 Found 193 hits with Last Name = 'kelland' and Initial = 'lr'
Found 193 hits with Last Name = 'kelland' and Initial = 'lr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50108806

(3,6,8,11,13-pentamethyl-8H-quino[4,3,2-kl]acridin-...)Show SMILES Cc1ccc2n(C)c3cc(C)cc4c5cc(C)ccc5[n+](C)c(c2c1)c34 Show InChI InChI=1S/C24H23N2/c1-14-6-8-20-17(10-14)18-12-16(3)13-22-23(18)24(26(20)5)19-11-15(2)7-9-21(19)25(22)4/h6-13H,1-5H3/q+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity using TRAP assay |
J Med Chem 45: 590-7 (2002)
BindingDB Entry DOI: 10.7270/Q2BR8RGQ |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50108803
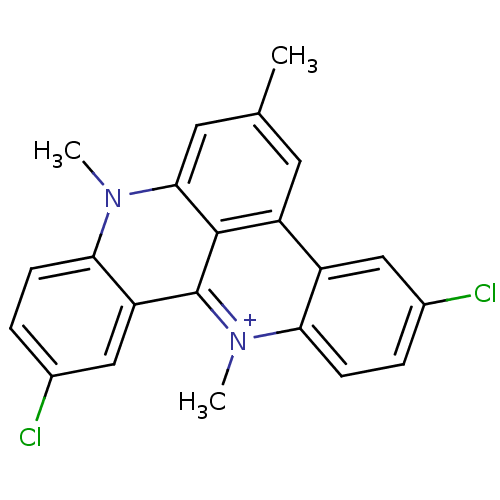
(3,11-dichloro-6,8,13-trimethyl-8H-quino[4,3,2-kl]a...)Show SMILES Cc1cc2n(C)c3ccc(Cl)cc3c3[n+](C)c4ccc(Cl)cc4c(c1)c23 Show InChI InChI=1S/C22H17Cl2N2/c1-12-8-16-15-10-13(23)4-6-18(15)26(3)22-17-11-14(24)5-7-19(17)25(2)20(9-12)21(16)22/h4-11H,1-3H3/q+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity using TRAP assay |
J Med Chem 45: 590-7 (2002)
BindingDB Entry DOI: 10.7270/Q2BR8RGQ |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50108802

(3,11-difluoro-6,8,13-trimethyl-8H-quino[4,3,2-kl]a...)Show SMILES Cc1cc2n(C)c3ccc(F)cc3c3[n+](C)c4ccc(F)cc4c(c1)c23 Show InChI InChI=1S/C22H17F2N2/c1-12-8-16-15-10-13(23)4-6-18(15)26(3)22-17-11-14(24)5-7-19(17)25(2)20(9-12)21(16)22/h4-11H,1-3H3/q+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity using TRAP assay |
J Med Chem 45: 590-7 (2002)
BindingDB Entry DOI: 10.7270/Q2BR8RGQ |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50108805
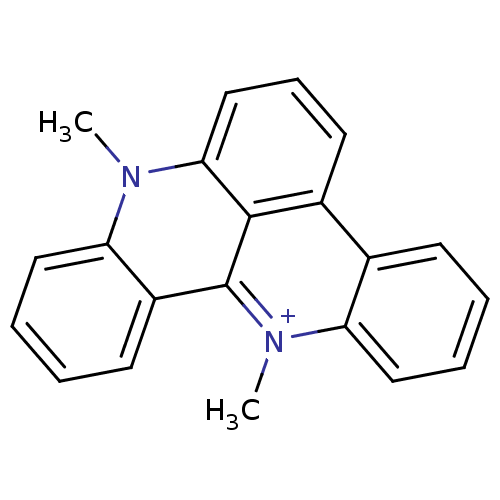
(8,13-Dimethyl-8H-8-aza-13-azonia-dibenzo[a,de]anth...)Show InChI InChI=1S/C21H17N2/c1-22-18-12-6-4-9-16(18)21-20-15(10-7-13-19(20)22)14-8-3-5-11-17(14)23(21)2/h3-13H,1-2H3/q+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity using TRAP assay |
J Med Chem 45: 590-7 (2002)
BindingDB Entry DOI: 10.7270/Q2BR8RGQ |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50108801

