

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301603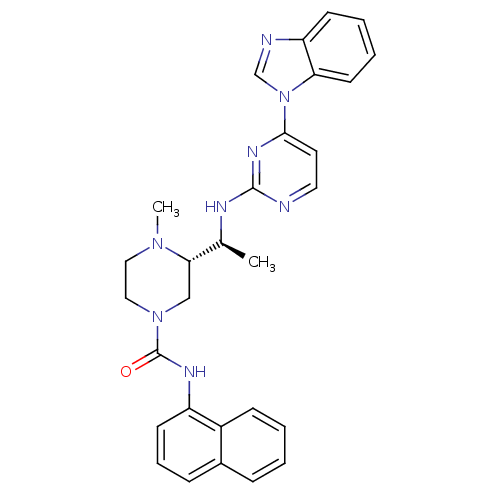 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301604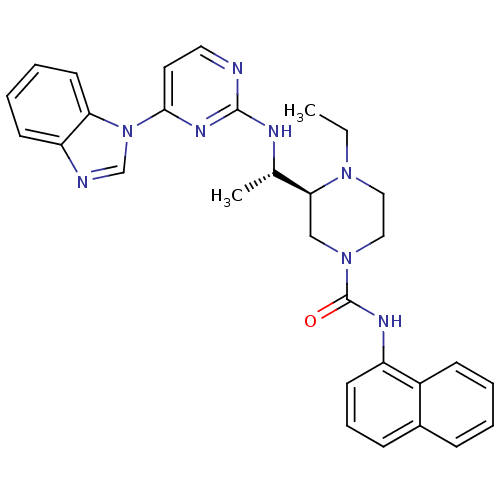 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301619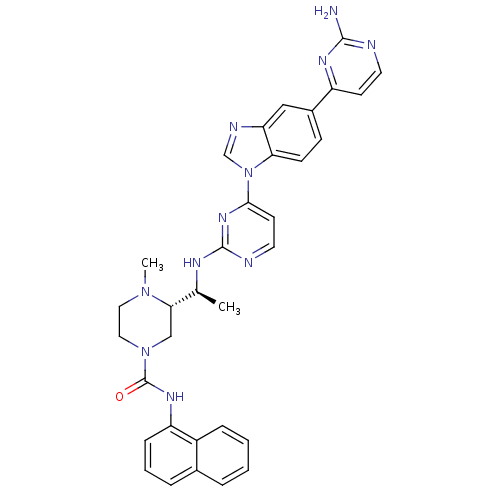 ((S)-3-((S)-1-(4-(5-(2-aminopyrimidin-4-yl)-1H-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301624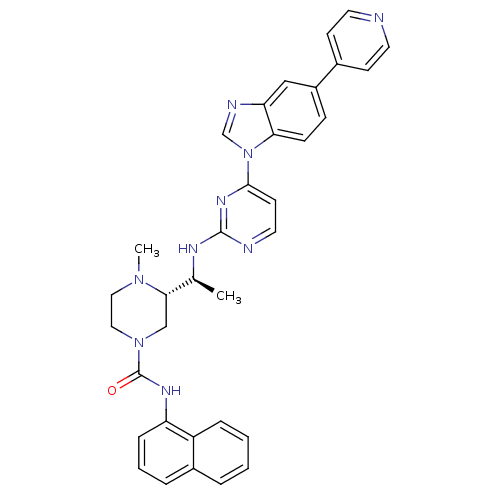 ((S)-4-methyl-N(S)-4-methyl-N-(naphthalen-1-yl)-3-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301605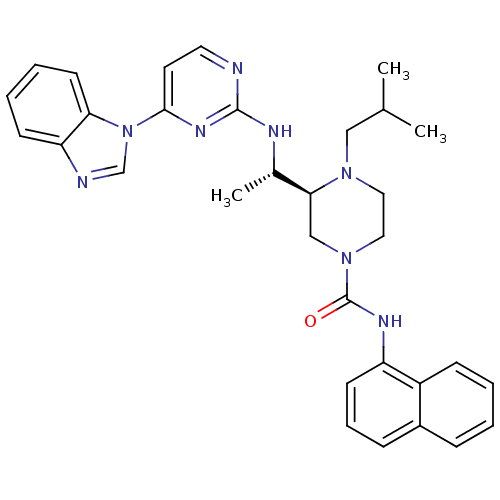 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301607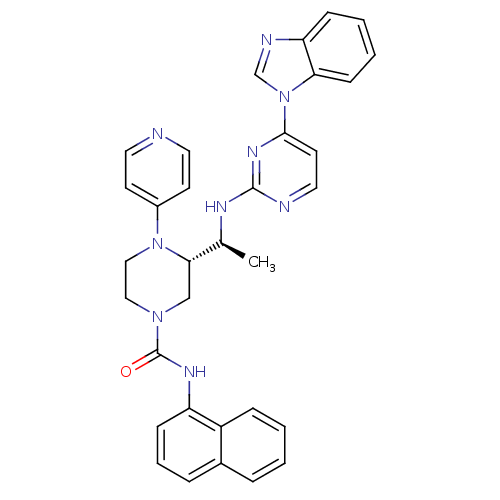 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301594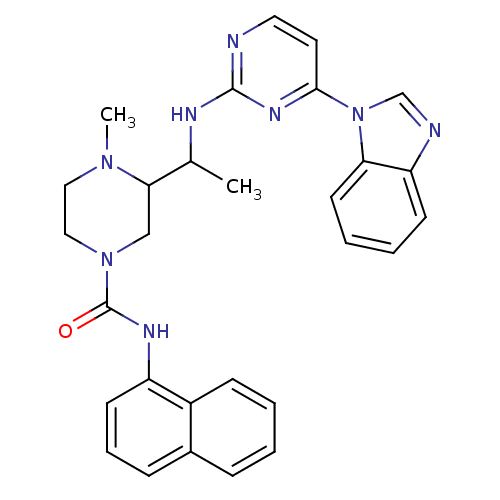 (3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301594 (3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301588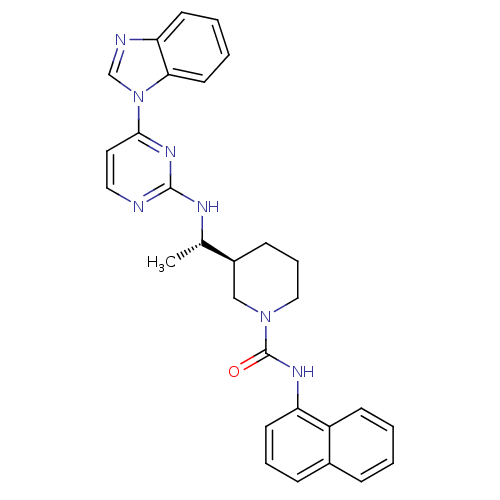 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301618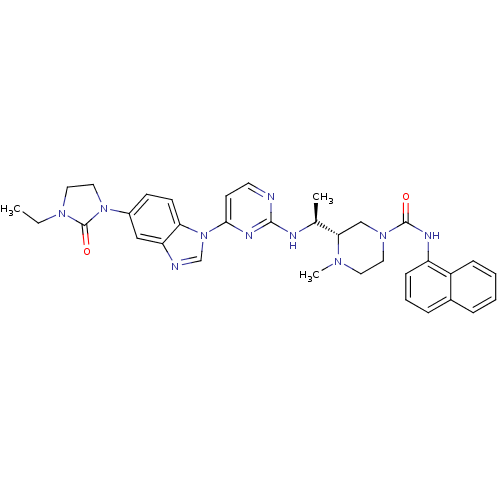 ((S)-3-((S)-1-(4-(5-(3-ethyl-2-oxoimidazolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301608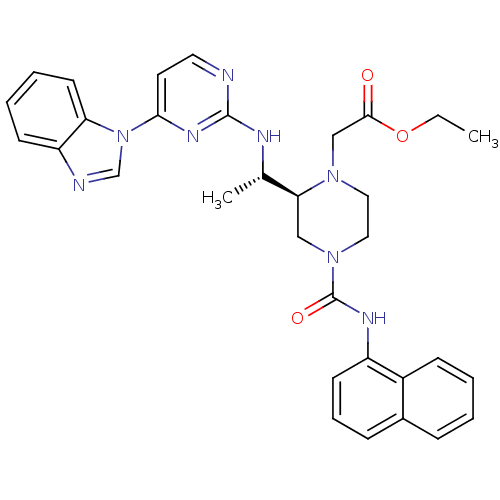 (CHEMBL566507 | ethyl 2-((S)-2-((S)-1-(4-(1H-benzo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98812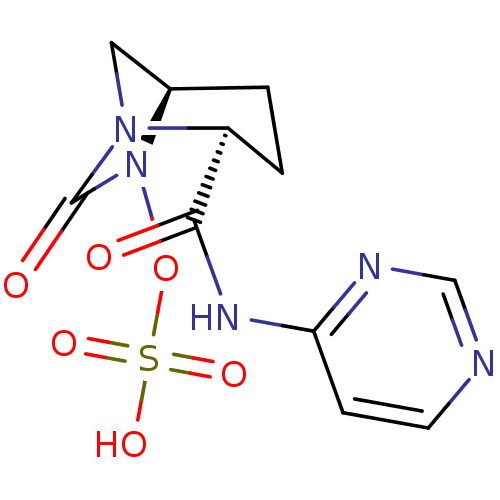 (US8487093, 204) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98802 (US8487093, 192) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301587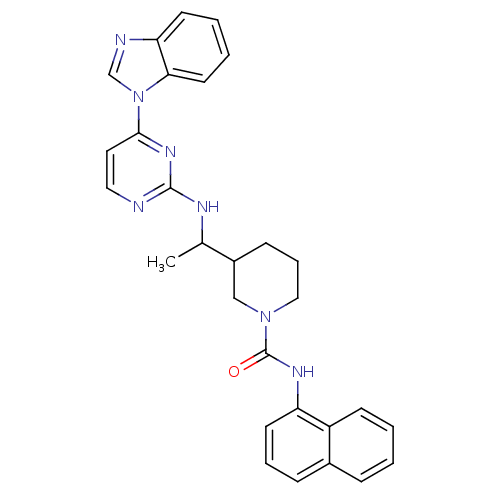 (CHEMBL567885 | rac 3-(1-(4-(1H-benzo[d]imidazol-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301617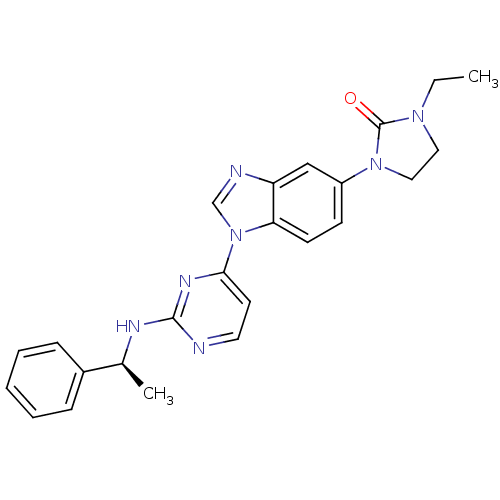 ((S)-1-ethyl-3-(1-(2-(1-phenylethylamino)pyrimidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301621 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| H2-K region expressed gene 2, rat orthologue (Rattus norvegicus) | BDBM50079829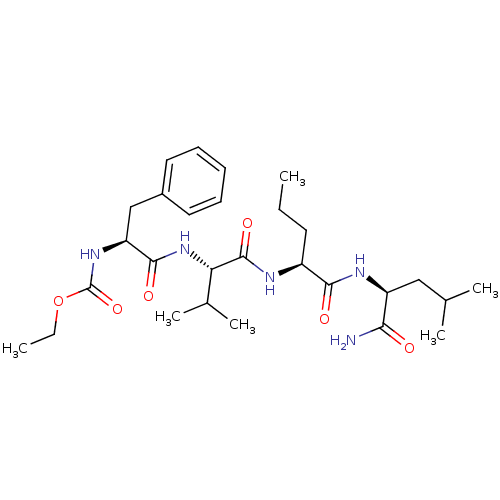 (((S)-1-{(S)-1-[(S)-1-((S)-1-Carbamoyl-3-methyl-but...