4/h5-10,12,26H,1-4H3,(H2,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR2 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193980

(3-{[[(2-bromophenyl)amino](cyanoimino)methyl]amino...)Show SMILES CN(C)S(=O)(=O)c1c(C)ccc(N=C(NC#N)Nc2ccccc2Br)c1O |w:12.11| Show InChI InChI=1S/C17H18BrN5O3S/c1-11-8-9-14(15(24)16(11)27(25,26)23(2)3)22-17(20-10-19)21-13-7-5-4-6-12(13)18/h4-9,24H,1-3H3,(H2,20,21,22) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193969

(1-(3-{[3-(4-aminopiperidin-1-yl)pentan-3-yl]sulfam...)Show SMILES CCC(CC)(NS(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2Br)c1O)N1CCC(N)CC1 |w:15.14| Show InChI InChI=1S/C24H31BrClN7O3S/c1-3-24(4-2,33-13-11-16(28)12-14-33)32-37(35,36)22-18(26)9-10-20(21(22)34)31-23(29-15-27)30-19-8-6-5-7-17(19)25/h5-10,16,32,34H,3-4,11-14,28H2,1-2H3,(H2,29,30,31) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193985

(3-{[[(2-bromophenyl)amino](cyanoimino)methyl]amino...)Show SMILES CN(C)S(=O)(=O)c1c(F)ccc(N=C(NC#N)Nc2ccccc2Br)c1O |w:12.11| Show InChI InChI=1S/C16H15BrFN5O3S/c1-23(2)27(25,26)15-11(18)7-8-13(14(15)24)22-16(20-9-19)21-12-6-4-3-5-10(12)17/h3-8,24H,1-2H3,(H2,20,21,22) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 192 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193978

(3-(2-bromophenyl)-1-[4-chloro-3-({3-[(2R,6S)-2,6-d...)Show SMILES CCC(CC)(NS(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2Br)c1O)N1C[C@H](C)O[C@H](C)C1 |w:15.14| Show InChI InChI=1S/C25H32BrClN6O4S/c1-5-25(6-2,33-13-16(3)37-17(4)14-33)32-38(35,36)23-19(27)11-12-21(22(23)34)31-24(29-15-28)30-20-10-8-7-9-18(20)26/h7-12,16-17,32,34H,5-6,13-14H2,1-4H3,(H2,29,30,31)/t16-,17+ | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193970

(3-[2-(benzyloxy)phenyl]-1-[4-chloro-3-(dimethylsul...)Show SMILES CN(C)S(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2OCc2ccccc2)c1O |w:12.11| Show InChI InChI=1S/C23H22ClN5O4S/c1-29(2)34(31,32)22-17(24)12-13-19(21(22)30)28-23(26-15-25)27-18-10-6-7-11-20(18)33-14-16-8-4-3-5-9-16/h3-13,30H,14H2,1-2H3,(H2,26,27,28) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 293 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193986

(6-chloro-3-({(cyanoimino)[(2-methylphenyl)amino]me...)Show SMILES CN(C)S(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2C)c1O |w:12.11| Show InChI InChI=1S/C17H18ClN5O3S/c1-11-6-4-5-7-13(11)21-17(20-10-19)22-14-9-8-12(18)16(15(14)24)27(25,26)23(2)3/h4-9,24H,1-3H3,(H2,20,21,22) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 378 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193981

(6-chloro-3-[((cyanoimino){[2-(trifluoromethyl)phen...)Show SMILES CN(C)S(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2C(F)(F)F)c1O |w:12.11| Show InChI InChI=1S/C17H15ClF3N5O3S/c1-26(2)30(28,29)15-11(18)7-8-13(14(15)27)25-16(23-9-22)24-12-6-4-3-5-10(12)17(19,20)21/h3-8,27H,1-2H3,(H2,23,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193984

