 Found 2092 hits with Last Name = 'culshaw' and Initial = 'j'
Found 2092 hits with Last Name = 'culshaw' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151885

(1-[4-(2,2-Diphenyl-ethylamino)-3-(morpholine-4-car...)Show SMILES CN(C)CCCN(CCCN(C)C)C(=O)C1CCN(CC1)S(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)C(=O)N1CCOCC1 Show InChI InChI=1S/C41H58N6O5S/c1-43(2)21-11-23-45(24-12-22-44(3)4)40(48)35-19-25-47(26-20-35)53(50,51)36-17-18-39(37(31-36)41(49)46-27-29-52-30-28-46)42-32-38(33-13-7-5-8-14-33)34-15-9-6-10-16-34/h5-10,13-18,31,35,38,42H,11-12,19-30,32H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151884
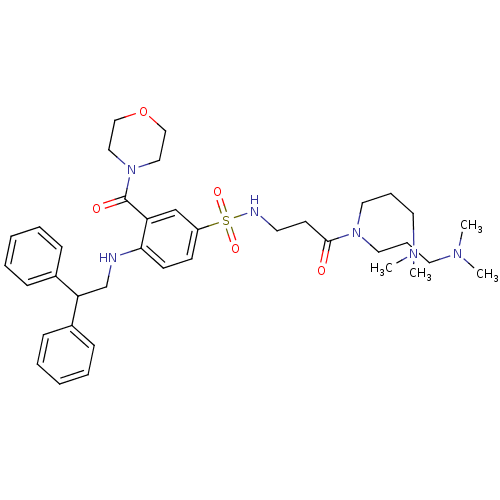
(CHEMBL185551 | N,N-Bis-(3-dimethylamino-propyl)-3-...)Show SMILES CN(C)CCCN(CCCN(C)C)C(=O)CCNS(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)C(=O)N1CCOCC1 Show InChI InChI=1S/C38H54N6O5S/c1-41(2)21-11-23-43(24-12-22-42(3)4)37(45)19-20-40-50(47,48)33-17-18-36(34(29-33)38(46)44-25-27-49-28-26-44)39-30-35(31-13-7-5-8-14-31)32-15-9-6-10-16-32/h5-10,13-18,29,35,39-40H,11-12,19-28,30H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151886
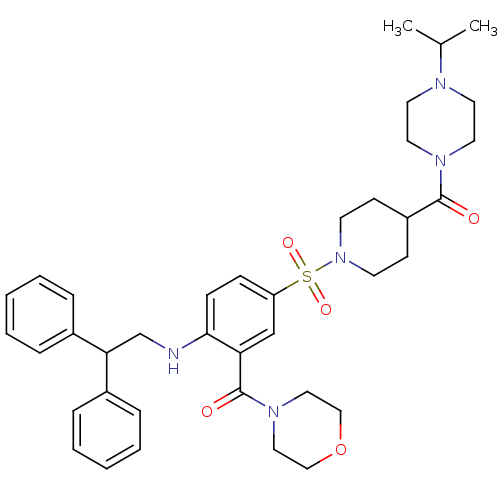
(CHEMBL181695 | {2-(2,2-Diphenyl-ethylamino)-5-[4-(...)Show SMILES CC(C)N1CCN(CC1)C(=O)C1CCN(CC1)S(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)C(=O)N1CCOCC1 Show InChI InChI=1S/C38H49N5O5S/c1-29(2)40-19-21-41(22-20-40)37(44)32-15-17-43(18-16-32)49(46,47)33-13-14-36(34(27-33)38(45)42-23-25-48-26-24-42)39-28-35(30-9-5-3-6-10-30)31-11-7-4-8-12-31/h3-14,27,29,32,35,39H,15-26,28H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151894
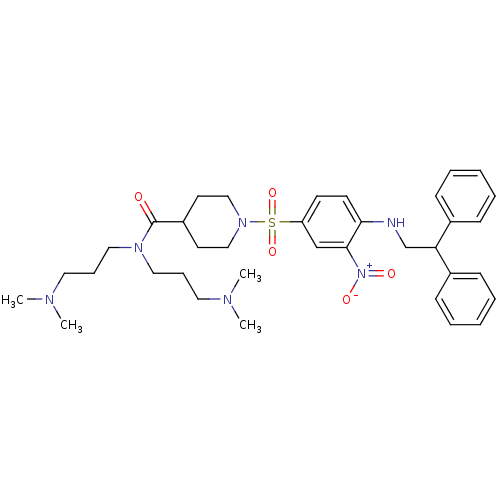
(1-[4-(2,2-Diphenyl-ethylamino)-3-nitro-benzenesulf...)Show SMILES CN(C)CCCN(CCCN(C)C)C(=O)C1CCN(CC1)S(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C36H50N6O5S/c1-38(2)21-11-23-40(24-12-22-39(3)4)36(43)31-19-25-41(26-20-31)48(46,47)32-17-18-34(35(27-32)42(44)45)37-28-33(29-13-7-5-8-14-29)30-15-9-6-10-16-30/h5-10,13-18,27,31,33,37H,11-12,19-26,28H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151891
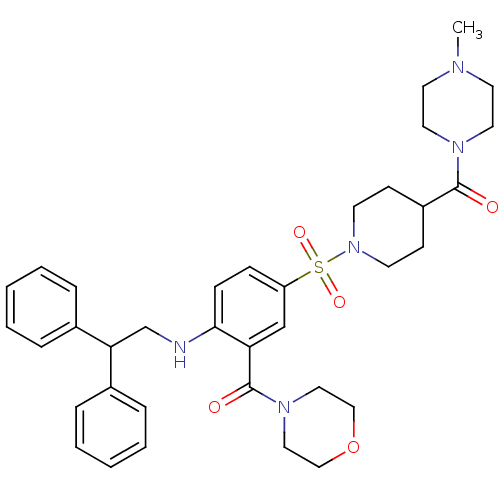
(CHEMBL273869 | {2-(2,2-Diphenyl-ethylamino)-5-[4-(...)Show SMILES CN1CCN(CC1)C(=O)C1CCN(CC1)S(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)C(=O)N1CCOCC1 Show InChI InChI=1S/C36H45N5O5S/c1-38-18-20-39(21-19-38)35(42)30-14-16-41(17-15-30)47(44,45)31-12-13-34(32(26-31)36(43)40-22-24-46-25-23-40)37-27-33(28-8-4-2-5-9-28)29-10-6-3-7-11-29/h2-13,26,30,33,37H,14-25,27H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151888
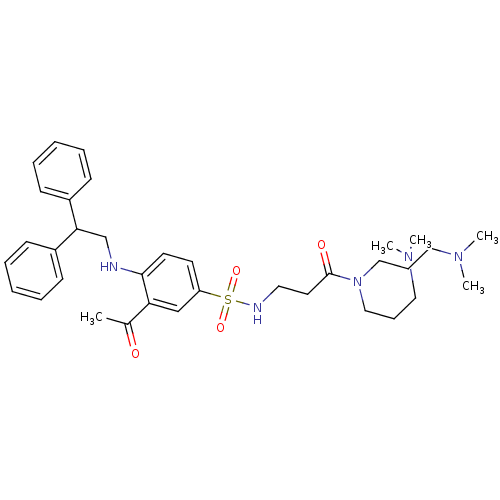
(3-[3-Acetyl-4-(2,2-diphenyl-ethylamino)-benzenesul...)Show SMILES CN(C)CCCN(CCCN(C)C)C(=O)CCNS(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)C(C)=O Show InChI InChI=1S/C35H49N5O4S/c1-28(41)32-26-31(18-19-34(32)36-27-33(29-14-8-6-9-15-29)30-16-10-7-11-17-30)45(43,44)37-21-20-35(42)40(24-12-22-38(2)3)25-13-23-39(4)5/h6-11,14-19,26,33,36-37H,12-13,20-25,27H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151889
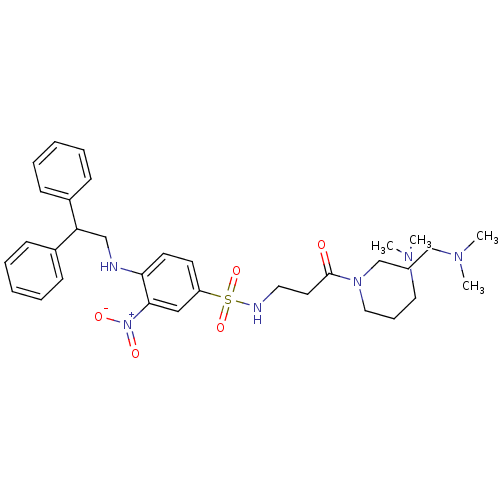
(CHEMBL180143 | N,N-Bis-(3-dimethylamino-propyl)-3-...)Show SMILES CN(C)CCCN(CCCN(C)C)C(=O)CCNS(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C33H46N6O5S/c1-36(2)21-11-23-38(24-12-22-37(3)4)33(40)19-20-35-45(43,44)29-17-18-31(32(25-29)39(41)42)34-26-30(27-13-7-5-8-14-27)28-15-9-6-10-16-28/h5-10,13-18,25,30,34-35H,11-12,19-24,26H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151890
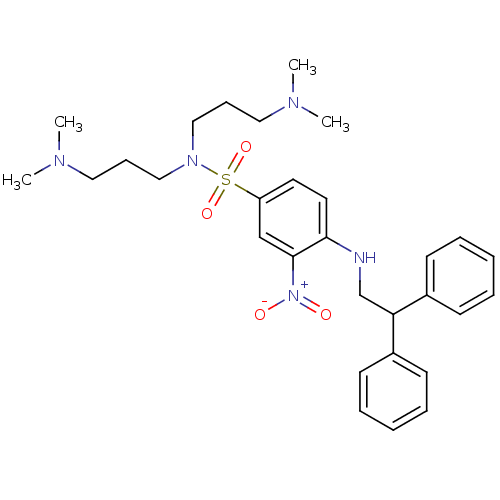