(6,8,13-trimethyl-8H-quino[4,3,2-kl]acridin-13-ium;...)Show SMILES Cc1cc2n(C)c3ccccc3c3[n+](C)c4ccccc4c(c1)c23 Show InChI InChI=1S/C22H19N2/c1-14-12-17-15-8-4-6-10-18(15)24(3)22-16-9-5-7-11-19(16)23(2)20(13-14)21(17)22/h4-13H,1-3H3/q+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity using TRAP assay |
J Med Chem 45: 590-7 (2002)
BindingDB Entry DOI: 10.7270/Q2BR8RGQ |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068324
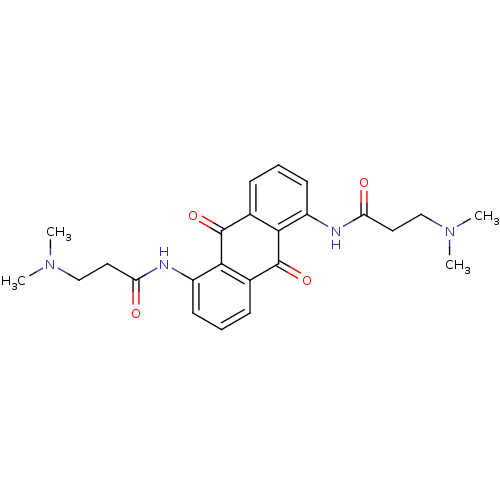
(3-Dimethylamino-N-[5-(3-dimethylamino-propionylami...)Show SMILES CN(C)CCC(=O)Nc1cccc2C(=O)c3c(NC(=O)CCN(C)C)cccc3C(=O)c12 Show InChI InChI=1S/C24H28N4O4/c1-27(2)13-11-19(29)25-17-9-5-7-15-21(17)23(31)16-8-6-10-18(22(16)24(15)32)26-20(30)12-14-28(3)4/h5-10H,11-14H2,1-4H3,(H,25,29)(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080842
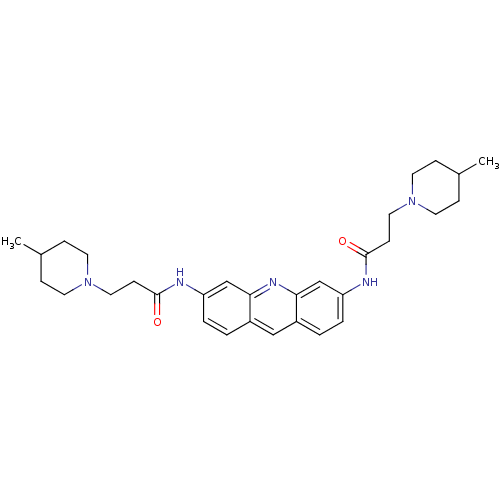
(3,6-Bis[3-(4-methylpiperidino)propionamido]acridin...)Show SMILES CC1CCN(CCC(=O)Nc2ccc3cc4ccc(NC(=O)CCN5CCC(C)CC5)cc4nc3c2)CC1 Show InChI InChI=1S/C31H41N5O2/c1-22-7-13-35(14-8-22)17-11-30(37)32-26-5-3-24-19-25-4-6-27(21-29(25)34-28(24)20-26)33-31(38)12-18-36-15-9-23(2)10-16-36/h3-6,19-23H,7-18H2,1-2H3,(H,32,37)(H,33,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit human telomerase in a modified cell-free TRAP assay using extracts from A2780 cell line |
Bioorg Med Chem Lett 9: 2463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2H41QN7 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50082524
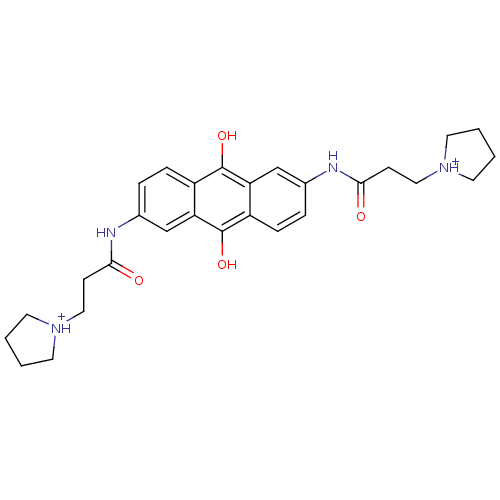
(2,6-Bis(3-pyrrolidinopropionamido)anthracene-9,10-...)Show SMILES Oc1c2ccc(NC(=O)CC[NH+]3CCCC3)cc2c(O)c2ccc(NC(=O)CC[NH+]3CCCC3)cc12 Show InChI InChI=1S/C28H34N4O4/c33-25(9-15-31-11-1-2-12-31)29-19-5-7-21-23(17-19)27(35)22-8-6-20(18-24(22)28(21)36)30-26(34)10-16-32-13-3-4-14-32/h5-8,17-18,35-36H,1-4,9-16H2,(H,29,33)(H,30,34)/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Telomerase evaluated by TRAP Assay studies. |
J Med Chem 42: 4538-46 (1999)
BindingDB Entry DOI: 10.7270/Q2J103VC |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068304
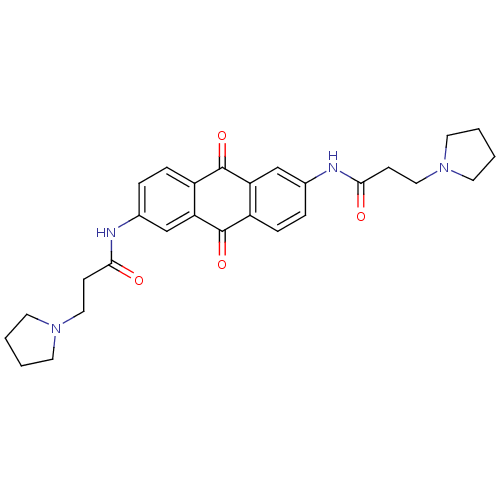
(CHEMBL343445 | N-[9,10-Dioxo-6-(3-pyrrolidin-1-yl-...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2C(=O)c3cc(NC(=O)CCN4CCCC4)ccc3C(=O)c2c1 Show InChI InChI=1S/C28H32N4O4/c33-25(9-15-31-11-1-2-12-31)29-19-5-7-21-23(17-19)27(35)22-8-6-20(18-24(22)28(21)36)30-26(34)10-16-32-13-3-4-14-32/h5-8,17-18H,1-4,9-16H2,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068320

(2,7-Bis[3-(pyrrolidino)propionamido]anthraquinone ...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2C(=O)c3ccc(NC(=O)CCN4CCCC4)cc3C(=O)c2c1 Show InChI InChI=1S/C28H32N4O4/c33-25(9-15-31-11-1-2-12-31)29-19-5-7-21-23(17-19)28(36)24-18-20(6-8-22(24)27(21)35)30-26(34)10-16-32-13-3-4-14-32/h5-8,17-18H,1-4,9-16H2,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50108804

(8,13-Diethyl-6-methyl-8H-8-aza-13-azonia-dibenzo[a...)Show SMILES CCn1c2ccccc2c2[n+](CC)c3ccccc3c3cc(C)cc1c23 Show InChI InChI=1S/C24H23N2/c1-4-25-21-13-9-7-11-18(21)24-23-19(14-16(3)15-22(23)25)17-10-6-8-12-20(17)26(24)5-2/h6-15H,4-5H2,1-3H3/q+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity using TRAP assay |
J Med Chem 45: 590-7 (2002)
BindingDB Entry DOI: 10.7270/Q2BR8RGQ |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068330