(6-chloro-3-({(cyanoimino)[(2-ethylphenyl)amino]met...)Show SMILES CCc1ccccc1NC(NC#N)=Nc1ccc(Cl)c(c1O)S(=O)(=O)N(C)C |w:13.14| Show InChI InChI=1S/C18H20ClN5O3S/c1-4-12-7-5-6-8-14(12)22-18(21-11-20)23-15-10-9-13(19)17(16(15)25)28(26,27)24(2)3/h5-10,25H,4H2,1-3H3,(H2,21,22,23) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 512 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193982

(3-(2-bromophenyl)-2-cyano-1-(2-hydroxy-3-{[3-(morp...)Show SMILES CCC(CC)(NS(=O)(=O)c1cccc(N=C(NC#N)Nc2ccccc2Br)c1O)N1CCOCC1 |w:14.13| Show InChI InChI=1S/C23H29BrN6O4S/c1-3-23(4-2,30-12-14-34-15-13-30)29-35(32,33)20-11-7-10-19(21(20)31)28-22(26-16-25)27-18-9-6-5-8-17(18)24/h5-11,29,31H,3-4,12-15H2,1-2H3,(H2,26,27,28) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 582 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193977

(1-[4-chloro-3-(dimethylsulfamoyl)-2-hydroxyphenyl]...)Show SMILES CN(C)S(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2-c2ccccc2)c1O |w:12.11| Show InChI InChI=1S/C22H20ClN5O3S/c1-28(2)32(30,31)21-17(23)12-13-19(20(21)29)27-22(25-14-24)26-18-11-7-6-10-16(18)15-8-4-3-5-9-15/h3-13,29H,1-2H3,(H2,25,26,27) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 752 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193976

(1-[4-chloro-3-({3-[(2R,6S)-2,6-dimethylmorpholin-4...)Show SMILES CCC(CC)(NS(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2C)c1O)N1C[C@H](C)O[C@H](C)C1 |w:15.14| Show InChI InChI=1S/C26H35ClN6O4S/c1-6-26(7-2,33-14-18(4)37-19(5)15-33)32-38(35,36)24-20(27)12-13-22(23(24)34)31-25(29-16-28)30-21-11-9-8-10-17(21)3/h8-13,18-19,32,34H,6-7,14-15H2,1-5H3,(H2,29,30,31)/t18-,19+ | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50451971
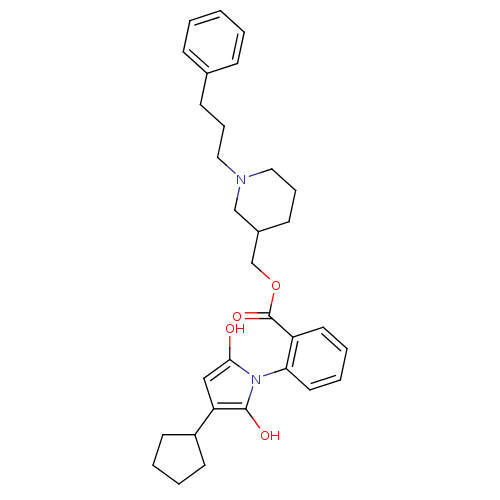
(CHEMBL330867)Show SMILES O=C(OCC1CCCN(CCCc2ccccc2)C1)c1ccccc1N1C(=O)CC(C2CCCC2)C1=O Show InChI InChI=1S/C31H38N2O4/c34-29-20-27(25-14-4-5-15-25)30(35)33(29)28-17-7-6-16-26(28)31(36)37-22-24-13-9-19-32(21-24)18-8-12-23-10-2-1-3-11-23/h1-3,6-7,10-11,16-17,24-25,27H,4-5,8-9,12-15,18-22H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50149051