(CHEMBL181098 | N,N-Bis-(3-dimethylamino-propyl)-4-...)Show SMILES CN(C)CCCN(CCCN(C)C)S(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C30H41N5O4S/c1-32(2)19-11-21-34(22-12-20-33(3)4)40(38,39)27-17-18-29(30(23-27)35(36)37)31-24-28(25-13-7-5-8-14-25)26-15-9-6-10-16-26/h5-10,13-18,23,28,31H,11-12,19-22,24H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151893
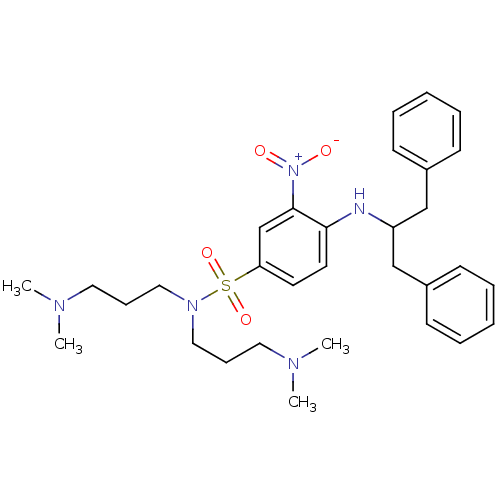
(4-(1-Benzyl-2-phenyl-ethylamino)-N,N-bis-(3-dimeth...)Show SMILES CN(C)CCCN(CCCN(C)C)S(=O)(=O)c1ccc(NC(Cc2ccccc2)Cc2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C31H43N5O4S/c1-33(2)19-11-21-35(22-12-20-34(3)4)41(39,40)29-17-18-30(31(25-29)36(37)38)32-28(23-26-13-7-5-8-14-26)24-27-15-9-6-10-16-27/h5-10,13-18,25,28,32H,11-12,19-24H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151892
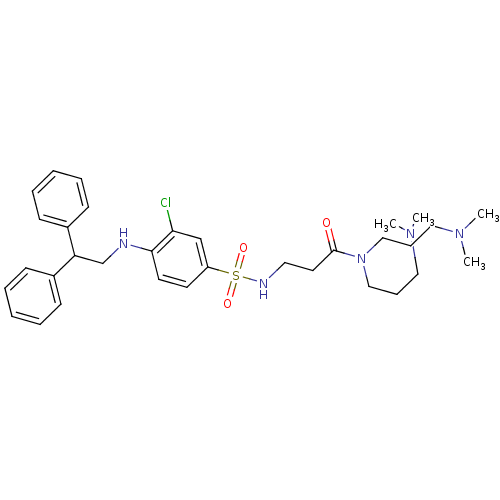
(3-[3-Chloro-4-(2,2-diphenyl-ethylamino)-benzenesul...)Show SMILES CN(C)CCCN(CCCN(C)C)C(=O)CCNS(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(Cl)c1 Show InChI InChI=1S/C33H46ClN5O3S/c1-37(2)21-11-23-39(24-12-22-38(3)4)33(40)19-20-36-43(41,42)29-17-18-32(31(34)25-29)35-26-30(27-13-7-5-8-14-27)28-15-9-6-10-16-28/h5-10,13-18,25,30,35-36H,11-12,19-24,26H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151887
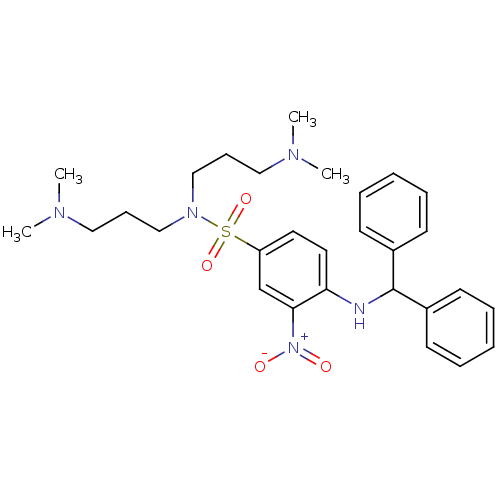
(4-(Benzhydryl-amino)-N,N-bis-(3-dimethylamino-prop...)Show SMILES CN(C)CCCN(CCCN(C)C)S(=O)(=O)c1ccc(NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C29H39N5O4S/c1-31(2)19-11-21-33(22-12-20-32(3)4)39(37,38)26-17-18-27(28(23-26)34(35)36)30-29(24-13-7-5-8-14-24)25-15-9-6-10-16-25/h5-10,13-18,23,29-30H,11-12,19-22H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B2 expressed in COS7 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151887

(4-(Benzhydryl-amino)-N,N-bis-(3-dimethylamino-prop...)Show SMILES CN(C)CCCN(CCCN(C)C)S(=O)(=O)c1ccc(NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C29H39N5O4S/c1-31(2)19-11-21-33(22-12-20-32(3)4)39(37,38)26-17-18-27(28(23-26)34(35)36)30-29(24-13-7-5-8-14-24)25-15-9-6-10-16-25/h5-10,13-18,23,29-30H,11-12,19-22H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from human CB1 receptor expressed in HEK293 cells |
J Med Chem 50: 3851-6 (2007)
Article DOI: 10.1021/jm070317a
BindingDB Entry DOI: 10.7270/Q2K64JWR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50263028
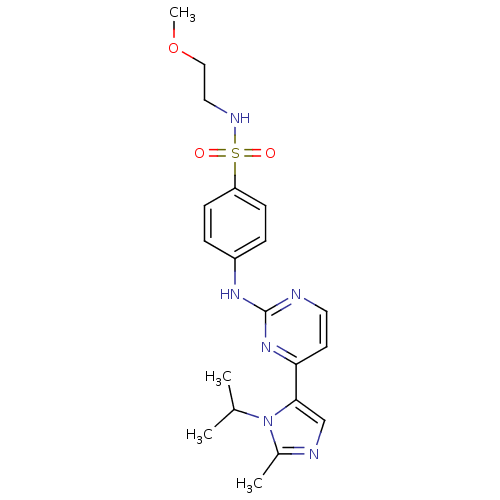
(4-(4-(1-isopropyl-2-methyl-1H-imidazol-5-yl)pyrimi...)Show SMILES COCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc(C)n2C(C)C)cc1 Show InChI InChI=1S/C20H26N6O3S/c1-14(2)26-15(3)22-13-19(26)18-9-10-21-20(25-18)24-16-5-7-17(8-6-16)30(27,28)23-11-12-29-4/h5-10,13-14,23H,11-12H2,1-4H3,(H,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 18: 5487-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.024
BindingDB Entry DOI: 10.7270/Q2J9667K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50072775

(2-((1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohe...)Show SMILES CCCCCCC(C)(C)c1ccc([C@@H]2C[C@H](O)CC[C@H]2CCCO)c(O)c1 |r| Show InChI InChI=1S/C24H40O3/c1-4-5-6-7-14-24(2,3)19-11-13-21(23(27)16-19)22-17-20(26)12-10-18(22)9-8-15-25/h11,13,16,18,20,22,25-27H,4-10,12,14-15,17H2,1-3H3/t18-,20-,22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 human CB2 receptor expressed in CHOK1 cells |
J Med Chem 50: 3851-6 (2007)
Article DOI: 10.1021/jm070317a
BindingDB Entry DOI: 10.7270/Q2K64JWR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50072961
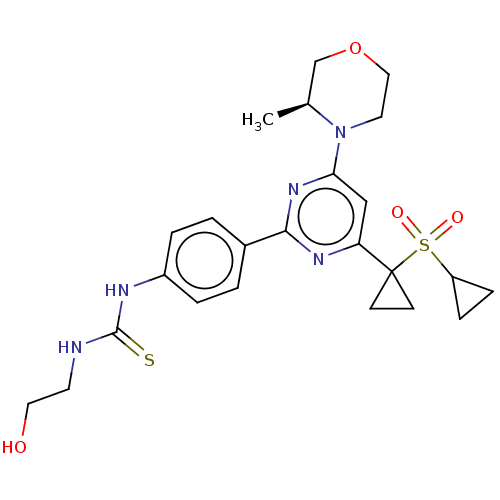
(CHEMBL3410672)Show SMILES C[C@H]1COCCN1c1cc(nc(n1)-c1ccc(NC(=S)NCCO)cc1)C1(CC1)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C24H31N5O4S2/c1-16-15-33-13-11-29(16)21-14-20(24(8-9-24)35(31,32)19-6-7-19)27-22(28-21)17-2-4-18(5-3-17)26-23(34)25-10-12-30/h2-5,14,16,19,30H,6-13,15H2,1H3,(H2,25,26,34)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant truncated FLAG-tagged mTOR (1362 to 2549 aa) (unknown origin) expressed in HEK293 cells using biotinylated p70 peptide as s... |