(CHEMBL144848 | N-[9,10-Dioxo-5-(3-piperidin-1-yl-p...)Show SMILES O=C(CCN1CCCCC1)Nc1cccc2C(=O)c3c(NC(=O)CCN4CCCCC4)cccc3C(=O)c12 Show InChI InChI=1S/C30H36N4O4/c35-25(13-19-33-15-3-1-4-16-33)31-23-11-7-9-21-27(23)29(37)22-10-8-12-24(28(22)30(21)38)32-26(36)14-20-34-17-5-2-6-18-34/h7-12H,1-6,13-20H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080848
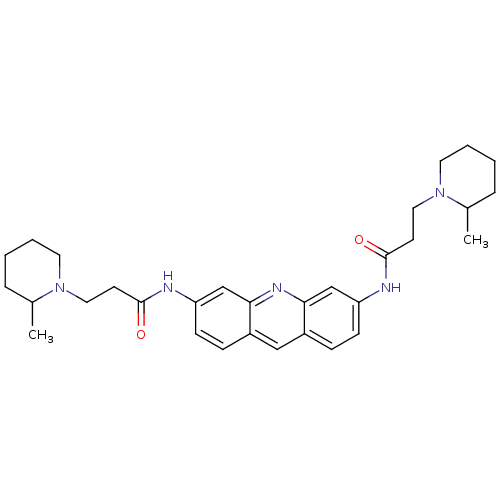
(3-(2-Methyl-piperidin-1-yl)-N-{6-[3-(2-methyl-pipe...)Show SMILES CC1CCCCN1CCC(=O)Nc1ccc2cc3ccc(NC(=O)CCN4CCCCC4C)cc3nc2c1 Show InChI InChI=1S/C31H41N5O2/c1-22-7-3-5-15-35(22)17-13-30(37)32-26-11-9-24-19-25-10-12-27(21-29(25)34-28(24)20-26)33-31(38)14-18-36-16-6-4-8-23(36)2/h9-12,19-23H,3-8,13-18H2,1-2H3,(H,32,37)(H,33,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit human telomerase in a modified cell-free TRAP assay using extracts from A2780 cell line |
Bioorg Med Chem Lett 9: 2463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2H41QN7 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068303
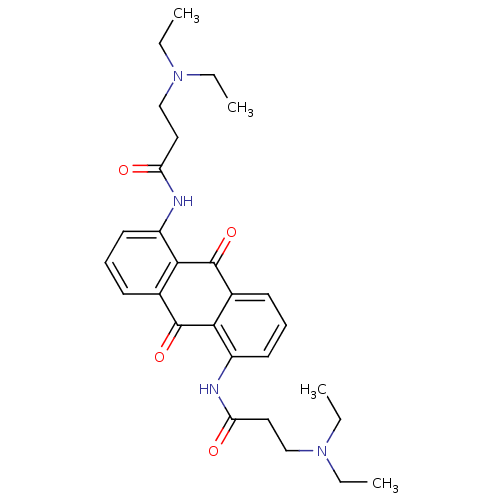
(1,5-BIS[3-(DIETHYLAMINO)PROPIONAMIDO]ANTHRACENE-9,...)Show SMILES CCN(CC)CCC(=O)Nc1cccc2C(=O)c3c(NC(=O)CCN(CC)CC)cccc3C(=O)c12 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)17-15-23(33)29-21-13-9-11-19-25(21)27(35)20-12-10-14-22(26(20)28(19)36)30-24(34)16-18-32(7-3)8-4/h9-14H,5-8,15-18H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080837

(3-(2-Ethyl-piperidin-1-yl)-N-{6-[3-(2-ethyl-piperi...)Show SMILES CCC1CCCCN1CCC(=O)Nc1ccc2cc3ccc(NC(=O)CCN4CCCCC4CC)cc3nc2c1 Show InChI InChI=1S/C33H45N5O2/c1-3-28-9-5-7-17-37(28)19-15-32(39)34-26-13-11-24-21-25-12-14-27(23-31(25)36-30(24)22-26)35-33(40)16-20-38-18-8-6-10-29(38)4-2/h11-14,21-23,28-29H,3-10,15-20H2,1-2H3,(H,34,39)(H,35,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit human telomerase in a modified cell-free TRAP assay using extracts from A2780 cell line |
Bioorg Med Chem Lett 9: 2463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2H41QN7 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080847

(3,6-Bis(3-piperidinopropionamido)acridine | 3-Pipe...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2cc3ccc(NC(=O)CCN4CCCCC4)cc3nc2c1 Show InChI InChI=1S/C29H37N5O2/c35-28(11-17-33-13-3-1-4-14-33)30-24-9-7-22-19-23-8-10-25(21-27(23)32-26(22)20-24)31-29(36)12-18-34-15-5-2-6-16-34/h7-10,19-21H,1-6,11-18H2,(H,30,35)(H,31,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Telomerase evaluated by TRAP Assay studies. |
J Med Chem 42: 4538-46 (1999)
BindingDB Entry DOI: 10.7270/Q2J103VC |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080847

(3,6-Bis(3-piperidinopropionamido)acridine | 3-Pipe...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2cc3ccc(NC(=O)CCN4CCCCC4)cc3nc2c1 Show InChI InChI=1S/C29H37N5O2/c35-28(11-17-33-13-3-1-4-14-33)30-24-9-7-22-19-23-8-10-25(21-27(23)32-26(22)20-24)31-29(36)12-18-34-15-5-2-6-16-34/h7-10,19-21H,1-6,11-18H2,(H,30,35)(H,31,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit human telomerase in a modified cell-free TRAP assay using extracts from A2780 cell line |
Bioorg Med Chem Lett 9: 2463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2H41QN7 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068321

(2,7-Bis[3-(piperidino)propionamido]anthraquinone |...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2C(=O)c3ccc(NC(=O)CCN4CCCCC4)cc3C(=O)c2c1 Show InChI InChI=1S/C30H36N4O4/c35-27(11-17-33-13-3-1-4-14-33)31-21-7-9-23-25(19-21)30(38)26-20-22(8-10-24(26)29(23)37)32-28(36)12-18-34-15-5-2-6-16-34/h7-10,19-20H,1-6,11-18H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068321

(2,7-Bis[3-(piperidino)propionamido]anthraquinone |...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2C(=O)c3ccc(NC(=O)CCN4CCCCC4)cc3C(=O)c2c1 Show InChI InChI=1S/C30H36N4O4/c35-27(11-17-33-13-3-1-4-14-33)31-21-7-9-23-25(19-21)30(38)26-20-22(8-10-24(26)29(23)37)32-28(36)12-18-34-15-5-2-6-16-34/h7-10,19-20H,1-6,11-18H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080840
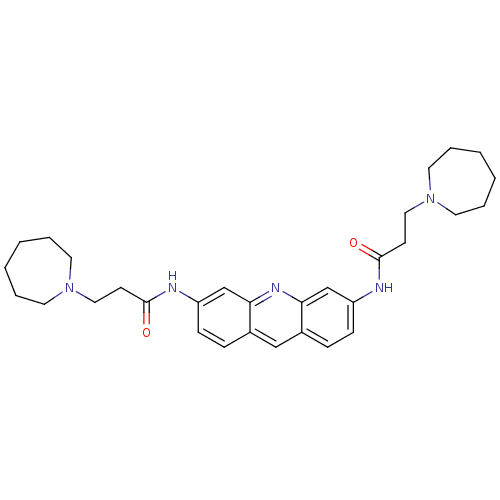
(3,6-Bis[3-(azepan-1-yl)propionamido]acridine | 3,6...)Show SMILES O=C(CCN1CCCCCC1)Nc1ccc2cc3ccc(NC(=O)CCN4CCCCCC4)cc3nc2c1 Show InChI InChI=1S/C31H41N5O2/c37-30(13-19-35-15-5-1-2-6-16-35)32-26-11-9-24-21-25-10-12-27(23-29(25)34-28(24)22-26)33-31(38)14-20-36-17-7-3-4-8-18-36/h9-12,21-23H,1-8,13-20H2,(H,32,37)(H,33,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit human telomerase in a modified cell-free TRAP assay using extracts from A2780 cell line |
Bioorg Med Chem Lett 9: 2463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2H41QN7 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080840

