

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293089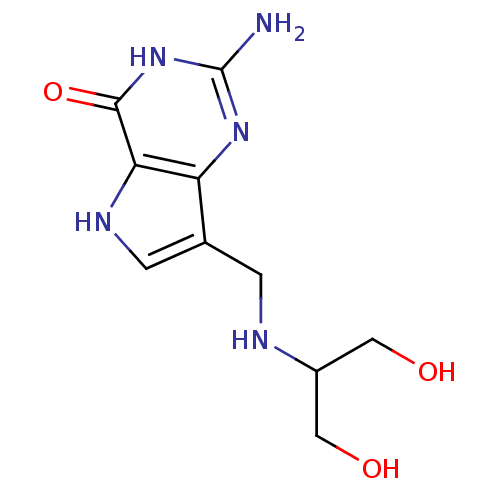 (2-Amino-7-{[(1,3-dihydroxypropan-2-yl)amino]methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293090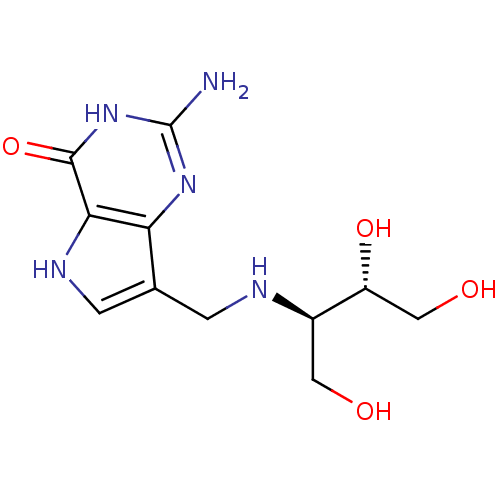 (2-Amino-7-({[(2R,3S)-1,3,4-trihydroxybutan-2-yl]am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50246593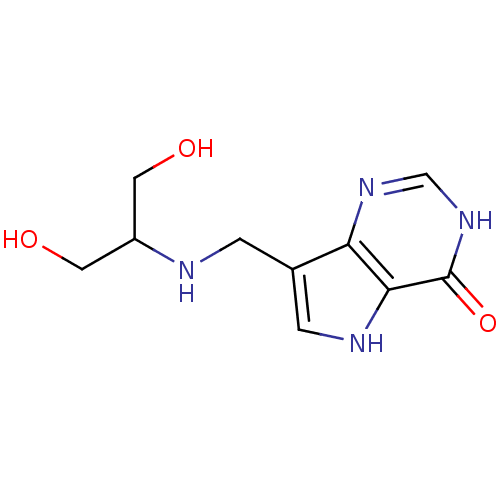 (7-((1,3-dihydroxypropan-2-ylamino)methyl)-3H-pyrro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50135920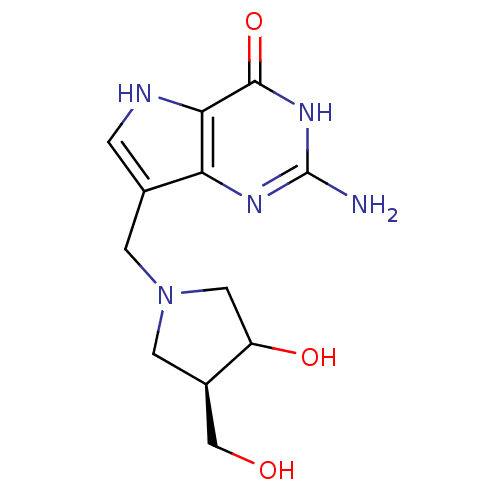 (2-Amino-7-(3-hydroxy-4-hydroxymethyl-pyrrolidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Dissociation constant against Human Purine Nucleoside Phosphorylase was reported | J Med Chem 46: 5271-6 (2003) Article DOI: 10.1021/jm030305z BindingDB Entry DOI: 10.7270/Q23N2449 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293087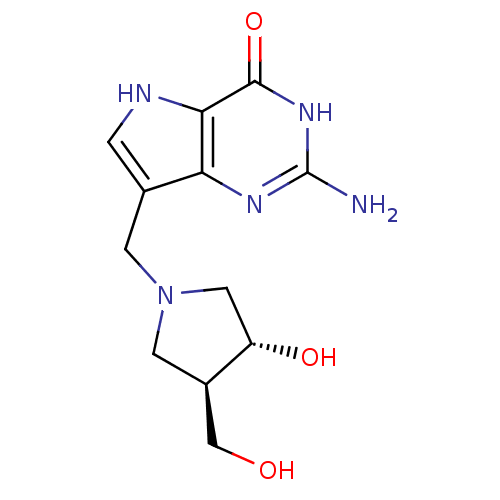 (2-amino-7-(((3R,4R)-3-hydroxy-4-(hydroxymethyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22109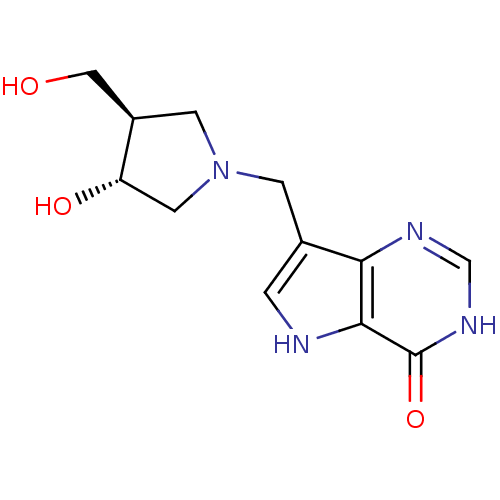 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293091 (7-({[(1R,2S)-2,3-DIHYDROXY-1-(HYDROXYMETHYL)PROPYL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50170092 (1-(4-Amino-4,5-dihydro-3H-pyrrolo[3,2-d]pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP as equilibrium dissociation constant | J Med Chem 48: 4679-89 (2005) Article DOI: 10.1021/jm050269z BindingDB Entry DOI: 10.7270/Q2FJ2HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22109 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Dissociation constant against Human Purine Nucleoside Phosphorylase was reported | J Med Chem 46: 5271-6 (2003) Article DOI: 10.1021/jm030305z BindingDB Entry DOI: 10.7270/Q23N2449 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50122726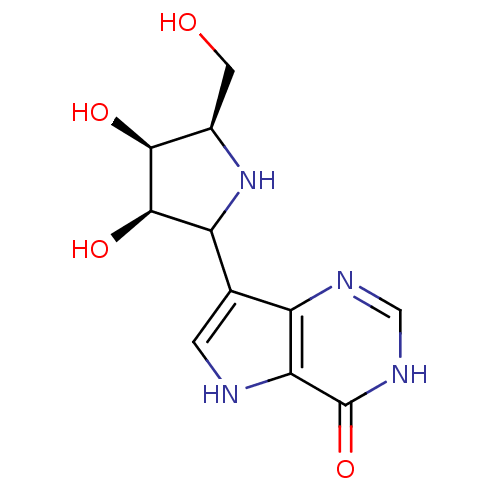 (2-Hydroxymethyl-5-(4-hydroxy-5H-pyrrolo[3,2-d]pyri...