J Med Chem 58: 2326-49 (2015)
Article DOI: 10.1021/jm501778s
BindingDB Entry DOI: 10.7270/Q2DF6SW8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7657
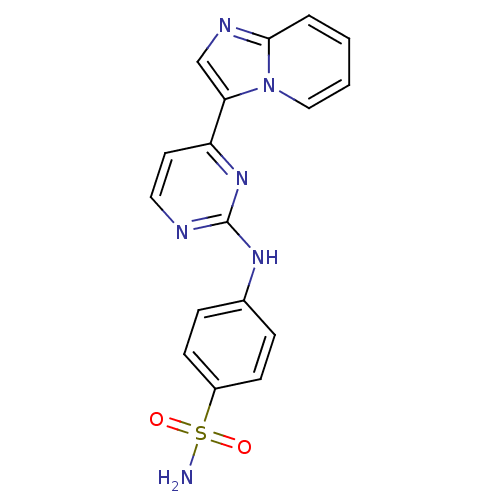
(4-[(4-{imidazo[1,2-a]pyridin-3-yl}pyrimidin-2-yl)a...)Show SMILES NS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3ccccn23)cc1 Show InChI InChI=1S/C17H14N6O2S/c18-26(24,25)13-6-4-12(5-7-13)21-17-19-9-8-14(22-17)15-11-20-16-3-1-2-10-23(15)16/h1-11H,(H2,18,24,25)(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 18: 5487-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.024
BindingDB Entry DOI: 10.7270/Q2J9667K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50246779

(CHEMBL4080574)Show SMILES CC(=O)N1CCN(CC1)[C@H]1CC[C@@H](CC1)Nc1ncnc2[nH]cc(C3CCOCC3)c12 |r,wU:12.16,wD:9.9,(19.88,-14.05,;18.55,-13.27,;18.56,-11.73,;17.21,-14.03,;17.2,-15.57,;15.87,-16.33,;14.54,-15.55,;14.54,-14.01,;15.88,-13.25,;13.21,-16.32,;11.88,-15.54,;10.54,-16.31,;10.55,-17.85,;11.88,-18.62,;13.2,-17.85,;9.22,-18.62,;9.22,-20.16,;10.55,-20.92,;10.56,-22.47,;9.22,-23.24,;7.89,-22.47,;6.42,-22.96,;5.51,-21.72,;6.41,-20.46,;5.92,-19,;6.94,-17.84,;6.46,-16.39,;4.95,-16.07,;3.93,-17.22,;4.41,-18.69,;7.88,-20.93,)| Show InChI InChI=1S/C23H34N6O2/c1-16(30)28-8-10-29(11-9-28)19-4-2-18(3-5-19)27-23-21-20(17-6-12-31-13-7-17)14-24-22(21)25-15-26-23/h14-15,17-19H,2-13H2,1H3,(H2,24,25,26,27)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human IRAK4 expressed in baculovirus expression system using 5-FAM-IPTSPITTTYFFFKKK-COOH as substrat... |
J Med Chem 60: 10071-10091 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01290
BindingDB Entry DOI: 10.7270/Q2T72KWX |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50246761
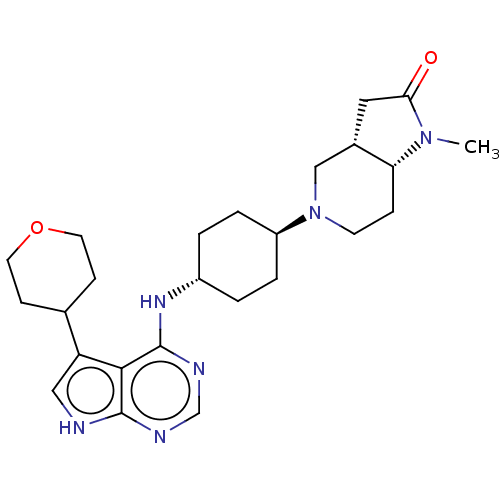
(CHEMBL4102501)Show SMILES [H][C@@]12CC(=O)N(C)[C@]1([H])CCN(C2)[C@H]1CC[C@@H](CC1)Nc1ncnc2[nH]cc(C3CCOCC3)c12 |r,wU:16.21,1.0,7.8,wD:13.14,(14.1,-14.84,;15.43,-15.62,;15.76,-14.11,;17.29,-13.95,;18.06,-12.62,;17.91,-15.36,;19.41,-15.68,;16.76,-16.39,;18.09,-17.15,;16.75,-17.93,;15.42,-18.69,;14.1,-17.92,;14.09,-16.38,;12.76,-18.68,;11.43,-17.91,;10.09,-18.68,;10.1,-20.22,;11.43,-20.99,;12.75,-20.22,;8.76,-20.99,;8.77,-22.53,;10.1,-23.29,;10.11,-24.84,;8.77,-25.61,;7.44,-24.84,;5.97,-25.33,;5.05,-24.09,;5.95,-22.83,;5.47,-21.37,;6.48,-20.21,;6,-18.76,;4.5,-18.44,;3.47,-19.59,;3.95,-21.06,;7.43,-23.3,)| Show InChI InChI=1S/C25H36N6O2/c1-30-21-6-9-31(14-17(21)12-22(30)32)19-4-2-18(3-5-19)29-25-23-20(16-7-10-33-11-8-16)13-26-24(23)27-15-28-25/h13,15-19,21H,2-12,14H2,1H3,(H2,26,27,28,29)/t17-,18-,19-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human IRAK4 expressed in baculovirus expression system using 5-FAM-IPTSPITTTYFFFKKK-COOH as substrat... |
J Med Chem 60: 10071-10091 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01290
BindingDB Entry DOI: 10.7270/Q2T72KWX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50246762