(3,6-Bis[3-(azepan-1-yl)propionamido]acridine | 3,6...)Show SMILES O=C(CCN1CCCCCC1)Nc1ccc2cc3ccc(NC(=O)CCN4CCCCCC4)cc3nc2c1 Show InChI InChI=1S/C31H41N5O2/c37-30(13-19-35-15-5-1-2-6-16-35)32-26-11-9-24-21-25-10-12-27(23-29(25)34-28(24)22-26)33-31(38)14-20-36-17-7-3-4-8-18-36/h9-12,21-23H,1-8,13-20H2,(H,32,37)(H,33,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Telomerase evaluated by TRAP Assay studies. |
J Med Chem 42: 4538-46 (1999)
BindingDB Entry DOI: 10.7270/Q2J103VC |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068328
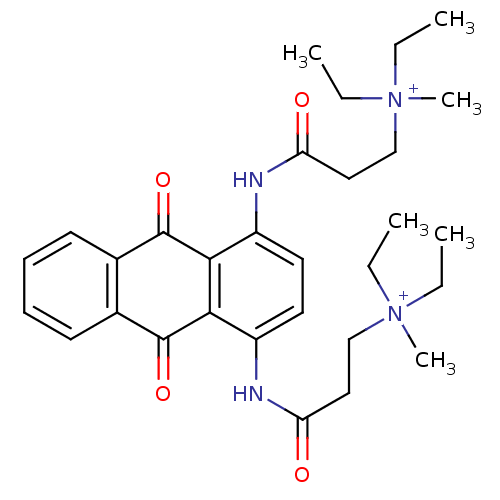
(CHEMBL144303 | N-[9,10-Dioxo-4-(3-(N,N-diethyl-N-m...)Show SMILES CC[N+](C)(CC)CCC(=O)Nc1ccc(NC(=O)CC[N+](C)(CC)CC)c2C(=O)c3ccccc3C(=O)c12 Show InChI InChI=1S/C30H40N4O4/c1-7-33(5,8-2)19-17-25(35)31-23-15-16-24(32-26(36)18-20-34(6,9-3)10-4)28-27(23)29(37)21-13-11-12-14-22(21)30(28)38/h11-16H,7-10,17-20H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50005750
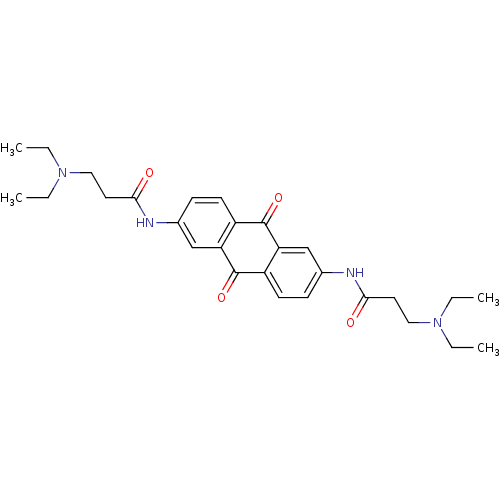
(3-Diethylamino-N-[6-(3-diethylamino-propionylamino...)Show SMILES CCN(CC)CCC(=O)Nc1ccc2C(=O)c3cc(NC(=O)CCN(CC)CC)ccc3C(=O)c2c1 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)15-13-25(33)29-19-9-11-21-23(17-19)27(35)22-12-10-20(18-24(22)28(21)36)30-26(34)14-16-32(7-3)8-4/h9-12,17-18H,5-8,13-16H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068332
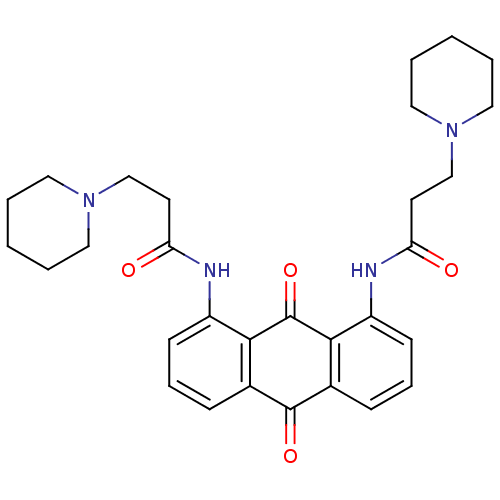
(CHEMBL144386 | N-[9,10-Dioxo-8-(3-piperidin-1-yl-p...)Show SMILES O=C(CCN1CCCCC1)Nc1cccc2C(=O)c3cccc(NC(=O)CCN4CCCCC4)c3C(=O)c12 Show InChI InChI=1S/C30H36N4O4/c35-25(13-19-33-15-3-1-4-16-33)31-23-11-7-9-21-27(23)30(38)28-22(29(21)37)10-8-12-24(28)32-26(36)14-20-34-17-5-2-6-18-34/h7-12H,1-6,13-20H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068311
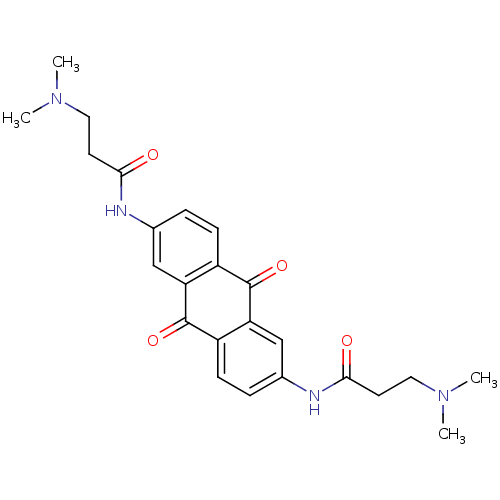
(3-Dimethylamino-N-[6-(3-dimethylamino-propionylami...)Show SMILES CN(C)CCC(=O)Nc1ccc2C(=O)c3cc(NC(=O)CCN(C)C)ccc3C(=O)c2c1 Show InChI InChI=1S/C24H28N4O4/c1-27(2)11-9-21(29)25-15-5-7-17-19(13-15)23(31)18-8-6-16(14-20(18)24(17)32)26-22(30)10-12-28(3)4/h5-8,13-14H,9-12H2,1-4H3,(H,25,29)(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080850

(3-[2-(2-Hydroxy-ethyl)-piperidin-1-yl]-N-(6-{3-[2-...)Show SMILES OCCC1CCCCN1CCC(=O)Nc1ccc2cc3ccc(NC(=O)CCN4CCCCC4CCO)cc3nc2c1 Show InChI InChI=1S/C33H45N5O4/c39-19-13-28-5-1-3-15-37(28)17-11-32(41)34-26-9-7-24-21-25-8-10-27(23-31(25)36-30(24)22-26)35-33(42)12-18-38-16-4-2-6-29(38)14-20-40/h7-10,21-23,28-29,39-40H,1-6,11-20H2,(H,34,41)(H,35,42) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit human telomerase in a modified cell-free TRAP assay using extracts from A2780 cell line |
Bioorg Med Chem Lett 9: 2463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2H41QN7 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068315