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibitory activity of compound against bovine purine nucleoside phosphorylase(PNP) | J Med Chem 46: 155-60 (2002) Article DOI: 10.1021/jm0203332 BindingDB Entry DOI: 10.7270/Q2DF6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293086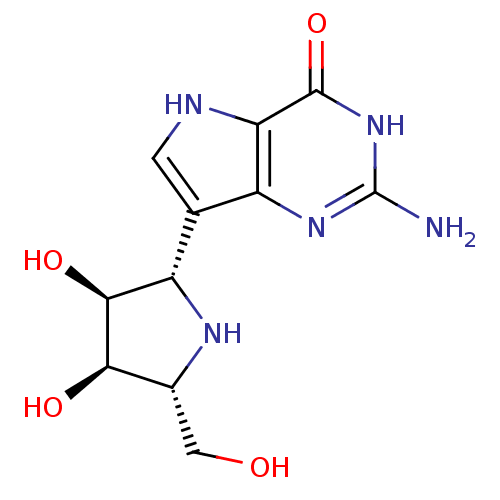 ((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibitory activity of compound against human purine nucleoside phosphorylase(PNP) | J Med Chem 46: 155-60 (2002) Article DOI: 10.1021/jm0203332 BindingDB Entry DOI: 10.7270/Q2DF6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50293086 ((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibitory activity of compound against bovine purine nucleoside phosphorylase(PNP) | J Med Chem 46: 155-60 (2002) Article DOI: 10.1021/jm0203332 BindingDB Entry DOI: 10.7270/Q2DF6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50170082 (1-(4-Amino-4,5-dihydro-3H-pyrrolo[3,2-d]pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP as equilibrium dissociation constant | J Med Chem 48: 4679-89 (2005) Article DOI: 10.1021/jm050269z BindingDB Entry DOI: 10.7270/Q2FJ2HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM50247150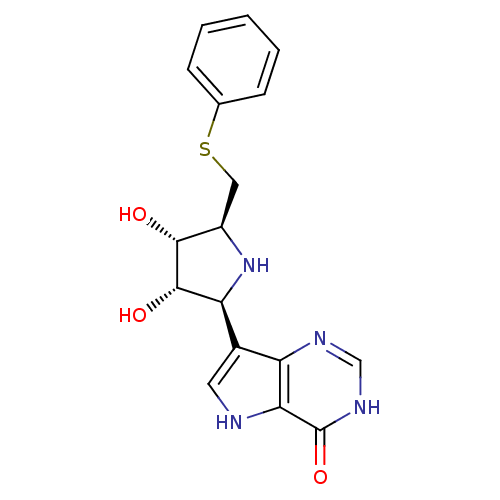 (5'-phenylthio-ImmH | CHEMBL474328 | US9290501, (B)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0350 | -59.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM218675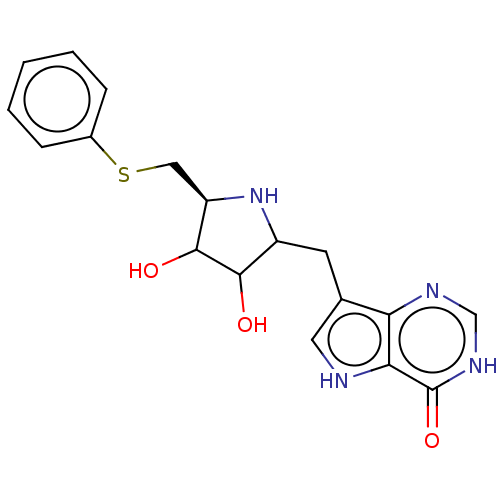 (US9290501, PT-ImmH) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0350 | -59.