(2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-benzoic ac...)Show SMILES O=C(OCC1CCCN(CCCc2ccccc2)C1)c1ccccc1N1C(=O)c2ccccc2C1=O Show InChI InChI=1S/C30H30N2O4/c33-28-24-14-4-5-15-25(24)29(34)32(28)27-17-7-6-16-26(27)30(35)36-21-23-13-9-19-31(20-23)18-8-12-22-10-2-1-3-11-22/h1-7,10-11,14-17,23H,8-9,12-13,18-21H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50451972
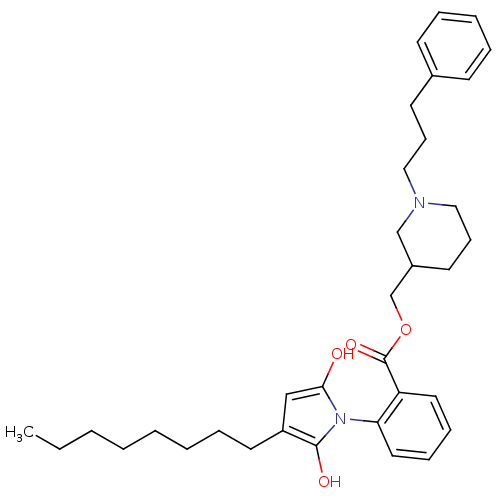
(CHEMBL125960)Show SMILES CCCCCCCCC1CC(=O)N(C1=O)c1ccccc1C(=O)OCC1CCCN(CCCc2ccccc2)C1 Show InChI InChI=1S/C34H46N2O4/c1-2-3-4-5-6-10-19-29-24-32(37)36(33(29)38)31-21-12-11-20-30(31)34(39)40-26-28-18-14-23-35(25-28)22-13-17-27-15-8-7-9-16-27/h7-9,11-12,15-16,20-21,28-29H,2-6,10,13-14,17-19,22-26H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50451974
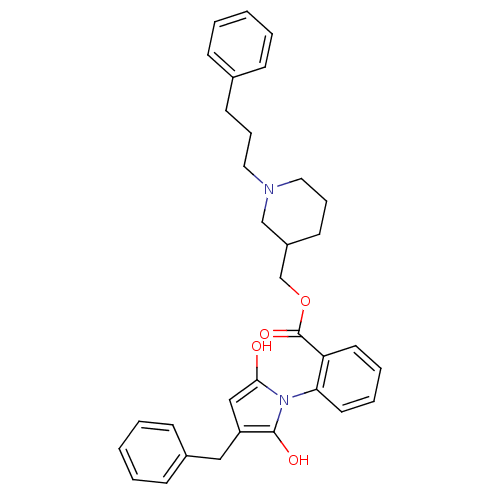
(CHEMBL340342)Show SMILES O=C(OCC1CCCN(CCCc2ccccc2)C1)c1ccccc1N1C(=O)CC(Cc2ccccc2)C1=O Show InChI InChI=1S/C33H36N2O4/c36-31-22-28(21-26-13-5-2-6-14-26)32(37)35(31)30-18-8-7-17-29(30)33(38)39-24-27-16-10-20-34(23-27)19-9-15-25-11-3-1-4-12-25/h1-8,11-14,17-18,27-28H,9-10,15-16,19-24H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50451977
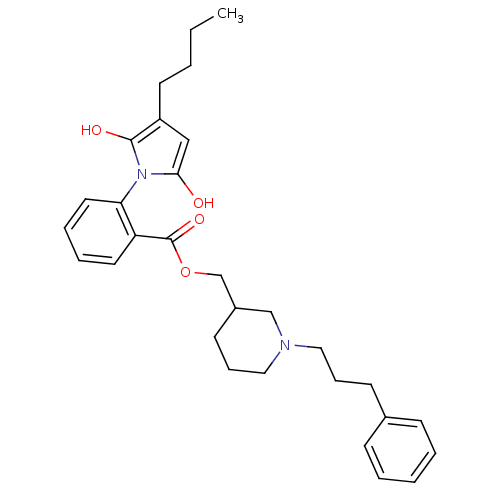
(CHEMBL122505)Show SMILES CCCCC1CC(=O)N(C1=O)c1ccccc1C(=O)OCC1CCCN(CCCc2ccccc2)C1 Show InChI InChI=1S/C30H38N2O4/c1-2-3-15-25-20-28(33)32(29(25)34)27-17-8-7-16-26(27)30(35)36-22-24-14-10-19-31(21-24)18-9-13-23-11-5-4-6-12-23/h4-8,11-12,16-17,24-25H,2-3,9-10,13-15,18-22H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50193974