(3-Diethylamino-N-[8-(3-diethylamino-propionylamino...)Show SMILES CCN(CC)CCC(=O)Nc1cccc2C(=O)c3cccc(NC(=O)CCN(CC)CC)c3C(=O)c12 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)17-15-23(33)29-21-13-9-11-19-25(21)28(36)26-20(27(19)35)12-10-14-22(26)30-24(34)16-18-32(7-3)8-4/h9-14H,5-8,15-18H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068312

(2,7-Bis[3-(diethylamino)propionamido]anthraquinone...)Show SMILES CCN(CC)CCC(=O)Nc1ccc2C(=O)c3ccc(NC(=O)CCN(CC)CC)cc3C(=O)c2c1 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)15-13-25(33)29-19-9-11-21-23(17-19)28(36)24-18-20(10-12-22(24)27(21)35)30-26(34)14-16-32(7-3)8-4/h9-12,17-18H,5-8,13-16H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068312

(2,7-Bis[3-(diethylamino)propionamido]anthraquinone...)Show SMILES CCN(CC)CCC(=O)Nc1ccc2C(=O)c3ccc(NC(=O)CCN(CC)CC)cc3C(=O)c2c1 Show InChI InChI=1S/C28H36N4O4/c1-5-31(6-2)15-13-25(33)29-19-9-11-21-23(17-19)28(36)24-18-20(10-12-22(24)27(21)35)30-26(34)14-16-32(7-3)8-4/h9-12,17-18H,5-8,13-16H2,1-4H3,(H,29,33)(H,30,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068335
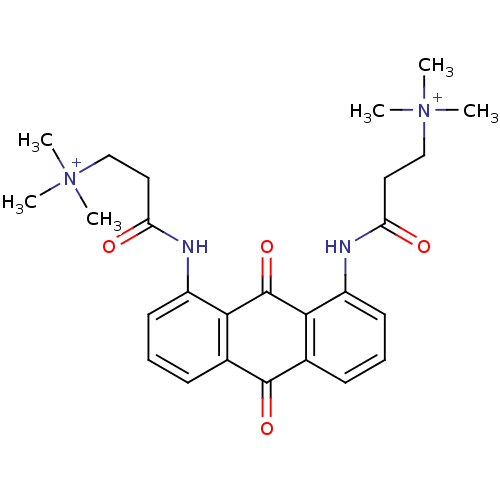
(CHEMBL422120 | N-[9,10-Dioxo-8-(3-(N,N,N-trimethyl...)Show SMILES C[N+](C)(C)CCC(=O)Nc1cccc2C(=O)c3cccc(NC(=O)CC[N+](C)(C)C)c3C(=O)c12 Show InChI InChI=1S/C26H32N4O4/c1-29(2,3)15-13-21(31)27-19-11-7-9-17-23(19)26(34)24-18(25(17)33)10-8-12-20(24)28-22(32)14-16-30(4,5)6/h7-12H,13-16H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080839

(3-(3-Methyl-piperidin-1-yl)-N-{6-[3-(3-methyl-pipe...)Show SMILES CC1CCCN(CCC(=O)Nc2ccc3cc4ccc(NC(=O)CCN5CCCC(C)C5)cc4nc3c2)C1 Show InChI InChI=1S/C31H41N5O2/c1-22-5-3-13-35(20-22)15-11-30(37)32-26-9-7-24-17-25-8-10-27(19-29(25)34-28(24)18-26)33-31(38)12-16-36-14-4-6-23(2)21-36/h7-10,17-19,22-23H,3-6,11-16,20-21H2,1-2H3,(H,32,37)(H,33,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit human telomerase in a modified cell-free TRAP assay using extracts from A2780 cell line |
Bioorg Med Chem Lett 9: 2463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2H41QN7 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50005746
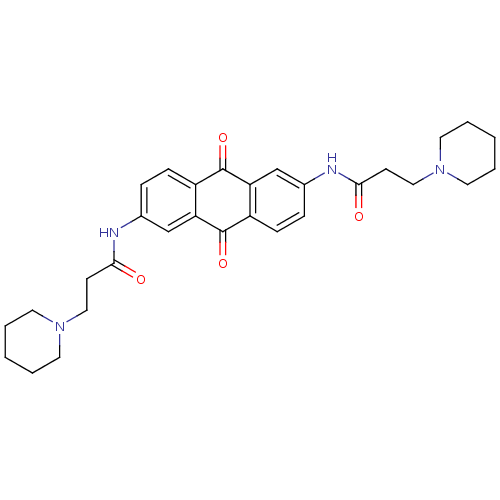
(CHEMBL109382 | CHEMBL33618 | N-[9,10-Dioxo-6-(3-pi...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2C(=O)c3cc(NC(=O)CCN4CCCCC4)ccc3C(=O)c2c1 Show InChI InChI=1S/C30H36N4O4/c35-27(11-17-33-13-3-1-4-14-33)31-21-7-9-23-25(19-21)29(37)24-10-8-22(20-26(24)30(23)38)32-28(36)12-18-34-15-5-2-6-16-34/h7-10,19-20H,1-6,11-18H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibition of telomerase activity |
J Med Chem 41: 3253-60 (1998)
Article DOI: 10.1021/jm9801105
BindingDB Entry DOI: 10.7270/Q2TX3G1R |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50082532

(2,6-Bis(3-piperidinopropionamido)anthracene-9,10-d...)Show SMILES Oc1c2ccc(NC(=O)CC[NH+]3CCCCC3)cc2c(O)c2ccc(NC(=O)CC[NH+]3CCCCC3)cc12 Show InChI InChI=1S/C30H38N4O4/c35-27(11-17-33-13-3-1-4-14-33)31-21-7-9-23-25(19-21)29(37)24-10-8-22(20-26(24)30(23)38)32-28(36)12-18-34-15-5-2-6-16-34/h7-10,19-20,37-38H,1-6,11-18H2,(H,31,35)(H,32,36)/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Telomerase evaluated by TRAP Assay studies. |
J Med Chem 42: 4538-46 (1999)
BindingDB Entry DOI: 10.7270/Q2J103VC |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50005746