(CHEMBL4078222)Show SMILES [H][C@]12CC(=O)N(C)[C@@]1([H])CCN(C2)[C@H]1CC[C@@H](CC1)Nc1ncnc2[nH]cc(C3CCOCC3)c12 |r,wU:16.21,wD:13.14,1.0,7.8,(12.7,-12.69,;14.04,-13.46,;14.36,-11.96,;15.89,-11.8,;16.66,-10.47,;16.52,-13.21,;18.02,-13.53,;15.37,-14.23,;16.7,-15,;15.36,-15.78,;14.03,-16.54,;12.7,-15.76,;12.7,-14.22,;11.37,-16.53,;10.03,-15.76,;8.7,-16.52,;8.7,-18.06,;10.03,-18.83,;11.36,-18.07,;7.37,-18.84,;7.37,-20.37,;8.71,-21.14,;8.71,-22.69,;7.38,-23.46,;6.04,-22.69,;4.57,-23.17,;3.66,-21.93,;4.56,-20.67,;4.07,-19.21,;5.09,-18.06,;4.61,-16.6,;3.11,-16.29,;2.08,-17.44,;2.56,-18.9,;6.04,-21.14,)| Show InChI InChI=1S/C25H36N6O2/c1-30-21-6-9-31(14-17(21)12-22(30)32)19-4-2-18(3-5-19)29-25-23-20(16-7-10-33-11-8-16)13-26-24(23)27-15-28-25/h13,15-19,21H,2-12,14H2,1H3,(H2,26,27,28,29)/t17-,18-,19-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human IRAK4 expressed in baculovirus expression system using 5-FAM-IPTSPITTTYFFFKKK-COOH as substrat... |
J Med Chem 60: 10071-10091 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01290
BindingDB Entry DOI: 10.7270/Q2T72KWX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7647
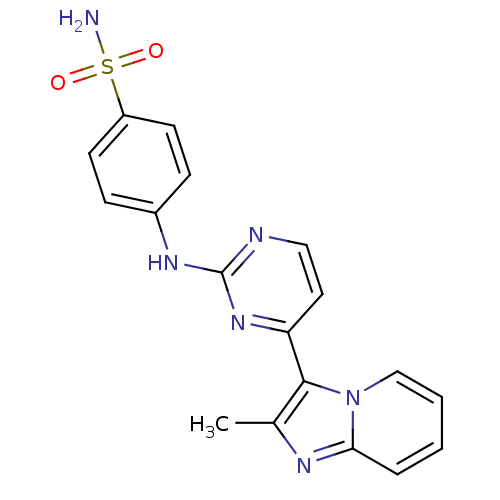
(4-[(4-{2-methylimidazo[1,2-a]pyridin-3-yl}pyrimidi...)Show SMILES Cc1nc2ccccn2c1-c1ccnc(Nc2ccc(cc2)S(N)(=O)=O)n1 Show InChI InChI=1S/C18H16N6O2S/c1-12-17(24-11-3-2-4-16(24)21-12)15-9-10-20-18(23-15)22-13-5-7-14(8-6-13)27(19,25)26/h2-11H,1H3,(H2,19,25,26)(H,20,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 13: 3021-6 (2003)
Article DOI: 10.1016/S0960-894X(03)00638-3
BindingDB Entry DOI: 10.7270/Q2610XJ5 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50246809
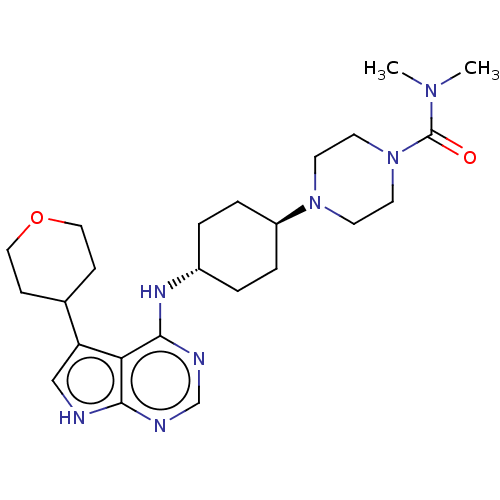
(CHEMBL4104802)Show SMILES CN(C)C(=O)N1CCN(CC1)[C@H]1CC[C@@H](CC1)Nc1ncnc2[nH]cc(C3CCOCC3)c12 |r,wU:14.18,wD:11.11,(20.28,-15.59,;20.29,-14.05,;21.63,-13.29,;18.96,-13.27,;18.97,-11.73,;17.62,-14.03,;17.61,-15.57,;16.28,-16.33,;14.96,-15.55,;14.95,-14.01,;16.3,-13.25,;13.62,-16.32,;12.29,-15.54,;10.95,-16.31,;10.96,-17.85,;12.29,-18.62,;13.62,-17.86,;9.63,-18.62,;9.63,-20.16,;10.96,-20.92,;10.97,-22.47,;9.63,-23.24,;8.3,-22.47,;6.83,-22.96,;5.92,-21.72,;6.82,-20.46,;6.33,-19,;7.35,-17.85,;6.87,-16.39,;5.36,-16.07,;4.34,-17.22,;4.82,-18.69,;8.29,-20.93,)| Show InChI InChI=1S/C24H37N7O2/c1-29(2)24(32)31-11-9-30(10-12-31)19-5-3-18(4-6-19)28-23-21-20(17-7-13-33-14-8-17)15-25-22(21)26-16-27-23/h15-19H,3-14H2,1-2H3,(H2,25,26,27,28)/t18-,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human IRAK4 expressed in baculovirus expression system using 5-FAM-IPTSPITTTYFFFKKK-COOH as substrat... |
J Med Chem 60: 10071-10091 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01290
BindingDB Entry DOI: 10.7270/Q2T72KWX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7658
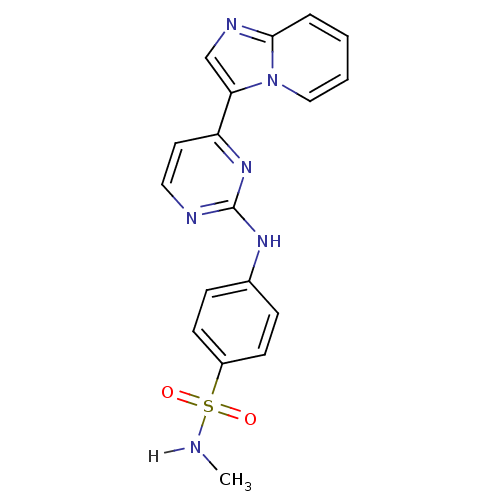
(4-[(4-{imidazo[1,2-a]pyridin-3-yl}pyrimidin-2-yl)a...)Show SMILES CNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3ccccn23)cc1 Show InChI InChI=1S/C18H16N6O2S/c1-19-27(25,26)14-7-5-13(6-8-14)22-18-20-10-9-15(23-18)16-12-21-17-4-2-3-11-24(16)17/h2-12,19H,1H3,(H,20,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.015
BindingDB Entry DOI: 10.7270/Q22805TT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7664
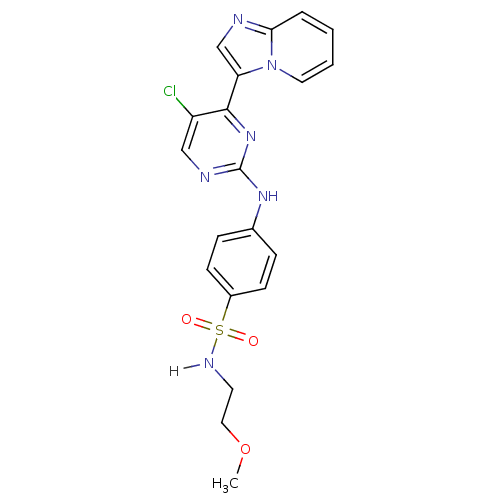
(4-[(5-chloro-4-{imidazo[1,2-a]pyridin-3-yl}pyrimid...)Show SMILES COCCNS(=O)(=O)c1ccc(Nc2ncc(Cl)c(n2)-c2cnc3ccccn23)cc1 Show InChI InChI=1S/C20H19ClN6O3S/c1-30-11-9-24-31(28,29)15-7-5-14(6-8-15)25-20-23-12-16(21)19(26-20)17-13-22-18-4-2-3-10-27(17)18/h2-8,10,12-13,24H,9,11H2,1H3,(H,23,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.015
BindingDB Entry DOI: 10.7270/Q22805TT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7665
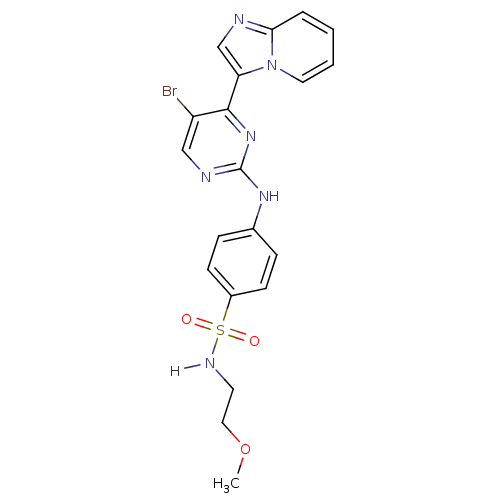
(4-[(5-bromo-4-{imidazo[1,2-a]pyridin-3-yl}pyrimidi...)Show SMILES COCCNS(=O)(=O)c1ccc(Nc2ncc(Br)c(n2)-c2cnc3ccccn23)cc1 Show InChI InChI=1S/C20H19BrN6O3S/c1-30-11-9-24-31(28,29)15-7-5-14(6-8-15)25-20-23-12-16(21)19(26-20)17-13-22-18-4-2-3-10-27(17)18/h2-8,10,12-13,24H,9,11H2,1H3,(H,23,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.015