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293086 ((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Dissociation constant against Human Purine Nucleoside Phosphorylase was reported | J Med Chem 46: 5271-6 (2003) Article DOI: 10.1021/jm030305z BindingDB Entry DOI: 10.7270/Q23N2449 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293086 ((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50422435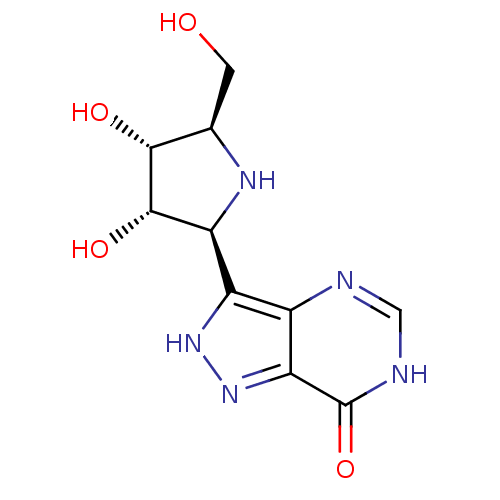 (CHEMBL2311112) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibitory activity of compound against bovine purine nucleoside phosphorylase(PNP) | J Med Chem 46: 155-60 (2002) Article DOI: 10.1021/jm0203332 BindingDB Entry DOI: 10.7270/Q2DF6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293086 ((1S)-1,4-dideoxy-1,4-imino-1-(9-deazaguanin-9-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibition | J Med Chem 46: 3412-23 (2003) Article DOI: 10.1021/jm030145r BindingDB Entry DOI: 10.7270/Q2X92C29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase (Escherichia coli (strain K12)) | BDBM22113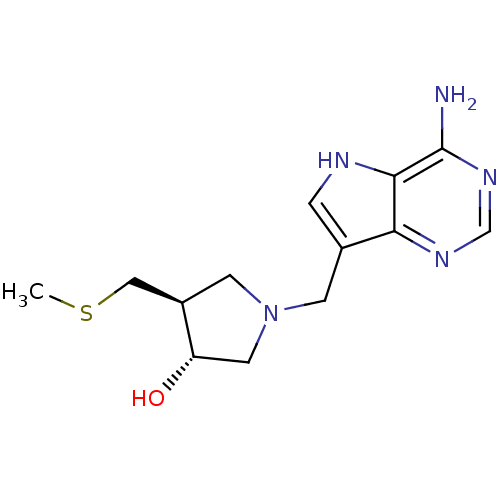 ((3R,4S)-1-({4-amino-5H-pyrrolo[3,2-d]pyrimidin-7-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0480 | -58.3 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Industrial Research Limited | Assay Description Enzyme activity was monitored by absorbance change in the xanthine oxidase coupled assay, which measures the formation of 2,8-dihydroxyadenine at 293... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50195587 (1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhbitory activity of compound against human purine nucleoside phosphorylase (PNP) | J Med Chem 47: 3275-81 (2004) Article DOI: 10.1021/jm0306475 BindingDB Entry DOI: 10.7270/Q2PZ59J8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50195587 (1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Dissociation