(CHEMBL109382 | CHEMBL33618 | N-[9,10-Dioxo-6-(3-pi...)Show SMILES O=C(CCN1CCCCC1)Nc1ccc2C(=O)c3cc(NC(=O)CCN4CCCCC4)ccc3C(=O)c2c1 Show InChI InChI=1S/C30H36N4O4/c35-27(11-17-33-13-3-1-4-14-33)31-21-7-9-23-25(19-21)29(37)24-10-8-22(20-26(24)30(23)38)32-28(36)12-18-34-15-5-2-6-16-34/h7-10,19-20H,1-6,11-18H2,(H,31,35)(H,32,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068336
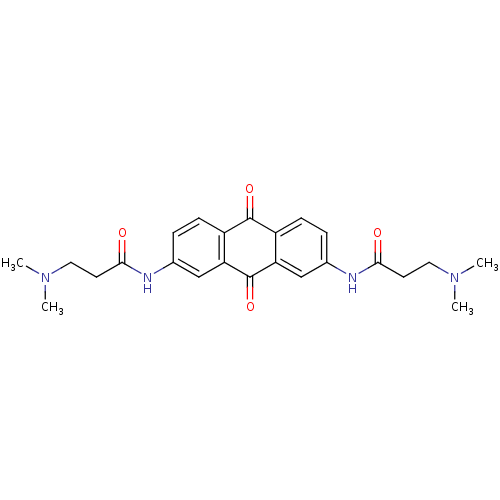
(2,7-Bis[3-(dimethylamino)propionamido]anthraquinon...)Show SMILES CN(C)CCC(=O)Nc1ccc2C(=O)c3ccc(NC(=O)CCN(C)C)cc3C(=O)c2c1 Show InChI InChI=1S/C24H28N4O4/c1-27(2)11-9-21(29)25-15-5-7-17-19(13-15)24(32)20-14-16(6-8-18(20)23(17)31)26-22(30)10-12-28(3)4/h5-8,13-14H,9-12H2,1-4H3,(H,25,29)(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068336

(2,7-Bis[3-(dimethylamino)propionamido]anthraquinon...)Show SMILES CN(C)CCC(=O)Nc1ccc2C(=O)c3ccc(NC(=O)CCN(C)C)cc3C(=O)c2c1 Show InChI InChI=1S/C24H28N4O4/c1-27(2)11-9-21(29)25-15-5-7-17-19(13-15)24(32)20-14-16(6-8-18(20)23(17)31)26-22(30)10-12-28(3)4/h5-8,13-14H,9-12H2,1-4H3,(H,25,29)(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
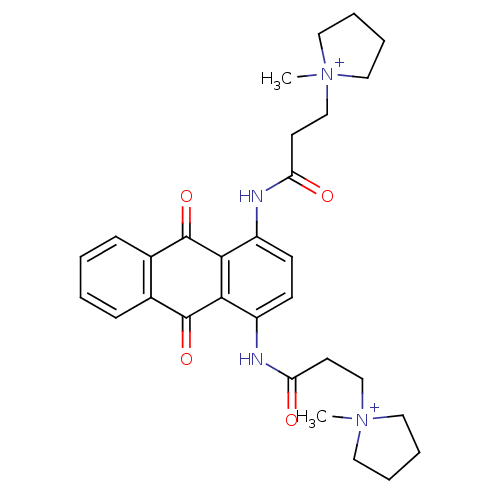
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068313
(CHEMBL143452 | N-[9,10-Dioxo-4-(3-(N-methylpyrroli...)Show SMILES C[N+]1(CCC(=O)Nc2ccc(NC(=O)CC[N+]3(C)CCCC3)c3C(=O)c4ccccc4C(=O)c23)CCCC1 Show InChI InChI=1S/C30H36N4O4/c1-33(15-5-6-16-33)19-13-25(35)31-23-11-12-24(32-26(36)14-20-34(2)17-7-8-18-34)28-27(23)29(37)21-9-3-4-10-22(21)30(28)38/h3-4,9-12H,5-8,13-20H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080849
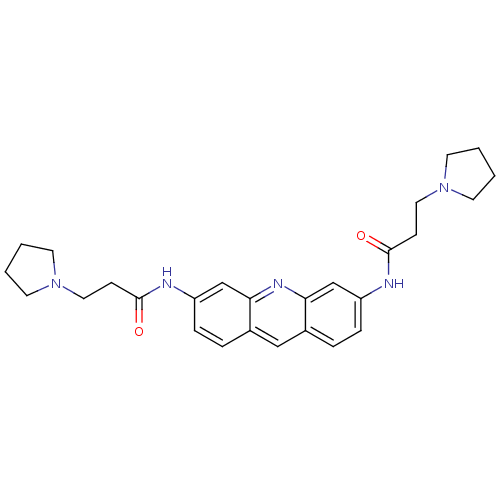
(3,6-Bis(3-pyrrolidinopropionamido)acridine | 3-PYR...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2cc3ccc(NC(=O)CCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C27H33N5O2/c33-26(9-15-31-11-1-2-12-31)28-22-7-5-20-17-21-6-8-23(19-25(21)30-24(20)18-22)29-27(34)10-16-32-13-3-4-14-32/h5-8,17-19H,1-4,9-16H2,(H,28,33)(H,29,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit human telomerase in a modified cell-free TRAP assay using extracts from A2780 cell line |
Bioorg Med Chem Lett 9: 2463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2H41QN7 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080849

(3,6-Bis(3-pyrrolidinopropionamido)acridine | 3-PYR...)Show SMILES O=C(CCN1CCCC1)Nc1ccc2cc3ccc(NC(=O)CCN4CCCC4)cc3nc2c1 Show InChI InChI=1S/C27H33N5O2/c33-26(9-15-31-11-1-2-12-31)28-22-7-5-20-17-21-6-8-23(19-25(21)30-24(20)18-22)29-27(34)10-16-32-13-3-4-14-32/h5-8,17-19H,1-4,9-16H2,(H,28,33)(H,29,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Telomerase evaluated by TRAP Assay studies. |
J Med Chem 42: 4538-46 (1999)
BindingDB Entry DOI: 10.7270/Q2J103VC |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080841