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to inhibit binding of biotinylated rat myelin basic protein 13-mer peptide (RMBP 90-102, Major histocompatibility complex class II to purifie... | Bioorg Med Chem Lett 9: 2109-14 (1999) BindingDB Entry DOI: 10.7270/Q2NZ86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| H2-K region expressed gene 2, rat orthologue (Rattus norvegicus) | BDBM50079824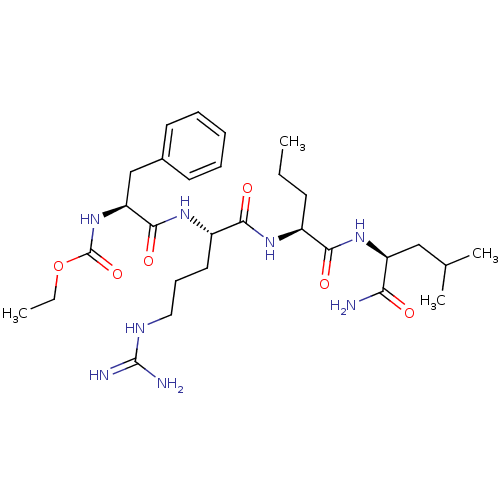 (((S)-1-{(S)-1-[(S)-(S)-1-(1-Carbamoyl-3-methyl-but...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Ability to inhibit binding of biotinylated rat myelin basic protein 13-mer peptide (RMBP 90-102, Major histocompatibility complex class II to purifie... | Bioorg Med Chem Lett 9: 2109-14 (1999) BindingDB Entry DOI: 10.7270/Q2NZ86TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98861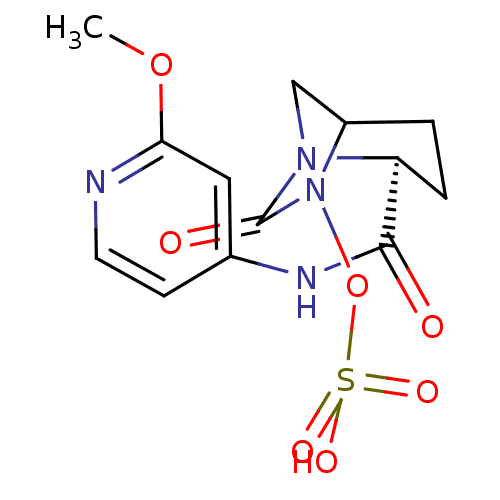 (US8487093, 7) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98792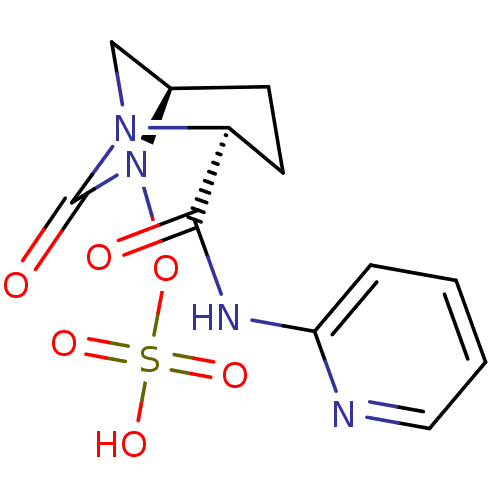 (US8487093, 180) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50079824 (((S)-1-{(S)-1-[(S)-(S)-1-(1-Carbamoyl-3-methyl-but...