constant against Human Purine Nucleoside Phosphorylase was reported | J Med Chem 46: 5271-6 (2003) Article DOI: 10.1021/jm030305z BindingDB Entry DOI: 10.7270/Q23N2449 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50195587 (1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibition | J Med Chem 46: 3412-23 (2003) Article DOI: 10.1021/jm030145r BindingDB Entry DOI: 10.7270/Q2X92C29 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50195587 (1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Bos taurus (bovine)) | BDBM50122724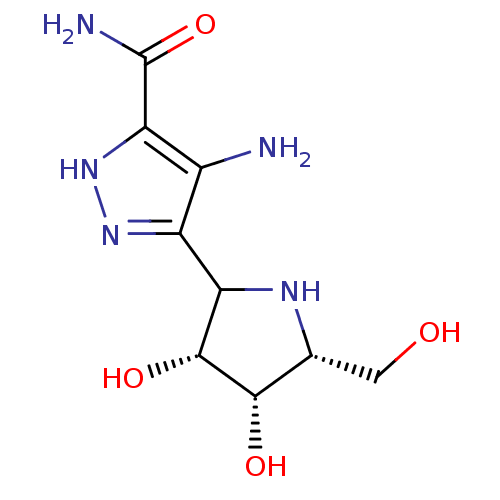 (4-Amino-5-(3,4-dihydroxy-5-hydroxymethyl-pyrrolidi...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibitory activity of compound against bovine purine nucleoside phosphorylase(PNP) | J Med Chem 46: 155-60 (2002) Article DOI: 10.1021/jm0203332 BindingDB Entry DOI: 10.7270/Q2DF6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM218673 (US9290501, PrT-DADMe-ImmH) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0720 | -57.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50122726 (2-Hydroxymethyl-5-(4-hydroxy-5H-pyrrolo[3,2-d]pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibitory activity of compound against human purine nucleoside phosphorylase(PNP) | J Med Chem 46: 155-60 (2002) Article DOI: 10.1021/jm0203332 BindingDB Entry DOI: 10.7270/Q2DF6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM50247149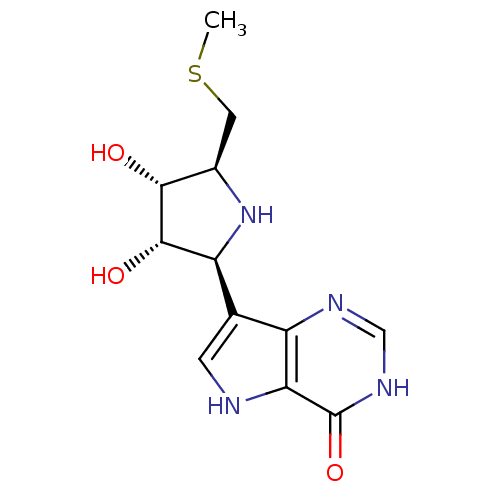 (5'-Methylthio-ImmH | CHEMBL473929 | US9290501, (A)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | US Patent | 0.0760 | -57.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM218674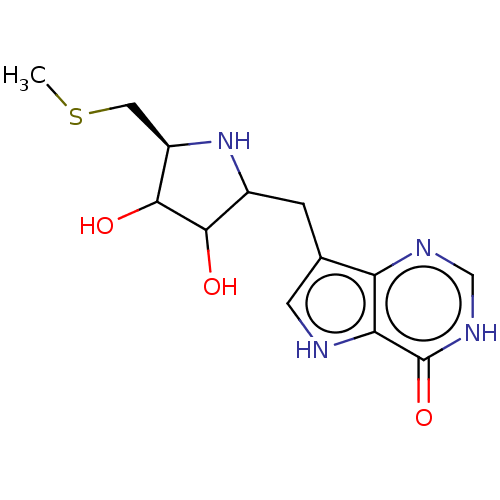 (US9290501, MT-ImmH) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0760 | -57.