

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18784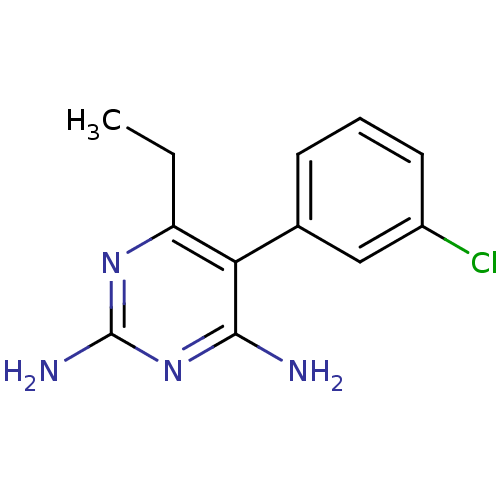 (5-(3-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of recombinant wild type (WT) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50064020 (6-Ethyl-5-(3-methoxy-phenyl)-pyrimidine-2,4-diamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of recombinant wild type (WT) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of recombinant wild type (WT) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18784 (5-(3-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of mutant (C59R + S108N) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18792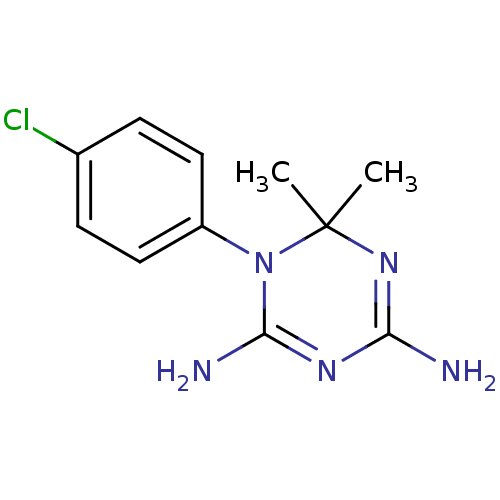 (1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of recombinant wild type (WT) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50192451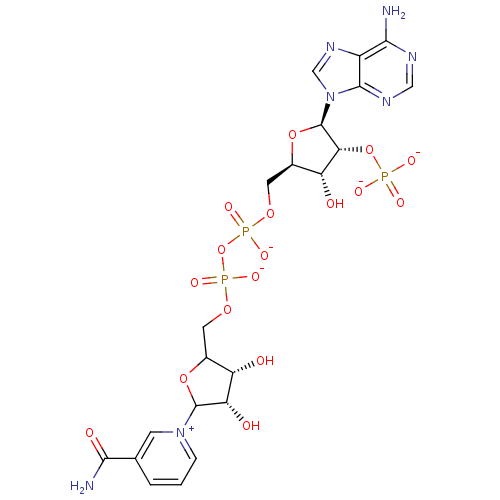 (CHEMBL213053 | NADP+) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of recombinant wild type (WT) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50064020 (6-Ethyl-5-(3-methoxy-phenyl)-pyrimidine-2,4-diamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of mutant (C59R + S108N) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50064020 (6-Ethyl-5-(3-methoxy-phenyl)-pyrimidine-2,4-diamin...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against mutant Plasmodium falciparum DHFR-TS | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM50192451 (CHEMBL213053 | NADP+) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of mutant (C59R + S108N) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50121753 (1-(5-Chloro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against human thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20079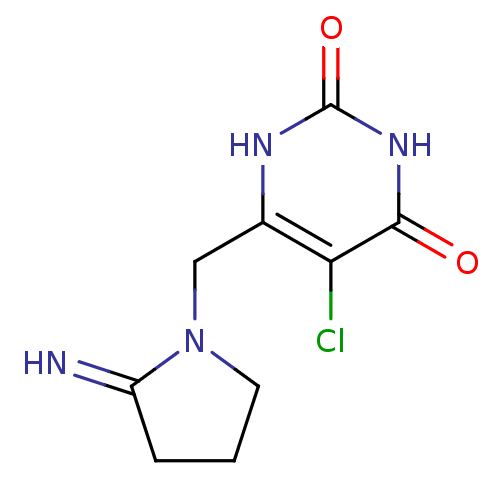 (5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against thymidine phosphorylase | J Med Chem 48: 392-402 (2005) Article DOI: 10.1021/jm049494r BindingDB Entry DOI: 10.7270/Q2445N8K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of mutant (C59R + S108N) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18792 (1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Binding affinity was evaluated as inhibition of mutant (C59R + S108N) Plasmodium falciparum DHFR-TS. | J Med Chem 41: 1367-70 (1998) Article DOI: 10.1021/jm970845u BindingDB Entry DOI: 10.7270/Q2ZC83JJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091160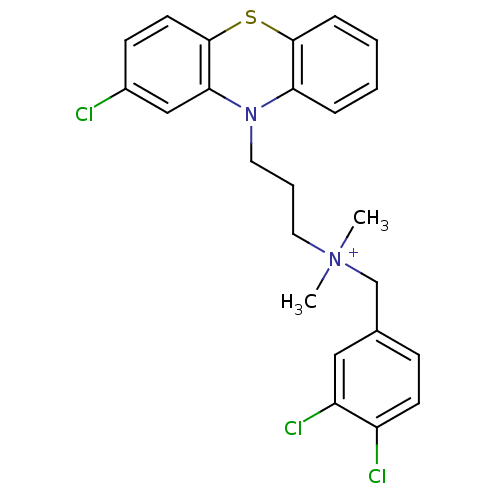 (CHEMBL106127 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178811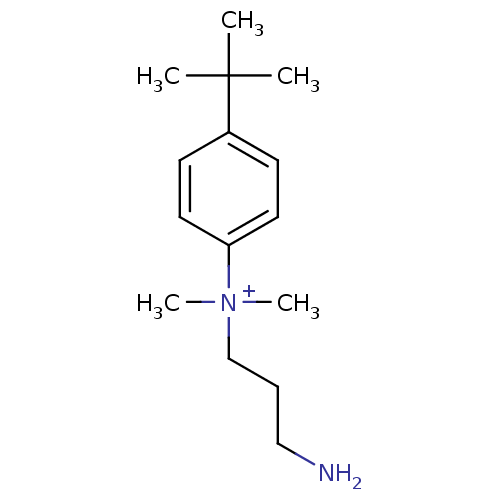 (CHEMBL199020 | N-(3-aminopropyl)-4-tert-butyl-N,N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Mixed inhibition of trypanothione reductase from Trypanosoma cruzi using TSST substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178803 (3,4-dichloro-N-(3-(2-chloro-10H-phenothiazin-10-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Linear competitive inhibition of trypanothione reductase from Trypanosoma cruzi using TSST substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178803 (3,4-dichloro-N-(3-(2-chloro-10H-phenothiazin-10-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Mixed inhibition of trypanothione reductase from Trypanosoma cruzi using TSST substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091165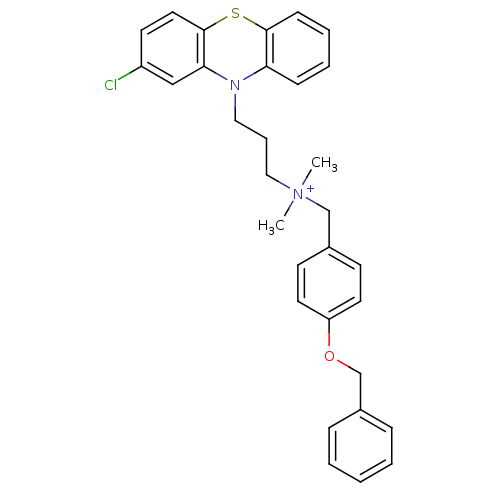 ((4-Benzyloxy-benzyl)-[3-(2-chloro-phenothiazin-10-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091158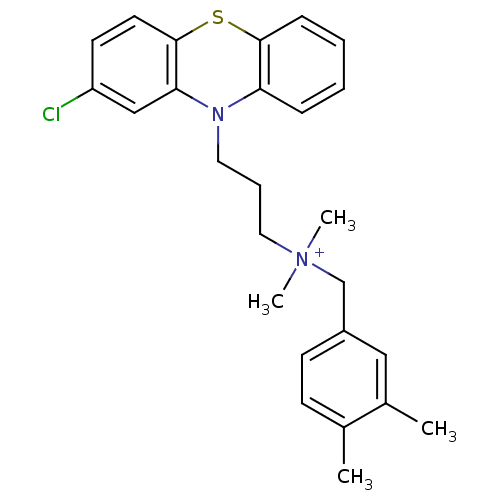 (CHEMBL322826 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091148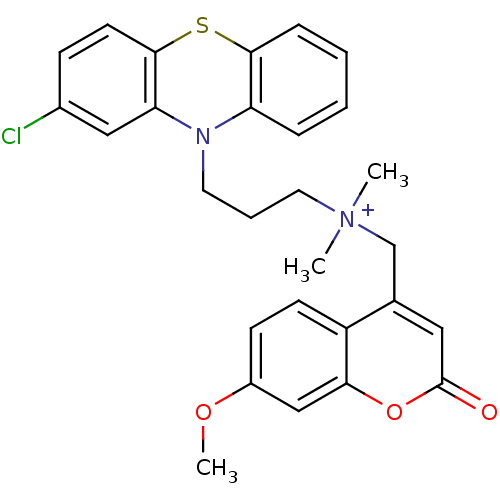 (CHEMBL106769 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178809 (4-tert-butyl-N-(3-(2-chloro-10H-phenothiazin-10-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Linear competitive inhibition of trypanothione reductase from Trypanosoma cruzi using TSST substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091162 ((4-tert-Butyl-benzyl)-[3-(2-chloro-phenothiazin-10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091163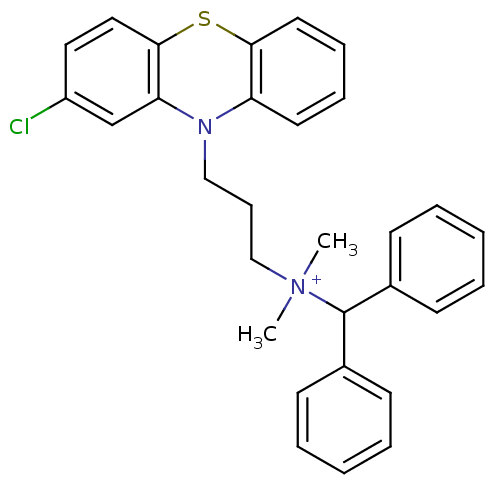 (Benzhydryl-[3-(2-chloro-phenothiazin-10-yl)-propyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091154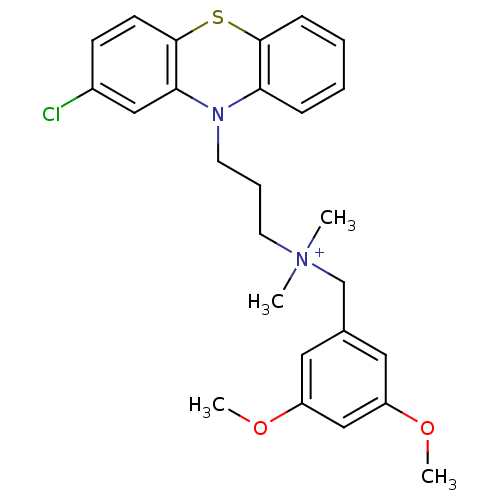 (CHEMBL106901 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Escherichia coli) | BDBM50122770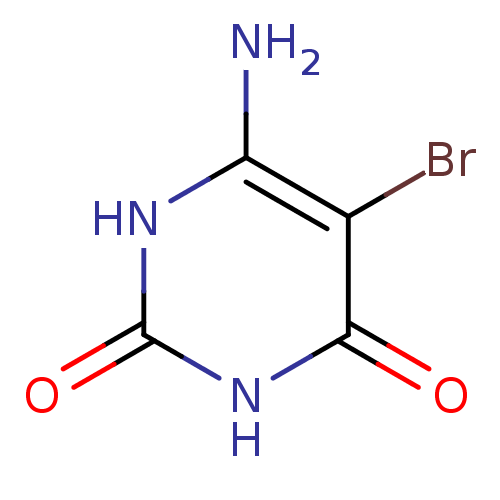 (6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli thymidine phosphorylase | J Med Chem 48: 392-402 (2005) Article DOI: 10.1021/jm049494r BindingDB Entry DOI: 10.7270/Q2445N8K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50019879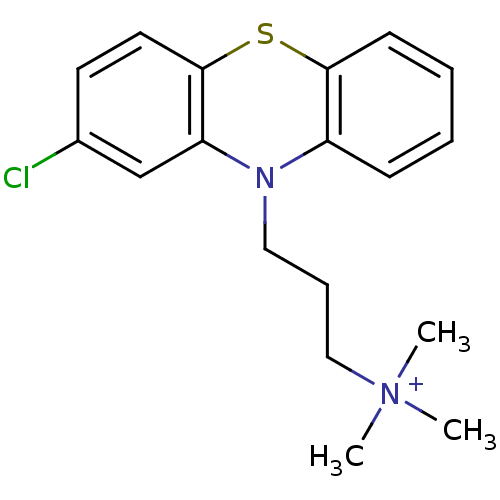 (CHEMBL279905 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091157 ((2-Adamantan-1-yl-2-oxo-ethyl)-[3-(2-chloro-phenot...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091153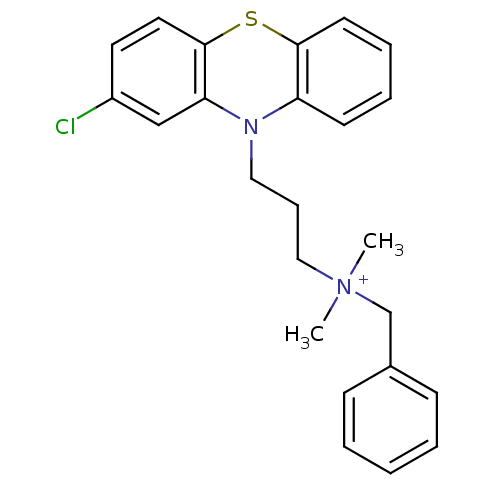 (Benzyl-[3-(2-chloro-phenothiazin-10-yl)-propyl]-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091159 (CHEMBL326458 | [2-(4-Chloro-3-methyl-phenyl)-2-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091156 ((4-Bromo-benzyl)-[3-(2-chloro-phenothiazin-10-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091155 (CHEMBL323271 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091164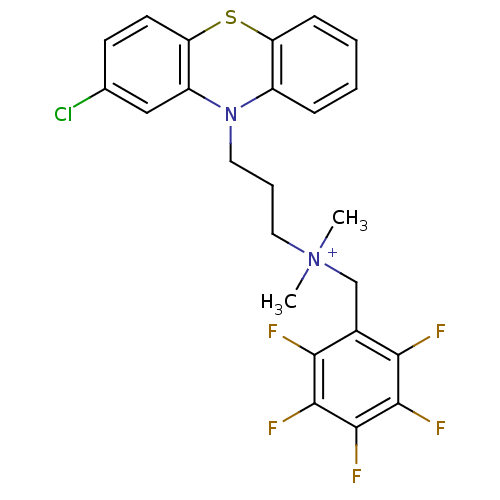 (CHEMBL106236 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178807 (3,4-dichloro-N-(3-(5-chloro-2-(phenylthio)phenylam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Linear competitive inhibition of trypanothione reductase from Trypanosoma cruzi using (ZCG.dmapa)2 substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091149 ((4-Chloro-benzyl)-[3-(2-chloro-phenothiazin-10-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091150 (CHEMBL323540 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091152 (CHEMBL106108 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091151 ((3-Chloro-benzyl)-[3-(2-chloro-phenothiazin-10-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122770 (6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178802 (4-tert-butyl-N-(3-(5-chloro-2-(phenylthio)phenylam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Linear competitive inhibition of trypanothione reductase from Trypanosoma cruzi using TSST substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50369578 (CHEMBL239370) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178805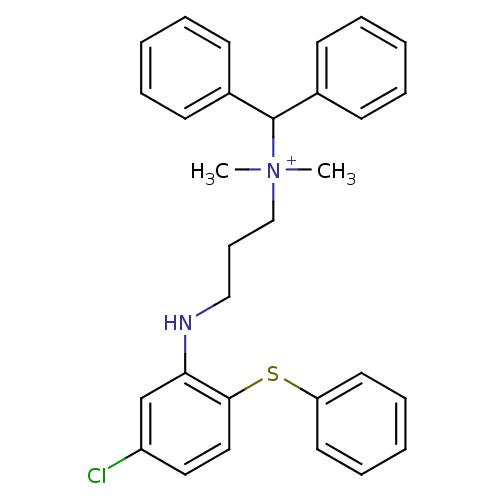 (CHEMBL436292 | N-benzhydryl-3-(5-chloro-2-(phenylt...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Linear competitive inhibition of trypanothione reductase from Trypanosoma cruzi using (ZCG.dmapa)2 substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178802 (4-tert-butyl-N-(3-(5-chloro-2-(phenylthio)phenylam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Linear competitive inhibition of trypanothione reductase from Trypanosoma cruzi using (ZCG.dmapa)2 substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178804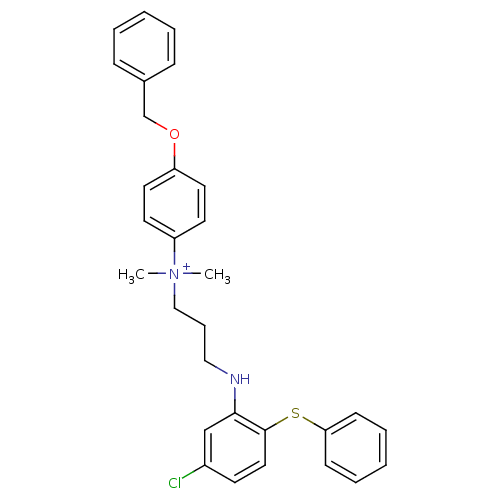 (4-(benzyloxy)-N-(3-(5-chloro-2-(phenylthio)phenyla...