(3-(2-Hydroxymethyl-piperidin-1-yl)-N-{6-[3-(2-hydr...)Show SMILES OCC1CCCCN1CCC(=O)Nc1ccc2cc3ccc(NC(=O)CCN4CCCCC4CO)cc3nc2c1 Show InChI InChI=1S/C31H41N5O4/c37-20-26-5-1-3-13-35(26)15-11-30(39)32-24-9-7-22-17-23-8-10-25(19-29(23)34-28(22)18-24)33-31(40)12-16-36-14-4-2-6-27(36)21-38/h7-10,17-19,26-27,37-38H,1-6,11-16,20-21H2,(H,32,39)(H,33,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit human telomerase in a modified cell-free TRAP assay using extracts from A2780 cell line |
Bioorg Med Chem Lett 9: 2463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2H41QN7 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080844
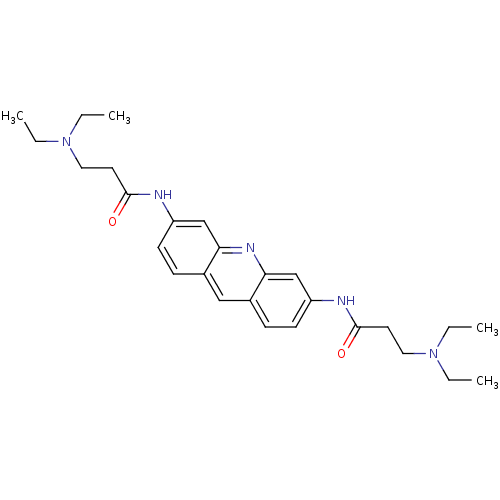
(3-Diethylamino-N-[6-(3-diethylamino-propionylamino...)Show SMILES CCN(CC)CCC(=O)Nc1ccc2cc3ccc(NC(=O)CCN(CC)CC)cc3nc2c1 Show InChI InChI=1S/C27H37N5O2/c1-5-31(6-2)15-13-26(33)28-22-11-9-20-17-21-10-12-23(19-25(21)30-24(20)18-22)29-27(34)14-16-32(7-3)8-4/h9-12,17-19H,5-8,13-16H2,1-4H3,(H,28,33)(H,29,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit human telomerase in a modified cell-free TRAP assay using extracts from A2780 cell line |
Bioorg Med Chem Lett 9: 2463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2H41QN7 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068323
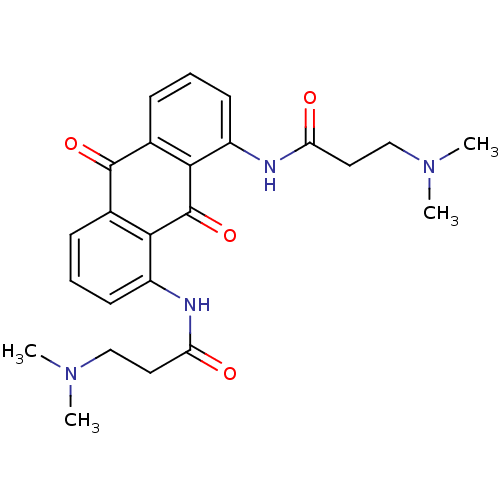
(3-Dimethylamino-N-[8-(3-dimethylamino-propionylami...)Show SMILES CN(C)CCC(=O)Nc1cccc2C(=O)c3cccc(NC(=O)CCN(C)C)c3C(=O)c12 Show InChI InChI=1S/C24H28N4O4/c1-27(2)13-11-19(29)25-17-9-5-7-15-21(17)24(32)22-16(23(15)31)8-6-10-18(22)26-20(30)12-14-28(3)4/h5-10H,11-14H2,1-4H3,(H,25,29)(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50066349
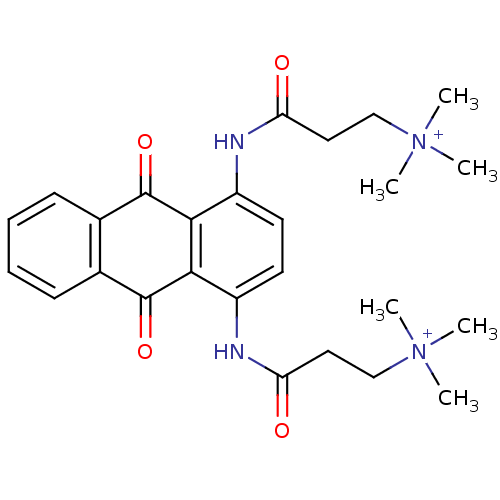
(3-Trimethylammonium-N-[4-(3-dimethylammonium-propi...)Show SMILES C[N+](C)(C)CCC(=O)Nc1ccc(NC(=O)CC[N+](C)(C)C)c2C(=O)c3ccccc3C(=O)c12 Show InChI InChI=1S/C26H32N4O4/c1-29(2,3)15-13-21(31)27-19-11-12-20(28-22(32)14-16-30(4,5)6)24-23(19)25(33)17-9-7-8-10-18(17)26(24)34/h7-12H,13-16H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
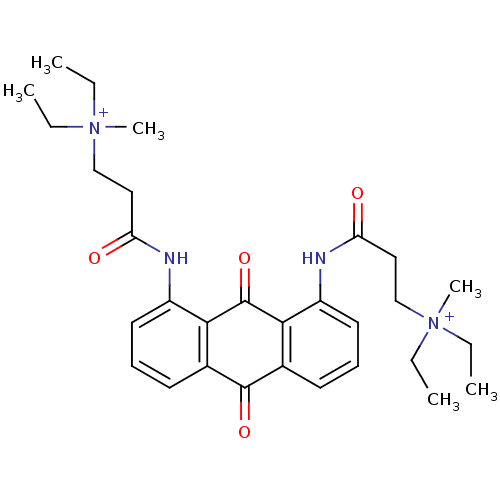
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068318
(CHEMBL145311 | N-[9,10-Dioxo-8-(3-(N,N-diethyl-N-m...)Show SMILES CC[N+](C)(CC)CCC(=O)Nc1cccc2C(=O)c3cccc(NC(=O)CC[N+](C)(CC)CC)c3C(=O)c12 Show InChI InChI=1S/C30H40N4O4/c1-7-33(5,8-2)19-17-25(35)31-23-15-11-13-21-27(23)30(38)28-22(29(21)37)14-12-16-24(28)32-26(36)18-20-34(6,9-3)10-4/h11-16H,7-10,17-20H2,1-6H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068308
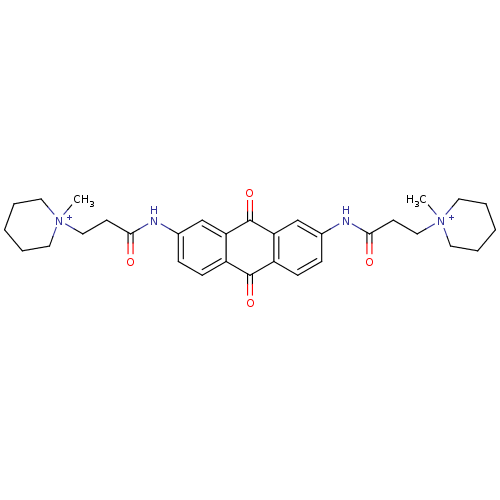
(CHEMBL14832 | CHEMBL90901 | N-[9,10-Dioxo-7-(3-(N-...)Show SMILES C[N+]1(CCC(=O)Nc2ccc3C(=O)c4ccc(NC(=O)CC[N+]5(C)CCCCC5)cc4C(=O)c3c2)CCCCC1 Show InChI InChI=1S/C32H40N4O4/c1-35(15-5-3-6-16-35)19-13-29(37)33-23-9-11-25-27(21-23)32(40)28-22-24(10-12-26(28)31(25)39)34-30(38)14-20-36(2)17-7-4-8-18-36/h9-12,21-22H,3-8,13-20H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068308