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50170089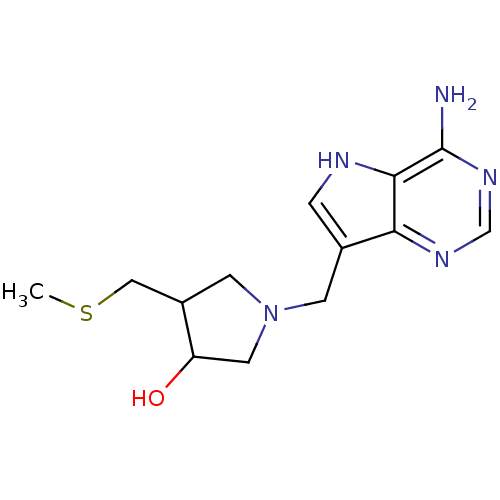 (1-(4-Amino-5H-pyrrolo[3,2-d]pyrimidin-7-ylmethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP as equilibrium dissociation constant | J Med Chem 48: 4679-89 (2005) Article DOI: 10.1021/jm050269z BindingDB Entry DOI: 10.7270/Q2FJ2HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM50246594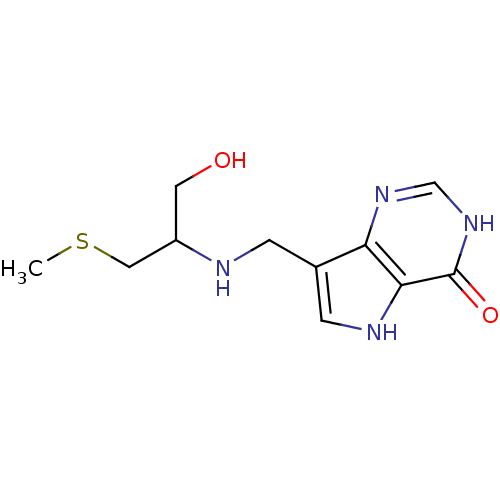 ((+/-)-7-((1-hydroxy-3-(methylthio)propan-2-ylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0960 | -57.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioinosine phosphorylase (Pseudomonas aeruginosa) | BDBM50246594 ((+/-)-7-((1-hydroxy-3-(methylthio)propan-2-ylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.0960 | -57.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Albert Einstein College of Medicine, Inc.; Victoria Link Limited US Patent | Assay Description Assays for slow-onset inhibitors were carried out by adding 1 nM PaMTIP into reaction mixtures at 25 °C. containing 100 mM Hepes, pH 7.4, 100 mM ... | US Patent US9290501 (2016) BindingDB Entry DOI: 10.7270/Q2639NKD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50122724 (4-Amino-5-(3,4-dihydroxy-5-hydroxymethyl-pyrrolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibitory activity of compound against human purine nucleoside phosphorylase(PNP) | J Med Chem 46: 155-60 (2002) Article DOI: 10.1021/jm0203332 BindingDB Entry DOI: 10.7270/Q2DF6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50422435 (CHEMBL2311112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibitory activity of compound against human purine nucleoside phosphorylase(PNP) | J Med Chem 46: 155-60 (2002) Article DOI: 10.1021/jm0203332 BindingDB Entry DOI: 