BindingDB Entry DOI: 10.7270/Q22805TT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7667

(4-[(4-{6-bromoimidazo[1,2-a]pyridin-3-yl}pyrimidin...)Show SMILES COCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3ccc(Br)cn23)cc1 Show InChI InChI=1S/C20H19BrN6O3S/c1-30-11-10-24-31(28,29)16-5-3-15(4-6-16)25-20-22-9-8-17(26-20)18-12-23-19-7-2-14(21)13-27(18)19/h2-9,12-13,24H,10-11H2,1H3,(H,22,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7668
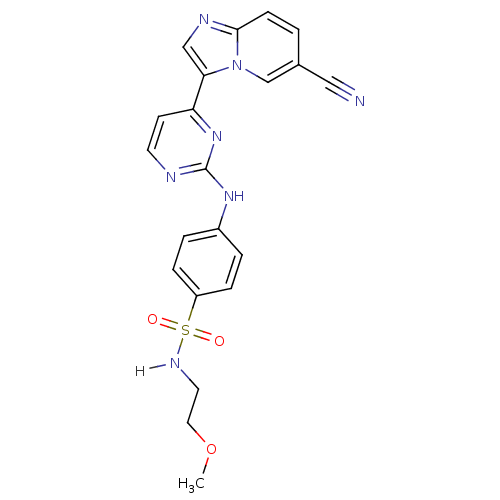
(4-[(4-{6-cyanoimidazo[1,2-a]pyridin-3-yl}pyrimidin...)Show SMILES COCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3ccc(cn23)C#N)cc1 Show InChI InChI=1S/C21H19N7O3S/c1-31-11-10-25-32(29,30)17-5-3-16(4-6-17)26-21-23-9-8-18(27-21)19-13-24-20-7-2-15(12-22)14-28(19)20/h2-9,13-14,25H,10-11H2,1H3,(H,23,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7669
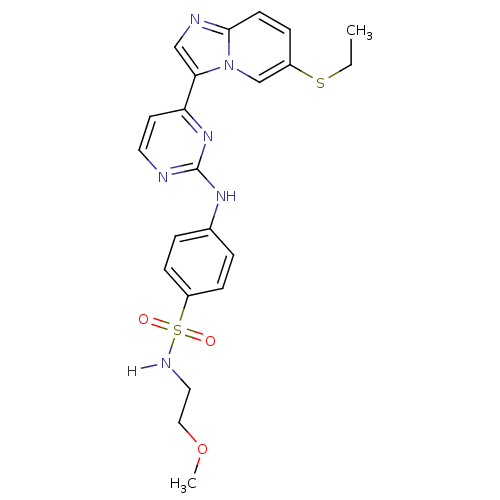
(4-({4-[6-(ethylsulfanyl)imidazo[1,2-a]pyridin-3-yl...)Show SMILES CCSc1ccc2ncc(-c3ccnc(Nc4ccc(cc4)S(=O)(=O)NCCOC)n3)n2c1 Show InChI InChI=1S/C22H24N6O3S2/c1-3-32-17-6-9-21-24-14-20(28(21)15-17)19-10-11-23-22(27-19)26-16-4-7-18(8-5-16)33(29,30)25-12-13-31-2/h4-11,14-15,25H,3,12-13H2,1-2H3,(H,23,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7671
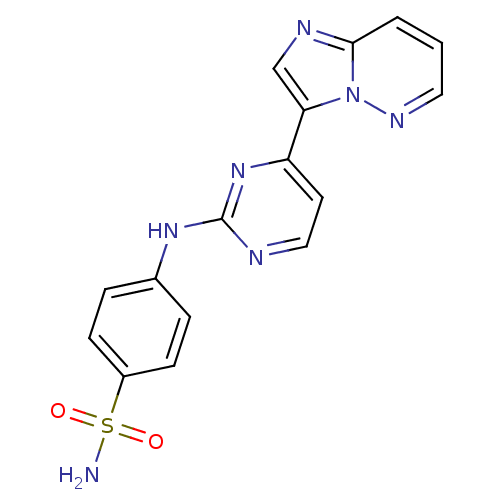
(4-[(4-{imidazo[1,2-a]pyridazin-3-yl}pyrimidin-2-yl...)Show SMILES NS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3cccnn23)cc1 Show InChI InChI=1S/C16H13N7O2S/c17-26(24,25)12-5-3-11(4-6-12)21-16-18-9-7-13(22-16)14-10-19-15-2-1-8-20-23(14)15/h1-10H,(H2,17,24,25)(H,18,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7672
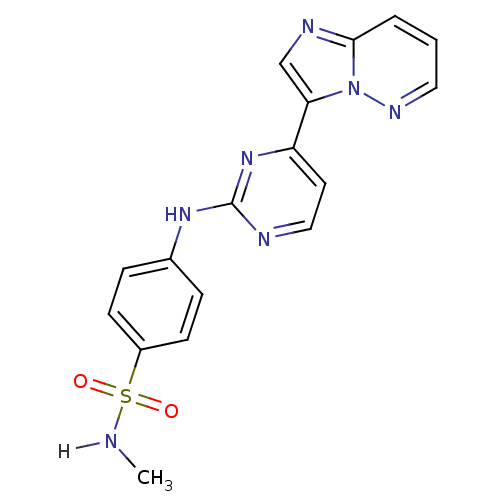
(4-[(4-{imidazo[1,2-a]pyridazin-3-yl}pyrimidin-2-yl...)Show SMILES CNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3cccnn23)cc1 Show InChI InChI=1S/C17H15N7O2S/c1-18-27(25,26)13-6-4-12(5-7-13)22-17-19-10-8-14(23-17)15-11-20-16-3-2-9-21-24(15)16/h2-11,18H,1H3,(H,19,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7673
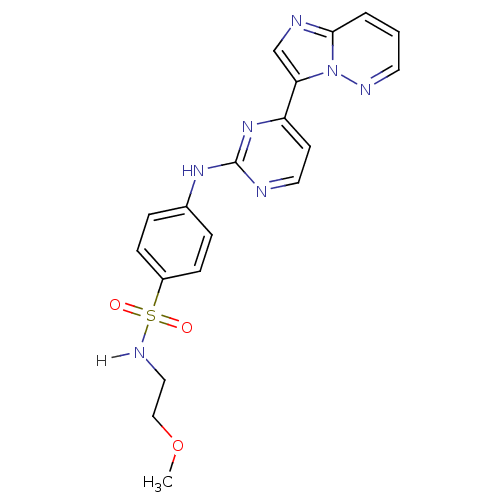
(4-[(4-{imidazo[1,2-a]pyridazin-3-yl}pyrimidin-2-yl...)Show SMILES COCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3cccnn23)cc1 Show InChI InChI=1S/C19H19N7O3S/c1-29-12-11-23-30(27,28)15-6-4-14(5-7-15)24-19-20-10-8-16(25-19)17-13-21-18-3-2-9-22-26(17)18/h2-10,13,23H,11-12H2,1H3,(H,20,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7675
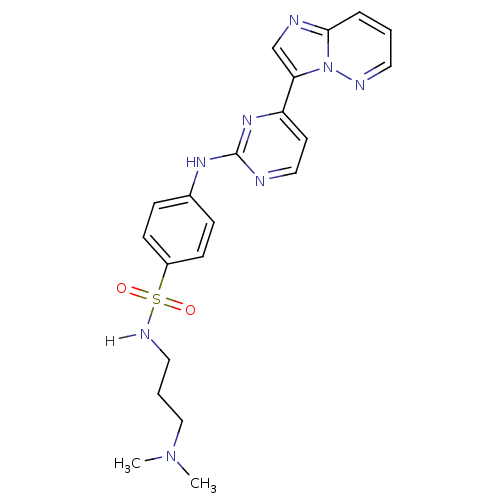
(Imidazo[1,2-b]pyridazine deriv. 2e | N-[3-(dimethy...)Show SMILES CN(C)CCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3cccnn23)cc1 Show InChI InChI=1S/C21H24N8O2S/c1-28(2)14-4-12-25-32(30,31)17-8-6-16(7-9-17)26-21-22-13-10-18(27-21)19-15-23-20-5-3-11-24-29(19)20/h3,5-11,13,15,25H,4,12,14H2,1-2H3,(H,22,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7679
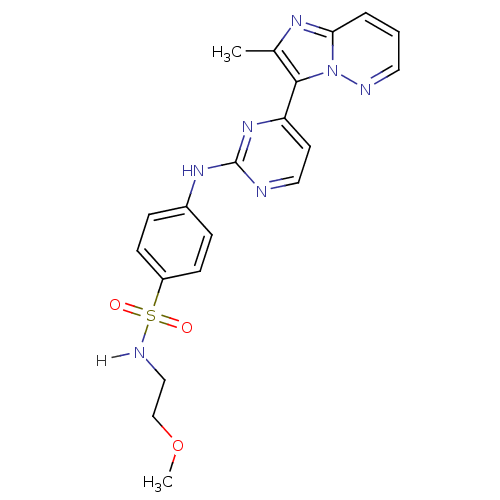
(Imidazo[1,2-b]pyridazine deriv. 4b | N-(2-methoxye...)Show SMILES COCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2c(C)nc3cccnn23)cc1 Show InChI InChI=1S/C20H21N7O3S/c1-14-19(27-18(24-14)4-3-10-22-27)17-9-11-21-20(26-17)25-15-5-7-16(8-6-15)31(28,29)23-12-13-30-2/h3-11,23H,12-13H2,1-2H3,(H,21,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2249-52 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.008
BindingDB Entry DOI: 10.7270/Q2XG9PCF |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50025069
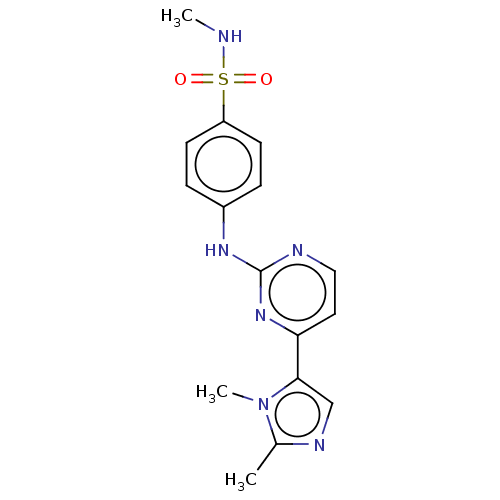
(CHEMBL485425)Show SMILES CNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc(C)n2C)cc1 Show InChI InChI=1S/C16H18N6O2S/c1-11-19-10-15(22(11)3)14-8-9-18-16(21-14)20-12-4-6-13(7-5-12)25(23,24)17-2/h4-10,17H,1-3H3,(H,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 18: 5487-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.024