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration to inhibit the binding of biotinylated rat myelin basic protein peptide (RMBP90-102) against DR1 allele of class II MHC for ... | Bioorg Med Chem Lett 7: 19-24 (1997) Article DOI: 10.1016/S0960-894X(96)00579-3 BindingDB Entry DOI: 10.7270/Q2Q52PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50289286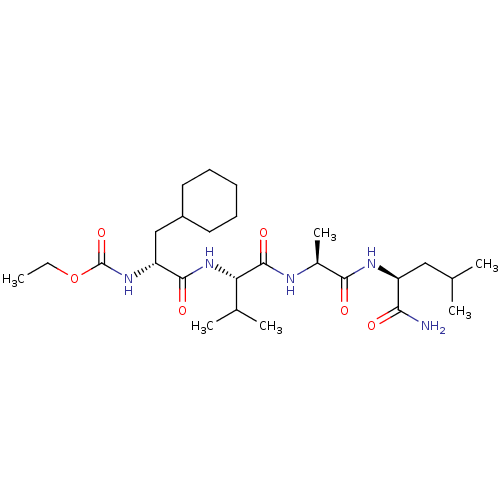 (((R)-1-{(S)-1-[(S)-1-((S)-1-Carbamoyl-3-methyl-but...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration to inhibit the binding of biotinylated rat myelin basic protein peptide (RMBP90-102) against DR1 allele of class II MHC for ... | Bioorg Med Chem Lett 7: 19-24 (1997) Article DOI: 10.1016/S0960-894X(96)00579-3 BindingDB Entry DOI: 10.7270/Q2Q52PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301616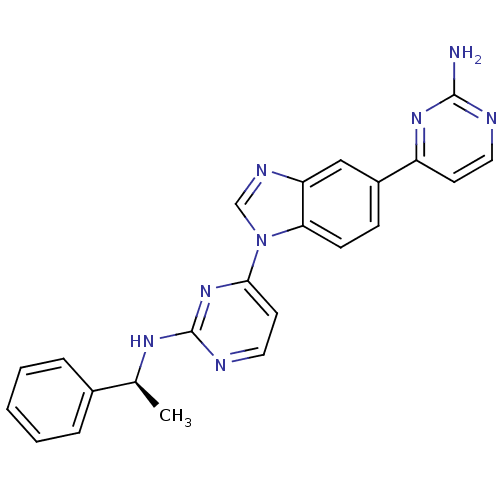 ((S)-4-(5-(2-aminopyrimidin-4-yl)-1H-benzo[d]imidaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98862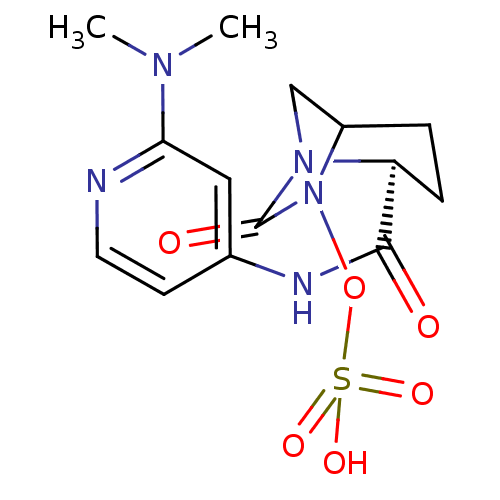 (US8487093, 8) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM98862 (US8487093, 8) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98819 (US8487093, 212) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301606 ((S)-3-((S)-1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM98847 (US8487093, 266) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98806 (US8487093, 196) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98799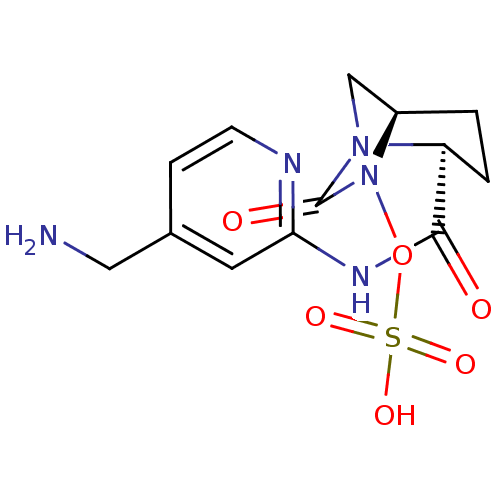 (US8487093, 188) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301595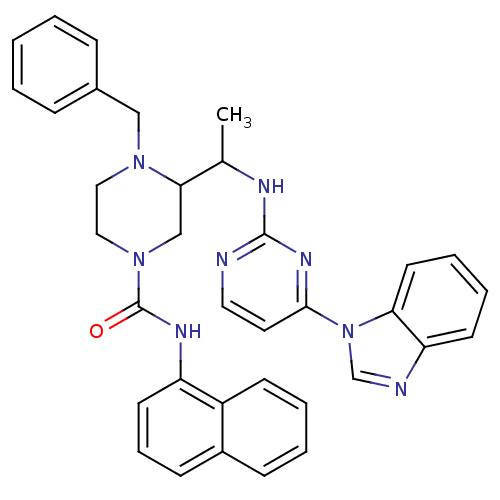 (3-(1-(4-(1H-benzo[d]imidazol-1-yl)pyrimidin-2-ylam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50301623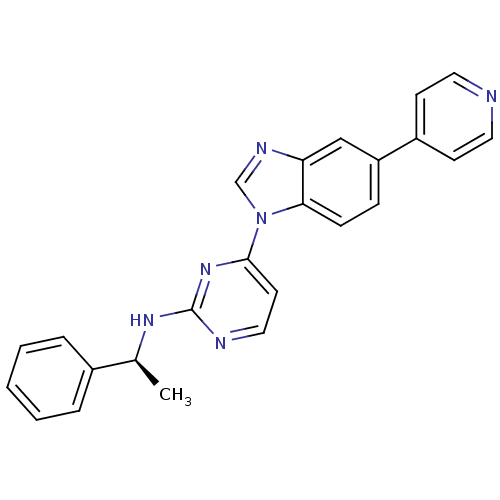 ((S)-N-(1-phenylethyl)-4-(5-(pyridin-4-yl)-1H-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Lck | Bioorg Med Chem Lett 19: 5440-3 (2009) Article DOI: 10.1016/j.bmcl.2009.07.102 BindingDB Entry DOI: 10.7270/Q2GX4BM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM98834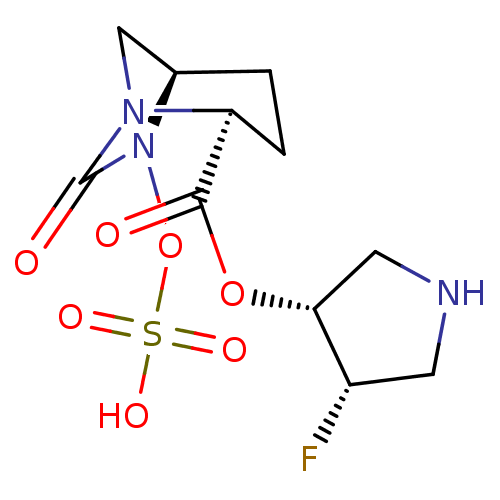 (US8487093, 242) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98800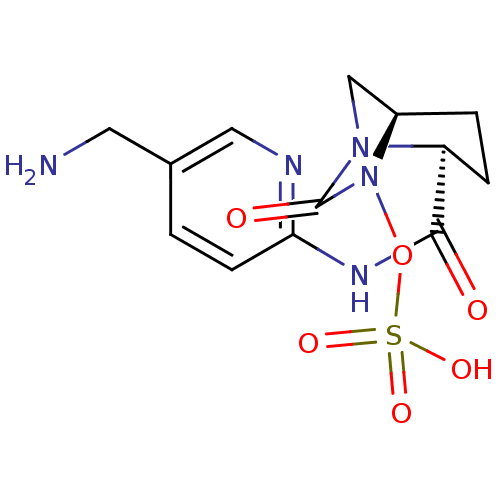 (US8487093, 190) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98798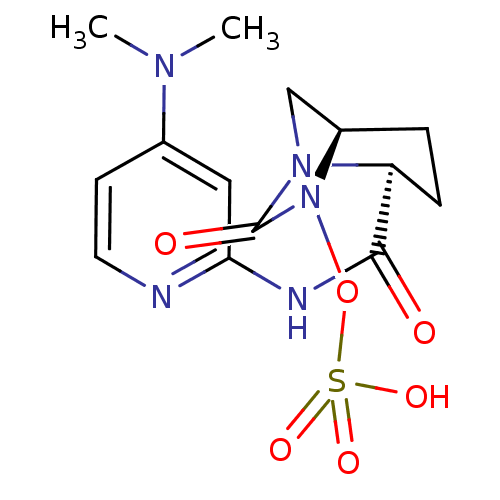 (US8487093, 186) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98790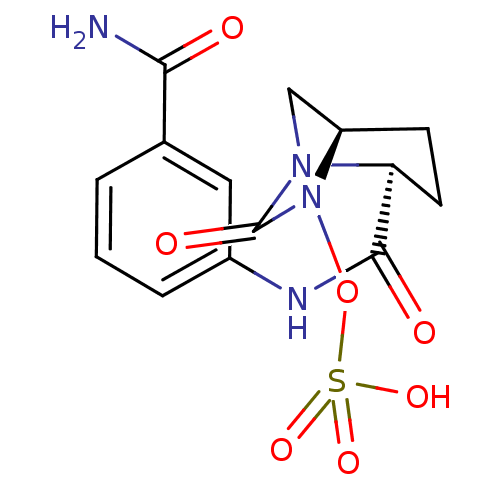 (US8487093, 176) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM98831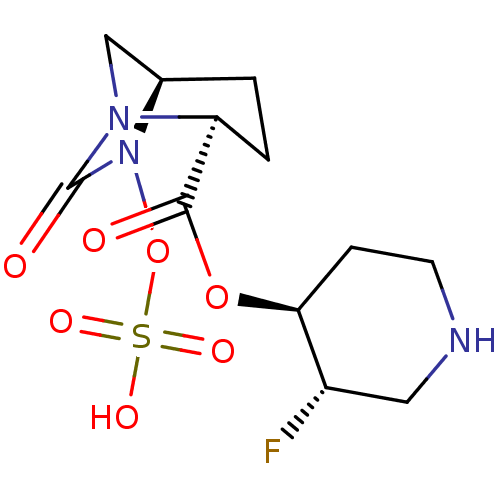 (US8487093, 236) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98810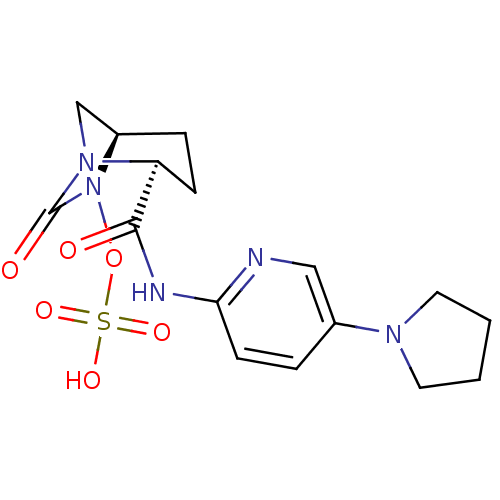 (US8487093, 200) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM98833 (US8487093, 240) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50347189 (CHEMBL1795572) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC | Bioorg Med Chem Lett 21: 4363-5 (2011) Article DOI: 10.1016/j.bmcl.2011.04.122 BindingDB Entry DOI: 10.7270/Q24Q7V94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98808 (US8487093, 198) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50289247 ((S)-2-((S)-3-Carbamoyl-2-{(S)-2-[((R)-2-ethoxycarb...