10.7270/Q2DF6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50170086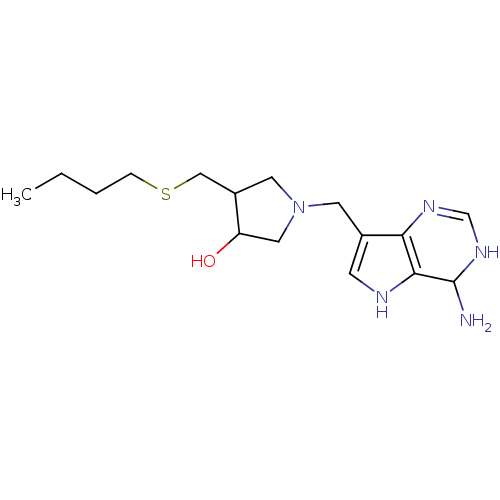 (1-(4-Amino-4,5-dihydro-3H-pyrrolo[3,2-d]pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP as equilibrium dissociation constant | J Med Chem 48: 4679-89 (2005) Article DOI: 10.1021/jm050269z BindingDB Entry DOI: 10.7270/Q2FJ2HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50170095 (1-(4-Amino-4,5-dihydro-3H-pyrrolo[3,2-d]pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP as equilibrium dissociation constant | J Med Chem 48: 4679-89 (2005) Article DOI: 10.1021/jm050269z BindingDB Entry DOI: 10.7270/Q2FJ2HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50170091 (1-(4-Amino-4,5-dihydro-3H-pyrrolo[3,2-d]pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP as equilibrium dissociation constant | J Med Chem 48: 4679-89 (2005) Article DOI: 10.1021/jm050269z BindingDB Entry DOI: 10.7270/Q2FJ2HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50170098 (1-(4-Amino-4,5-dihydro-3H-pyrrolo[3,2-d]pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP as equilibrium dissociation constant | J Med Chem 48: 4679-89 (2005) Article DOI: 10.1021/jm050269z BindingDB Entry DOI: 10.7270/Q2FJ2HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50135920 (2-Amino-7-(3-hydroxy-4-hydroxymethyl-pyrrolidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Binding affinity towards Human Purine Nucleoside Phosphorylase was reported | J Med Chem 46: 5271-6 (2003) Article DOI: 10.1021/jm030305z BindingDB Entry DOI: 10.7270/Q23N2449 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM36496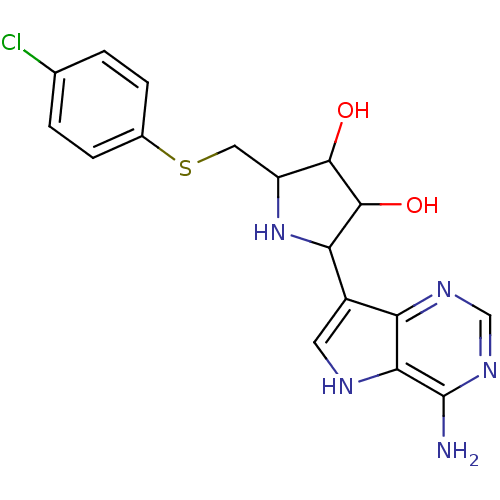 (CHEMBL552894 | ImmA-pClPh) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Equilibrium dissociation constant towards human 5'-methylthioadenosine