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Linear competitive inhibition of trypanothione reductase from Trypanosoma cruzi using (ZCG.dmapa)2 substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (T. cruzi) Trypanothione reductase (linear competitive type) | J Med Chem 41: 148-56 (1998) Article DOI: 10.1021/jm960814j BindingDB Entry DOI: 10.7270/Q2ZP457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178810 (CHEMBL371088 | N-(5-chloro-2-(phenylthio)phenyl)bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Mixed inhibition of trypanothione reductase from Trypanosoma cruzi using TSST substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178814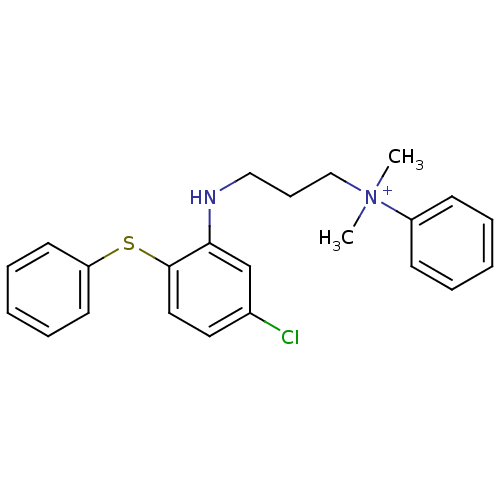 (CHEMBL199067 | N-(3-(5-chloro-2-(phenylthio)phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Linear competitive inhibition of trypanothione reductase from Trypanosoma cruzi using (ZCG.dmapa)2 substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50062260 (CHEMBL38403 | [3-(2-Chloro-phenothiazin-10-yl)-pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (T. cruzi) Trypanothione reductase (linear competitive type) | J Med Chem 41: 148-56 (1998) Article DOI: 10.1021/jm960814j BindingDB Entry DOI: 10.7270/Q2ZP457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178808 (CHEMBL372379 | N-(5-chloro-2-(phenylthio)phenyl)pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Mixed inhibition of trypanothione reductase from Trypanosoma cruzi using TSST substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM78433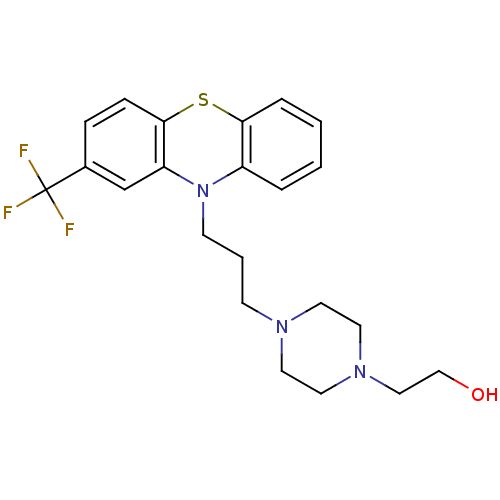 (2-[4-[3-[2-(trifluoromethyl)-10-phenothiazinyl]pro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against recombinant Trypanosoma cruzi (T. cruzi) Trypanothione reductase (linear competitive type) | J Med Chem 41: 148-56 (1998) Article DOI: 10.1021/jm960814j BindingDB Entry DOI: 10.7270/Q2ZP457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 162 total ) | Next | Last >> |