(CHEMBL14832 | CHEMBL90901 | N-[9,10-Dioxo-7-(3-(N-...)Show SMILES C[N+]1(CCC(=O)Nc2ccc3C(=O)c4ccc(NC(=O)CC[N+]5(C)CCCCC5)cc4C(=O)c3c2)CCCCC1 Show InChI InChI=1S/C32H40N4O4/c1-35(15-5-3-6-16-35)19-13-29(37)33-23-9-11-25-27(21-23)32(40)28-22-24(10-12-26(28)31(25)39)34-30(38)14-20-36(2)17-7-4-8-18-36/h9-12,21-22H,3-8,13-20H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068337

(CHEMBL343238 | N-[9,10-Dioxo-8-(3-(N-methylpiperid...)Show SMILES C[N+]1(CCC(=O)Nc2cccc3C(=O)c4cccc(NC(=O)CC[N+]5(C)CCCCC5)c4C(=O)c23)CCCCC1 Show InChI InChI=1S/C32H40N4O4/c1-35(17-5-3-6-18-35)21-15-27(37)33-25-13-9-11-23-29(25)32(40)30-24(31(23)39)12-10-14-26(30)34-28(38)16-22-36(2)19-7-4-8-20-36/h9-14H,3-8,15-22H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50080845
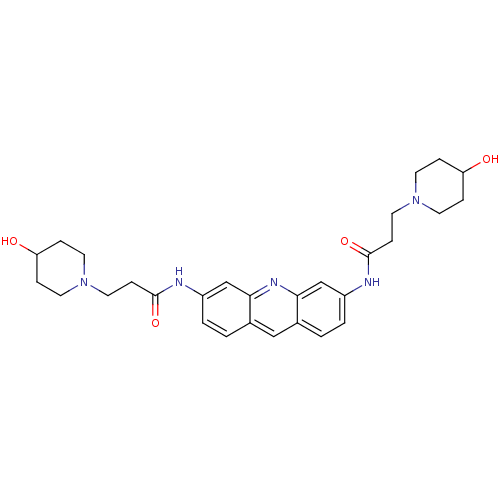
(3-(4-Hydroxy-piperidin-1-yl)-N-{6-[3-(4-hydroxy-pi...)Show SMILES OC1CCN(CCC(=O)Nc2ccc3cc4ccc(NC(=O)CCN5CCC(O)CC5)cc4nc3c2)CC1 Show InChI InChI=1S/C29H37N5O4/c35-24-5-11-33(12-6-24)15-9-28(37)30-22-3-1-20-17-21-2-4-23(19-27(21)32-26(20)18-22)31-29(38)10-16-34-13-7-25(36)8-14-34/h1-4,17-19,24-25,35-36H,5-16H2,(H,30,37)(H,31,38) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Ability to inhibit human telomerase in a modified cell-free TRAP assay using extracts from A2780 cell line |
Bioorg Med Chem Lett 9: 2463-8 (1999)
BindingDB Entry DOI: 10.7270/Q2H41QN7 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50079139
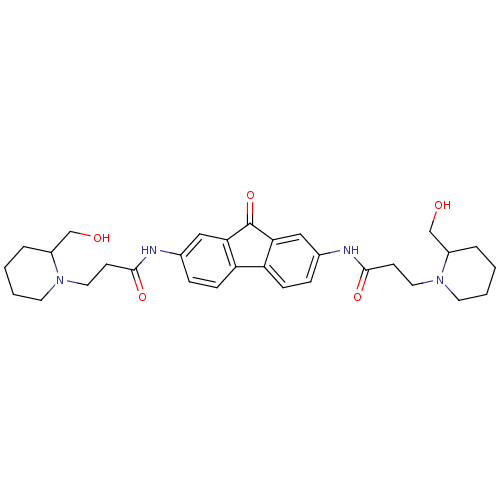
(3-(2-Hydroxymethyl-piperidin-1-yl)-N-{7-[3-(2-hydr...)Show SMILES OCC1CCCCN1CCC(=O)Nc1ccc2-c3ccc(NC(=O)CCN4CCCCC4CO)cc3C(=O)c2c1 Show InChI InChI=1S/C31H40N4O5/c36-19-23-5-1-3-13-34(23)15-11-29(38)32-21-7-9-25-26-10-8-22(18-28(26)31(40)27(25)17-21)33-30(39)12-16-35-14-4-2-6-24(35)20-37/h7-10,17-18,23-24,36-37H,1-6,11-16,19-20H2,(H,32,38)(H,33,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against telomerase |
J Med Chem 42: 2679-84 (1999)
Article DOI: 10.1021/jm990084q
BindingDB Entry DOI: 10.7270/Q2DF6RX9 |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50068322
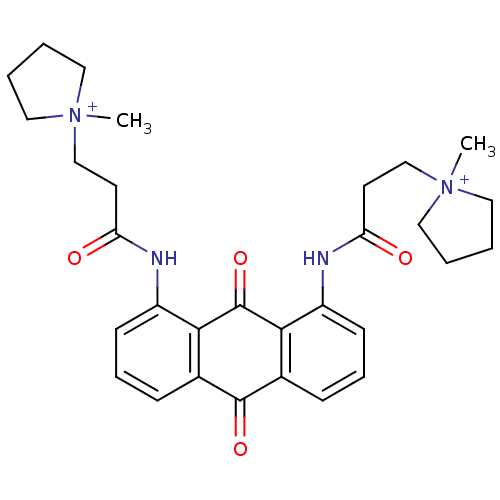
(CHEMBL144219 | N-[9,10-Dioxo-8-(3-(N-methylpyrroli...)Show SMILES C[N+]1(CCC(=O)Nc2cccc3C(=O)c4cccc(NC(=O)CC[N+]5(C)CCCC5)c4C(=O)c23)CCCC1 Show InChI InChI=1S/C30H36N4O4/c1-33(15-3-4-16-33)19-13-25(35)31-23-11-7-9-21-27(23)30(38)28-22(29(21)37)10-8-12-24(28)32-26(36)14-20-34(2)17-5-6-18-34/h7-12H,3-6,13-20H2,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Cancer Research
Curated by ChEMBL
| Assay Description
Inhibitory activity of telomerase was measured using the TRAP assay. |
J Med Chem 41: 4873-84 (1998)
Article DOI: 10.1021/jm981067o
BindingDB Entry DOI: 10.7270/Q2WW7GSD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data