phosphorylase | J Med Chem 47: 3275-81 (2004) Article DOI: 10.1021/jm0306475 BindingDB Entry DOI: 10.7270/Q2PZ59J8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50170083 (1-(4-Amino-4,5-dihydro-3H-pyrrolo[3,2-d]pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human MTAP as equilibrium dissociation constant | J Med Chem 48: 4679-89 (2005) Article DOI: 10.1021/jm050269z BindingDB Entry DOI: 10.7270/Q2FJ2HKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50422435 (CHEMBL2311112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Dissociation constant against Human Purine Nucleoside Phosphorylase was reported | J Med Chem 46: 5271-6 (2003) Article DOI: 10.1021/jm030305z BindingDB Entry DOI: 10.7270/Q23N2449 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50422435 (CHEMBL2311112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Equilibrium dissociation constant determined against human purine nucleoside phosphorylase (PNP) after slow-onset inhibition | J Med Chem 46: 3412-23 (2003) Article DOI: 10.1021/jm030145r BindingDB Entry DOI: 10.7270/Q2X92C29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30369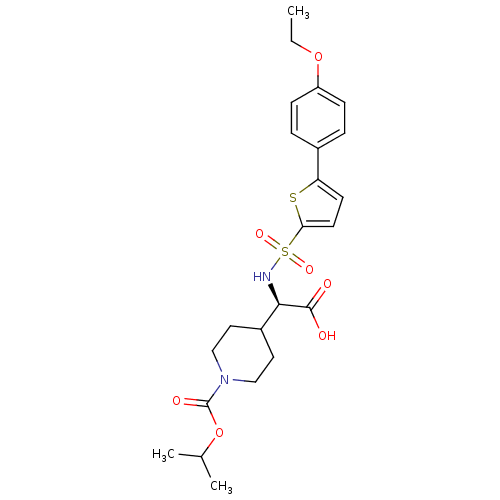 (piperidinyl glycine derivative, 24f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.190 | -54.9 | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293060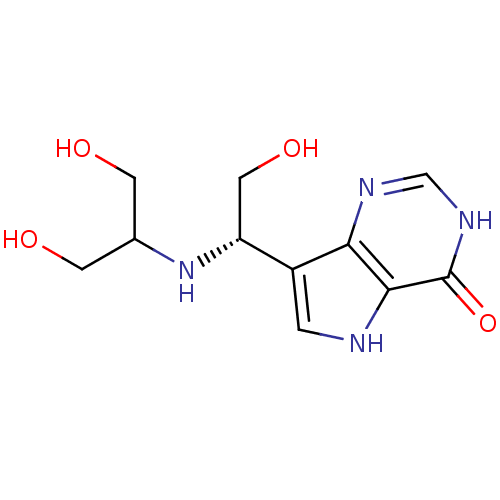 (7-{(1S)-1-[(1,3-Dihydroxypropan-2-yl)amino]-2-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-methyl-5'-thioadenosine phosphorylase (Homo sapiens (Human)) | BDBM50148131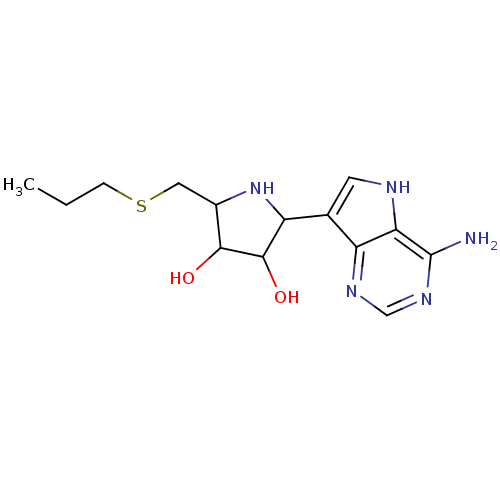 (2-(4-Amino-5H-pyrrolo[3,2-d]pyrimidin-7-yl)-5-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.214 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Equilibrium dissociation constant