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit the binding of biotinylated rat myelin basic protein peptide (RMBP90-102) against DR1 allele of class II... | Bioorg Med Chem Lett 7: 19-24 (1997) Article DOI: 10.1016/S0960-894X(96)00579-3 BindingDB Entry DOI: 10.7270/Q2Q52PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98811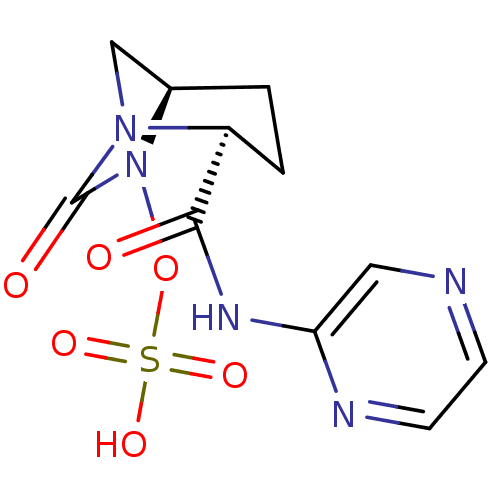 (US8487093, 202) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98854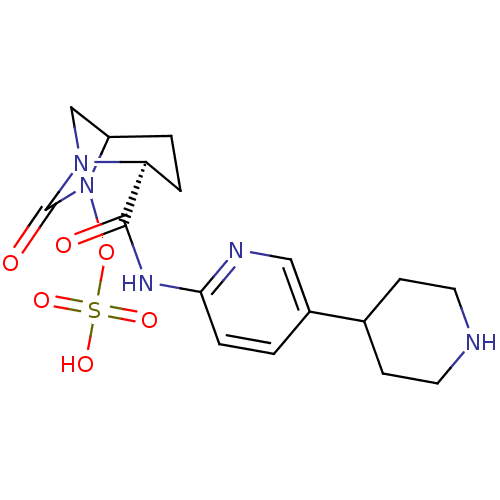 (US8487093, 17) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Klebsiella pneumoniae) | BDBM98822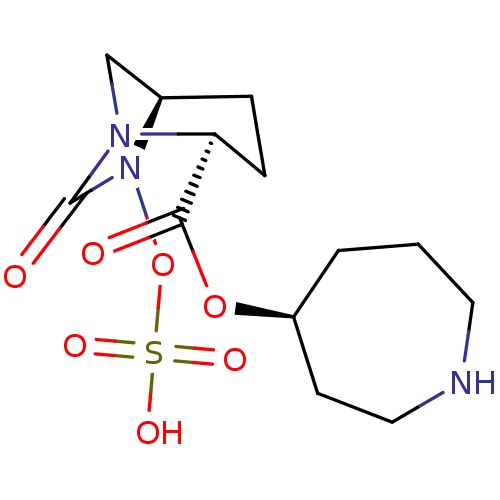 (US8487093, 218) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98860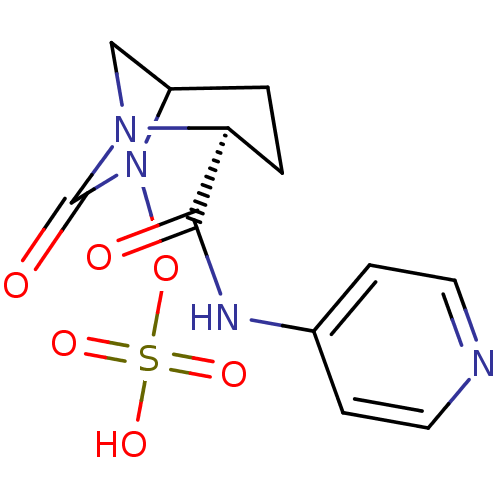 (US8487093, 6) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| MHC class II antigen (Homo sapiens (Human)) | BDBM50289278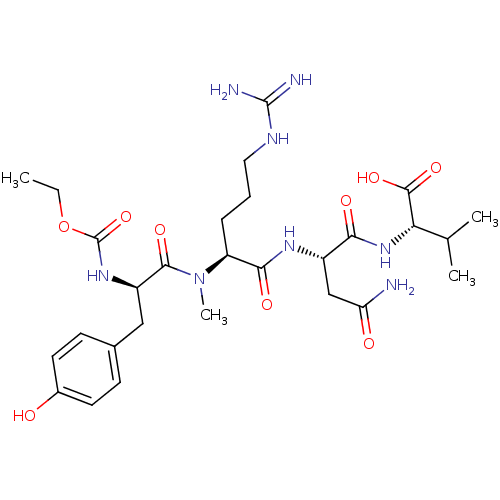 ((S)-2-[(S)-3-Carbamoyl-2-((S)-2-{[(R)-2-ethoxycarb...