BindingDB Entry DOI: 10.7270/Q2J9667K |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50025068
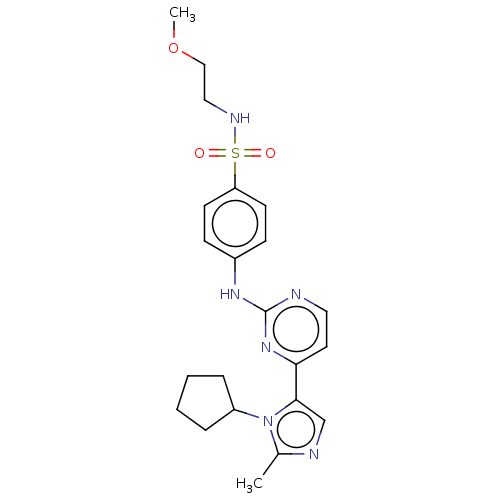
(CHEMBL484555)Show SMILES COCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc(C)n2C2CCCC2)cc1 Show InChI InChI=1S/C22H28N6O3S/c1-16-24-15-21(28(16)18-5-3-4-6-18)20-11-12-23-22(27-20)26-17-7-9-19(10-8-17)32(29,30)25-13-14-31-2/h7-12,15,18,25H,3-6,13-14H2,1-2H3,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 18: 5487-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.024
BindingDB Entry DOI: 10.7270/Q2J9667K |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7672

(4-[(4-{imidazo[1,2-a]pyridazin-3-yl}pyrimidin-2-yl...)Show SMILES CNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3cccnn23)cc1 Show InChI InChI=1S/C17H15N7O2S/c1-18-27(25,26)13-6-4-12(5-7-13)22-17-19-10-8-14(23-17)15-11-20-16-3-2-9-21-24(15)16/h2-11,18H,1H3,(H,19,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 18: 5487-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.024
BindingDB Entry DOI: 10.7270/Q2J9667K |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin S
(Homo sapiens (Human)) | BDBM50263555
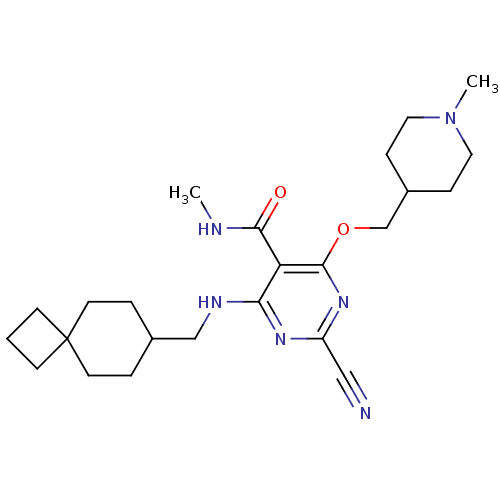
(2-cyano-N-methyl-4-((1-methylpiperidin-4-yl)methox...)Show SMILES CNC(=O)c1c(NCC2CCC3(CCC3)CC2)nc(nc1OCC1CCN(C)CC1)C#N Show InChI InChI=1S/C24H36N6O2/c1-26-22(31)20-21(27-15-17-4-10-24(11-5-17)8-3-9-24)28-19(14-25)29-23(20)32-16-18-6-12-30(2)13-7-18/h17-18H,3-13,15-16H2,1-2H3,(H,26,31)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S by fluorometric assay |
Bioorg Med Chem Lett 18: 5280-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.067
BindingDB Entry DOI: 10.7270/Q2736QQ6 |
More data for this
Ligand-Target Pair | |
Cathepsin S
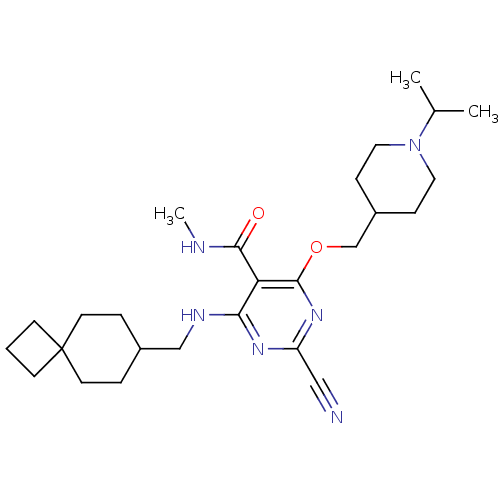
(Homo sapiens (Human)) | BDBM50263557
(2-cyano-4-((1-isopropylpiperidin-4-yl)methoxy)-N-m...)Show SMILES CNC(=O)c1c(NCC2CCC3(CCC3)CC2)nc(nc1OCC1CCN(CC1)C(C)C)C#N Show InChI InChI=1S/C26H40N6O2/c1-18(2)32-13-7-20(8-14-32)17-34-25-22(24(33)28-3)23(30-21(15-27)31-25)29-16-19-5-11-26(12-6-19)9-4-10-26/h18-20H,4-14,16-17H2,1-3H3,(H,28,33)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S by fluorometric assay |
Bioorg Med Chem Lett 18: 5280-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.067
BindingDB Entry DOI: 10.7270/Q2736QQ6 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50263609

(2-cyano-4-((1-(2-hydroxyethyl)piperidin-4-yl)metho...)Show SMILES CNC(=O)c1c(NCC2CCC3(CCC3)CC2)nc(nc1OCC1CCN(CCO)CC1)C#N Show InChI InChI=1S/C25H38N6O3/c1-27-23(33)21-22(28-16-18-3-9-25(10-4-18)7-2-8-25)29-20(15-26)30-24(21)34-17-19-5-11-31(12-6-19)13-14-32/h18-19,32H,2-14,16-17H2,1H3,(H,27,33)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S by fluorometric assay |
Bioorg Med Chem Lett 18: 5280-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.067
BindingDB Entry DOI: 10.7270/Q2736QQ6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
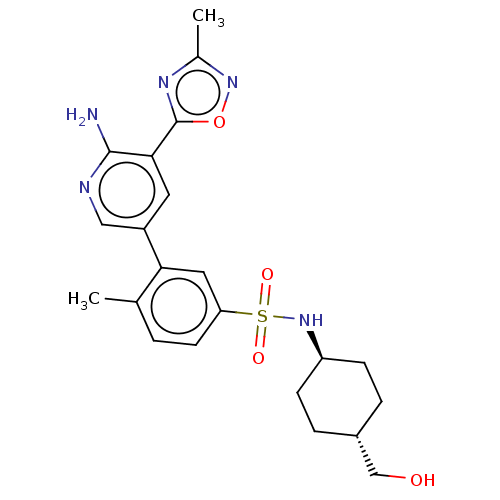
(Homo sapiens (Human)) | BDBM295065
(US10112926, Example 19 | trans 3-[6-Amino-5-(3-met...)Show SMILES Cc1noc(n1)-c1cc(cnc1N)-c1cc(ccc1C)S(=O)(=O)N[C@H]1CC[C@H](CO)CC1 |r,wU:24.26,wD:27.30,(5.5,-.1,;4.73,1.23,;5.2,2.69,;3.96,3.6,;2.71,2.69,;3.19,1.23,;1.38,3.47,;.04,2.69,;-1.29,3.47,;-1.29,5,;.04,5.78,;1.38,5,;2.71,5.78,;-2.62,2.69,;-2.62,1.15,;-3.96,.38,;-5.29,1.15,;-5.29,2.69,;-3.96,3.47,;-3.96,5,;-3.96,-1.15,;-2.42,-1.15,;-5.5,-1.15,;-3.96,-2.69,;-2.62,-3.47,;-1.29,-2.69,;.04,-3.47,;.04,-5,;1.38,-5.78,;2.71,-5,;-1.29,-5.78,;-2.62,-5,)| Show InChI InChI=1S/C22H27N5O4S/c1-13-3-8-18(32(29,30)27-17-6-4-15(12-28)5-7-17)10-19(13)16-9-20(21(23)24-11-16)22-25-14(2)26-31-22/h3,8-11,15,17,27-28H,4-7,12H2,1-2H3,(H2,23,24)/t15-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG
US Patent
| Assay Description
The TR-FRET Adapta Universal Kinase Assay Kit was purchased from Invitrogen Corporation (Carlsbad, Calif., USA) (Cat. No. PV5099). The kit contains t... |
US Patent US10112926 (2018)
BindingDB Entry DOI: 10.7270/Q22R3TQ9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
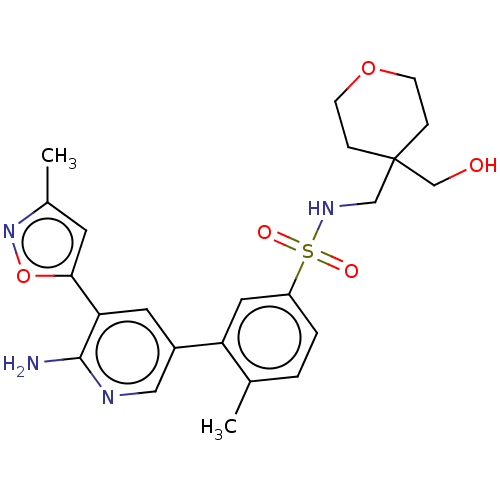
(Homo sapiens (Human)) | BDBM295114
(3-(6-Amino-5-(3-methylisoxazol-5-yl)pyridin-3-yl)-...)Show SMILES Cc1cc(on1)-c1cc(cnc1N)-c1cc(ccc1C)S(=O)(=O)NCC1(CO)CCOCC1 Show InChI InChI=1S/C23H28N4O5S/c1-15-3-4-18(33(29,30)26-13-23(14-28)5-7-31-8-6-23)11-19(15)17-10-20(22(24)25-12-17)21-9-16(2)27-32-21/h3-4,9-12,26,28H,5-8,13-14H2,1-2H3,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG
US Patent
| Assay Description
The TR-FRET Adapta Universal Kinase Assay Kit was purchased from Invitrogen Corporation (Carlsbad, Calif., USA) (Cat. No. PV5099). The kit contains t... |
US Patent US10112926 (2018)
BindingDB Entry DOI: 10.7270/Q22R3TQ9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM295115
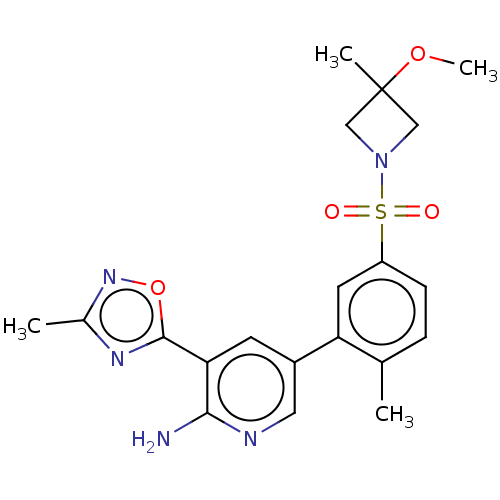
(5-(5-((3-Methoxy-3-methylazetidin-1-yl)sulfonyl)-2...)Show SMILES COC1(C)CN(C1)S(=O)(=O)c1ccc(C)c(c1)-c1cnc(N)c(c1)-c1nc(C)no1 Show InChI InChI=1S/C20H23N5O4S/c1-12-5-6-15(30(26,27)25-10-20(3,11-25)28-4)8-16(12)14-7-17(18(21)22-9-14)19-23-13(2)24-29-19/h5-9H,10-11H2,1-4H3,(H2,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG
US Patent
| Assay Description
The TR-FRET Adapta Universal Kinase Assay Kit was purchased from Invitrogen Corporation (Carlsbad, Calif., USA) (Cat. No. PV5099). The kit contains t... |
US Patent US10112926 (2018)
BindingDB Entry DOI: 10.7270/Q22R3TQ9 |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50246810

(CHEMBL4078625)Show SMILES CN(C)[C@H]1CC[C@@H](CC1)Nc1ncnc2[nH]cc(C3CCC3)c12 |r,wU:6.9,wD:3.2,(14.47,-18.02,;13.14,-17.24,;13.15,-15.7,;11.81,-18.01,;10.48,-17.24,;9.14,-18,;9.15,-19.55,;10.47,-20.31,;11.8,-19.55,;7.81,-20.32,;7.81,-21.86,;9.15,-22.62,;9.15,-24.17,;7.82,-24.94,;6.49,-24.17,;5.02,-24.65,;4.1,-23.41,;5,-22.15,;4.52,-20.69,;5.2,-19.31,;3.82,-18.63,;3.13,-20,;6.48,-22.62,)| Show InChI InChI=1S/C18H27N5/c1-23(2)14-8-6-13(7-9-14)22-18-16-15(12-4-3-5-12)10-19-17(16)20-11-21-18/h10-14H,3-9H2,1-2H3,(H2,19,20,21,22)/t13-,14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human IRAK4 expressed in baculovirus expression system using 5-FAM-IPTSPITTTYFFFKKK-COOH as substrat... |
J Med Chem 60: 10071-10091 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01290
BindingDB Entry DOI: 10.7270/Q2T72KWX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7657

(4-[(4-{imidazo[1,2-a]pyridin-3-yl}pyrimidin-2-yl)a...)Show SMILES NS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3ccccn23)cc1 Show InChI InChI=1S/C17H14N6O2S/c18-26(24,25)13-6-4-12(5-7-13)21-17-19-9-8-14(22-17)15-11-20-16-3-1-2-10-23(15)16/h1-11H,(H2,18,24,25)(H,19,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.015
BindingDB Entry DOI: 10.7270/Q22805TT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7662
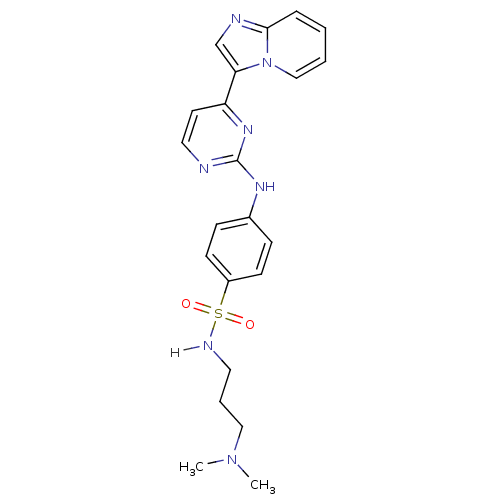
(N-[3-(dimethylamino)propyl]-4-[(4-{imidazo[1,2-a]p...)Show SMILES CN(C)CCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3ccccn23)cc1 Show InChI InChI=1S/C22H25N7O2S/c1-28(2)14-5-12-25-32(30,31)18-9-7-17(8-10-18)26-22-23-13-11-19(27-22)20-16-24-21-6-3-4-15-29(20)21/h3-4,6-11,13,15-16,25H,5,12,14H2,1-2H3,(H,23,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.015