(6-chloro-3-({(cyanoimino)[(2-fluorophenyl)amino]me...)Show SMILES CN(C)S(=O)(=O)c1c(Cl)ccc(N=C(NC#N)Nc2ccccc2F)c1O |w:12.11| Show InChI InChI=1S/C16H15ClFN5O3S/c1-23(2)27(25,26)15-10(17)7-8-13(14(15)24)22-16(20-9-19)21-12-6-4-3-5-11(12)18/h3-8,24H,1-2H3,(H2,20,21,22) | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]IL8 from CXCR1 expressed in CHO cells |
Bioorg Med Chem Lett 16: 5513-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.042
BindingDB Entry DOI: 10.7270/Q2NV9HWD |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50149040

(Biphenyl-2-carboxylic acid 1-(3-phenyl-propyl)-pip...)Show SMILES O=C(OCC1CCCN(CCCc2ccccc2)C1)c1ccccc1-c1ccccc1 Show InChI InChI=1S/C28H31NO2/c30-28(27-18-8-7-17-26(27)25-15-5-2-6-16-25)31-22-24-14-10-20-29(21-24)19-9-13-23-11-3-1-4-12-23/h1-8,11-12,15-18,24H,9-10,13-14,19-22H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50451973

(CHEMBL332769)Show SMILES C=CCC1CC(=O)N(C1=O)c1ccccc1C(=O)OCC1CCCN(CCCc2ccccc2)C1 Show InChI InChI=1S/C29H34N2O4/c1-2-10-24-19-27(32)31(28(24)33)26-16-7-6-15-25(26)29(34)35-21-23-14-9-18-30(20-23)17-8-13-22-11-4-3-5-12-22/h2-7,11-12,15-16,23-24H,1,8-10,13-14,17-21H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50366799
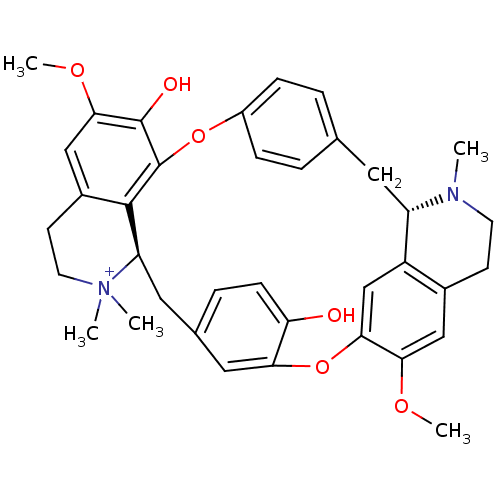
(TUBOCURARINE | TUBOCURARINE CHLORIDE)Show SMILES COc1cc2CC[N+](C)(C)[C@@H]3Cc4ccc(O)c(Oc5cc6[C@H](Cc7ccc(Oc(c1O)c23)cc7)N(C)CCc6cc5OC)c4 |r| Show InChI InChI=1S/C37H40N2O6/c1-38-14-12-24-19-32(42-4)33-21-27(24)28(38)16-22-6-9-26(10-7-22)44-37-35-25(20-34(43-5)36(37)41)13-15-39(2,3)29(35)17-23-8-11-30(40)31(18-23)45-33/h6-11,18-21,28-29H,12-17H2,1-5H3,(H-,40,41)/p+1/t28-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50451978
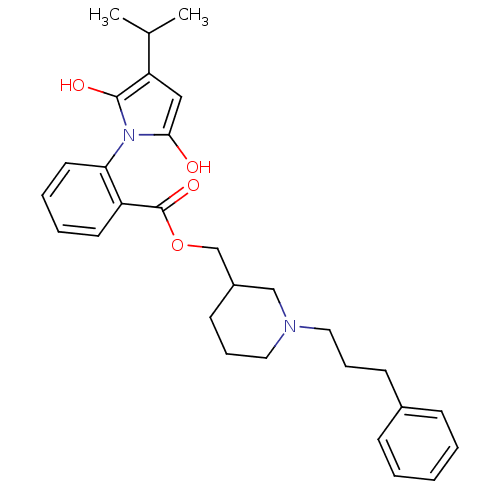
(CHEMBL334123)Show SMILES CC(C)C1CC(=O)N(C1=O)c1ccccc1C(=O)OCC1CCCN(CCCc2ccccc2)C1 Show InChI InChI=1S/C29H36N2O4/c1-21(2)25-18-27(32)31(28(25)33)26-15-7-6-14-24(26)29(34)35-20-23-13-9-17-30(19-23)16-8-12-22-10-4-3-5-11-22/h3-7,10-11,14-15,21,23,25H,8-9,12-13,16-20H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50366779