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit the binding of biotinylated rat myelin basic protein peptide (RMBP90-102) against DR1 allele of class II... | Bioorg Med Chem Lett 7: 19-24 (1997) Article DOI: 10.1016/S0960-894X(96)00579-3 BindingDB Entry DOI: 10.7270/Q2Q52PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98788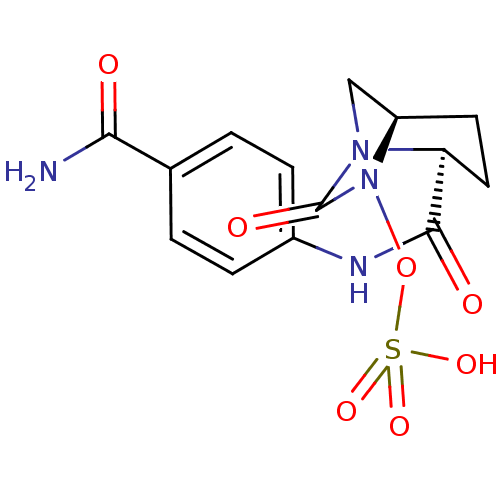 (US8487093, 172) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa) | BDBM98789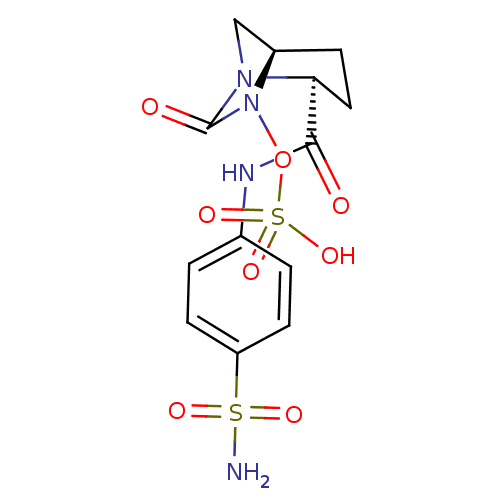 (US8487093, 174) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Enzyme activities were measured in the presence of the test inhibitor in spectrophotometric assay. | US Patent US8487093 (2013) BindingDB Entry DOI: 10.7270/Q2057DKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Pseudomonas aeruginosa (PAO1)) | BDBM50347184 (CHEMBL1795567) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co. Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa beta lactamase AmpC | Bioorg Med Chem Lett 21: 4363-5 (2011) Article DOI: 10.1016/j.bmcl.2011.04.122 BindingDB Entry DOI: 10.7270/Q24Q7V94 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 547 total ) | Next | Last >> |