BindingDB Entry DOI: 10.7270/Q22805TT |
More data for this
Ligand-Target Pair | |
Interleukin-1 receptor-associated kinase 4
(Homo sapiens (Human)) | BDBM50246777

(CHEMBL4067181)Show SMILES COC(=O)N1CCN(CC1)[C@H]1CC[C@@H](CC1)Nc1ncnc2[nH]cc(C3CCOCC3)c12 |r,wU:13.17,wD:10.10,(21.79,-16.28,;21.8,-14.75,;20.47,-13.96,;20.48,-12.42,;19.14,-14.73,;19.13,-16.27,;17.79,-17.02,;16.47,-16.25,;16.47,-14.71,;17.81,-13.95,;15.14,-17.01,;13.8,-16.24,;12.46,-17.01,;12.47,-18.55,;13.8,-19.32,;15.13,-18.55,;11.14,-19.32,;11.14,-20.86,;12.48,-21.62,;12.48,-23.17,;11.15,-23.94,;9.81,-23.17,;8.35,-23.65,;7.43,-22.41,;8.33,-21.15,;7.85,-19.7,;8.87,-18.54,;8.38,-17.09,;6.88,-16.77,;5.85,-17.92,;6.33,-19.39,;9.81,-21.62,)| Show InChI InChI=1S/C23H34N6O3/c1-31-23(30)29-10-8-28(9-11-29)18-4-2-17(3-5-18)27-22-20-19(16-6-12-32-13-7-16)14-24-21(20)25-15-26-22/h14-18H,2-13H2,1H3,(H2,24,25,26,27)/t17-,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human IRAK4 expressed in baculovirus expression system using 5-FAM-IPTSPITTTYFFFKKK-COOH as substrat... |
J Med Chem 60: 10071-10091 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01290
BindingDB Entry DOI: 10.7270/Q2T72KWX |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7655
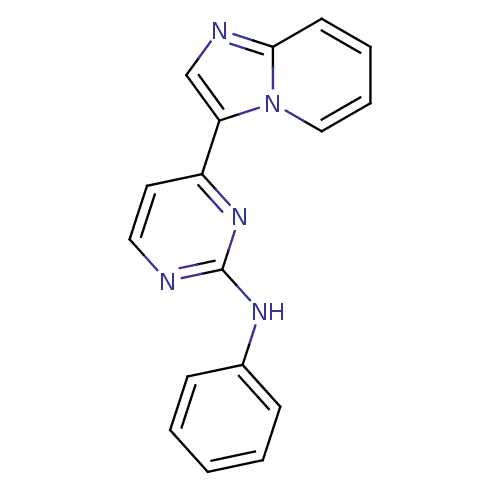
(4-{imidazo[1,2-a]pyridin-3-yl}-N-phenylpyrimidin-2...)Show InChI InChI=1S/C17H13N5/c1-2-6-13(7-3-1)20-17-18-10-9-14(21-17)15-12-19-16-8-4-5-11-22(15)16/h1-12H,(H,18,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.015
BindingDB Entry DOI: 10.7270/Q22805TT |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1
(Homo sapiens (Human)) | BDBM7660
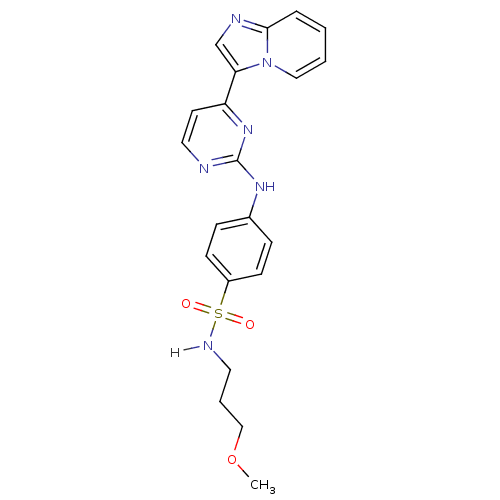
(4-[(4-{imidazo[1,2-a]pyridin-3-yl}pyrimidin-2-yl)a...)Show SMILES COCCCNS(=O)(=O)c1ccc(Nc2nccc(n2)-c2cnc3ccccn23)cc1 Show InChI InChI=1S/C21H22N6O3S/c1-30-14-4-11-24-31(28,29)17-8-6-16(7-9-17)25-21-22-12-10-18(26-21)19-15-23-20-5-2-3-13-27(19)20/h2-3,5-10,12-13,15,24H,4,11,14H2,1H3,(H,22,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.0 | 22 |
AstraZeneca
| Assay Description
The kinase activity was assayed using an in vitro scintillation proximity assay (SPA) for measuring incorporation of [gamma-33P] ATP into GST-Rb. |
Bioorg Med Chem Lett 14: 2245-8 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.015
BindingDB Entry DOI: 10.7270/Q22805TT |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50263554
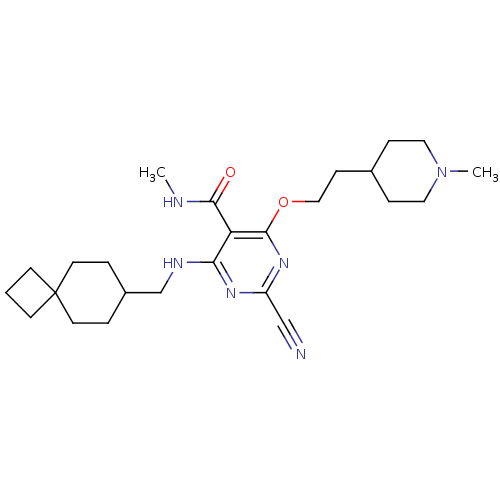
(2-cyano-N-methyl-4-(2-(1-methylpiperidin-4-yl)etho...)Show SMILES CNC(=O)c1c(NCC2CCC3(CCC3)CC2)nc(nc1OCCC1CCN(C)CC1)C#N Show InChI InChI=1S/C25H38N6O2/c1-27-23(32)21-22(28-17-19-4-11-25(12-5-19)9-3-10-25)29-20(16-26)30-24(21)33-15-8-18-6-13-31(2)14-7-18/h18-19H,3-15,17H2,1-2H3,(H,27,32)(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant cathepsin S by fluorometric assay |
Bioorg Med Chem Lett 18: 5280-4 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.067
BindingDB Entry DOI: 10.7270/Q2736QQ6 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50274698
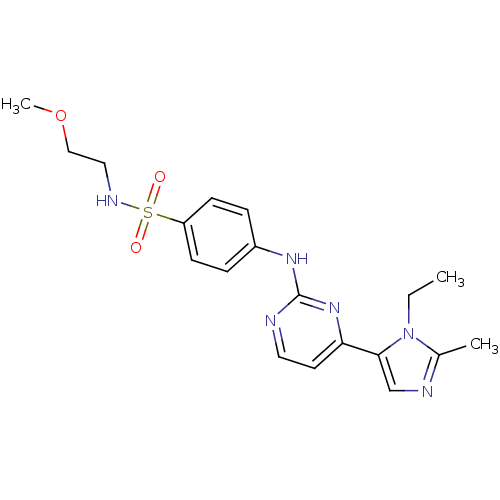
(4-(4-(1-ethyl-2-methyl-1H-imidazol-5-yl)pyrimidin-...)Show SMILES CCn1c(C)ncc1-c1ccnc(Nc2ccc(cc2)S(=O)(=O)NCCOC)n1 Show InChI InChI=1S/C19H24N6O3S/c1-4-25-14(2)21-13-18(25)17-9-10-20-19(24-17)23-15-5-7-16(8-6-15)29(26,27)22-11-12-28-3/h5-10,13,22H,4,11-12H2,1-3H3,(H,20,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 18: 5487-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.024
BindingDB Entry DOI: 10.7270/Q2J9667K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data