(METHYLLYCACONITINE)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)[C@@]34[C@@H]5C[C@H]6[C@H](OC)[C@@H]5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)[C@H]23)[C@H]14 |r,wU:4.4,48.54,44.48,42.46,36.39,39.43,wD:28.52,35.36,31.32,29.31,47.50,25.27,32.34,TLB:23:4:28:44.42,4:47:36.29.35:48,1:2:28:44.42,45:44:36.29.35:48,44:47:24.23.25:3.48.2,42:36:32:29.30,THB:37:36:32:29.30,40:39:32:29.30,5:4:28:44.42,(5.29,-1.99,;6.48,-1.02,;7.91,-1.57,;7.91,-4.71,;8.6,-3.19,;9.67,-4.27,;9.27,-5.75,;10.35,-6.84,;11.83,-6.45,;9.94,-8.32,;8.46,-8.7,;8.06,-10.18,;9.14,-11.27,;10.63,-10.87,;11.02,-9.39,;12.51,-8.99,;13.7,-9.95,;13.62,-11.48,;14.98,-9.12,;14.58,-7.64,;15.55,-6.45,;13.05,-7.56,;11.52,-7.55,;7.27,-2.43,;7.27,-.88,;8.6,-.11,;8.59,1.42,;7.27,2.19,;9.93,-.88,;11.13,.09,;10.97,1.62,;12.38,2.24,;13.41,1.1,;17.14,1.1,;17.91,-.25,;12.64,-.24,;13.31,-1.62,;14.39,-2.7,;15.18,-1.64,;15.11,2.18,;16.19,3.26,;15.8,4.74,;12.66,-3.02,;13.74,-4.1,;11.15,-3.38,;10.82,-4.87,;12.29,-5.26,;9.94,-2.42,;9.88,1.15,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22-,24+,25+,27-,28+,29-,30+,33-,34+,35-,36+,37+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50149039