towards human 5'-methylthioadenosine phosphorylase | J Med Chem 47: 3275-81 (2004) Article DOI: 10.1021/jm0306475 BindingDB Entry DOI: 10.7270/Q2PZ59J8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50370253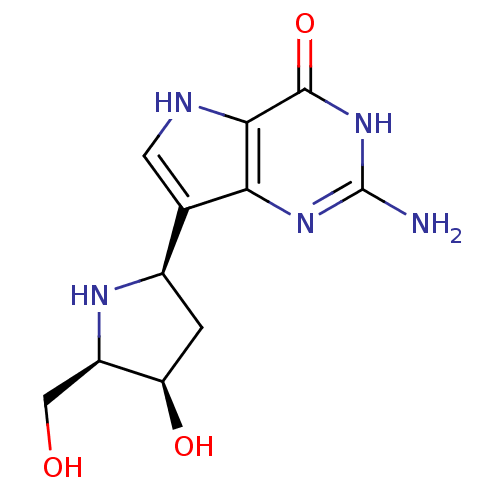 (CHEMBL114781) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human purine nucleoside phosphorylase; Initial rate. | J Med Chem 46: 3412-23 (2003) Article DOI: 10.1021/jm030145r BindingDB Entry DOI: 10.7270/Q2X92C29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22103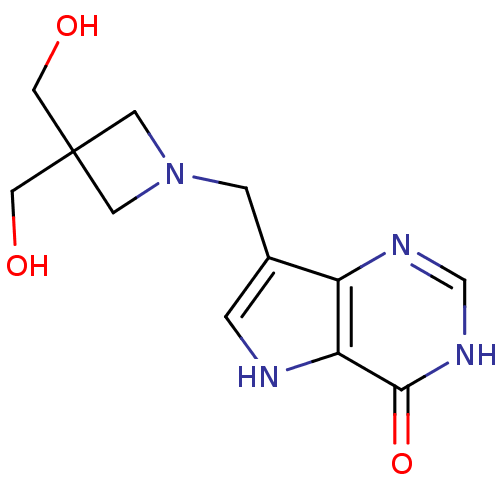 (7-{[3,3-bis(hydroxymethyl)azetidin-1-yl]methyl}-3H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human PNP by xanthine-oxidase coupled assay | J Med Chem 52: 1126-43 (2009) Article DOI: 10.1021/jm801421q BindingDB Entry DOI: 10.7270/Q2QR4Z18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22103 (7-{[3,3-bis(hydroxymethyl)azetidin-1-yl]methyl}-3H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.229 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.7 | 22 |
Industrial Research Limited | Assay Description PNP activity was monitored by absorbance change in a coupled assay. In the assay, inosine was converted to hypoxanthine, and then hypoxanthine was co... | J Med Chem 51: 948-56 (2008) Article DOI: 10.1021/jm701265n BindingDB Entry DOI: 10.7270/Q2QC01T3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50370255 (CHEMBL115146) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Industrial Research Limited Curated by ChEMBL | Assay Description Inhibition of human purine nucleoside phosphorylase; Initial rate. | J Med Chem 46: 3412-23 (2003) Article DOI: 10.1021/jm030145r BindingDB Entry DOI: 10.7270/Q2X92C29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4176 total ) | Next | Last >> |