(Biphenyl-4-carboxylic acid 1-(3-phenyl-propyl)-pip...)Show SMILES O=C(OCC1CCCN(CCCc2ccccc2)C1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C28H31NO2/c30-28(27-17-15-26(16-18-27)25-13-5-2-6-14-25)31-22-24-12-8-20-29(21-24)19-7-11-23-9-3-1-4-10-23/h1-6,9-10,13-18,24H,7-8,11-12,19-22H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50149050
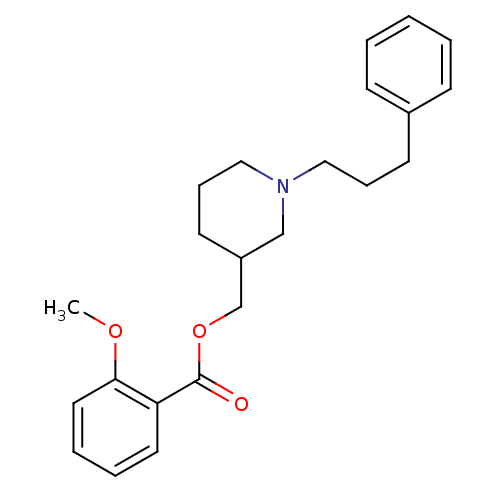
(2-Methoxy-benzoic acid 1-(3-phenyl-propyl)-piperid...)Show InChI InChI=1S/C23H29NO3/c1-26-22-14-6-5-13-21(22)23(25)27-18-20-12-8-16-24(17-20)15-7-11-19-9-3-2-4-10-19/h2-6,9-10,13-14,20H,7-8,11-12,15-18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50149043
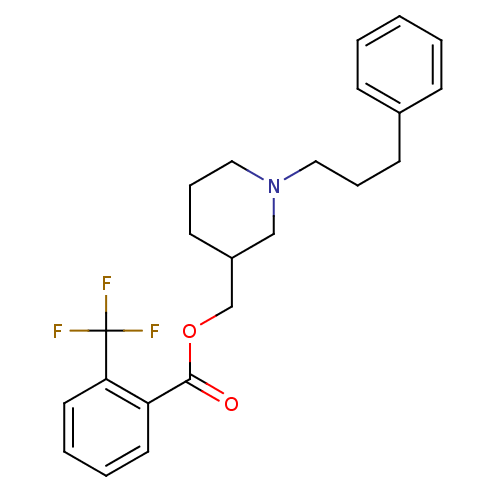
(2-Trifluoromethyl-benzoic acid 1-(3-phenyl-propyl)...)Show SMILES FC(F)(F)c1ccccc1C(=O)OCC1CCCN(CCCc2ccccc2)C1 Show InChI InChI=1S/C23H26F3NO2/c24-23(25,26)21-13-5-4-12-20(21)22(28)29-17-19-11-7-15-27(16-19)14-6-10-18-8-2-1-3-9-18/h1-5,8-9,12-13,19H,6-7,10-11,14-17H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3/beta-4
(Homo sapiens (Human)) | BDBM50149041
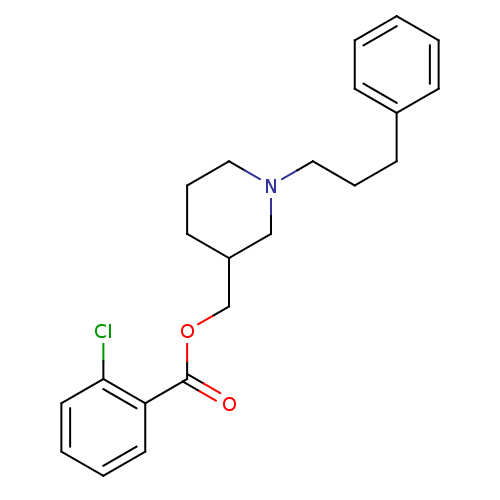
(2-Chloro-benzoic acid 1-(3-phenyl-propyl)-piperidi...)Show InChI InChI=1S/C22H26ClNO2/c23-21-13-5-4-12-20(21)22(25)26-17-19-11-7-15-24(16-19)14-6-10-18-8-2-1-3-9-18/h1-5,8-9,12-13,19H,6-7,10-11,14-17H2 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ohio University
Curated by ChEMBL
| Assay Description
Effect on alpha3-beta4 neuronal nicotinic acetylcholine receptor-stimulated adrenal catecholamine secretion |
Bioorg Med Chem Lett 14: 3739-42 (2004)
Article DOI: 10.1016/j.bmcl.2004.05.001
BindingDB Entry DOI: 10.7270/Q28G8M80 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data