 Found 756 hits with Last Name = 'leblond' and Initial = 'b'
Found 756 hits with Last Name = 'leblond' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176918

((2S)-aminobutyryl-(2S)-indoline carboxylic acid pr...)Show SMILES CCCNC(=O)[C@@H]1Cc2ccccc2N1C(=O)[C@@H](N)CC |r| Show InChI InChI=1S/C16H23N3O2/c1-3-9-18-15(20)14-10-11-7-5-6-8-13(11)19(14)16(21)12(17)4-2/h5-8,12,14H,3-4,9-10,17H2,1-2H3,(H,18,20)/t12-,14-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50121282
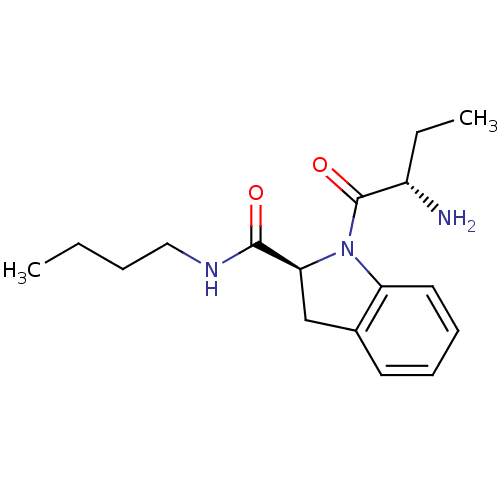
((S)-1-((S)-2-aminobutanoyl)-N-butylindoline-2-carb...)Show SMILES CCCCNC(=O)[C@@H]1Cc2ccccc2N1C(=O)[C@@H](N)CC Show InChI InChI=1S/C17H25N3O2/c1-3-5-10-19-16(21)15-11-12-8-6-7-9-14(12)20(15)17(22)13(18)4-2/h6-9,13,15H,3-5,10-11,18H2,1-2H3,(H,19,21)/t13-,15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176940
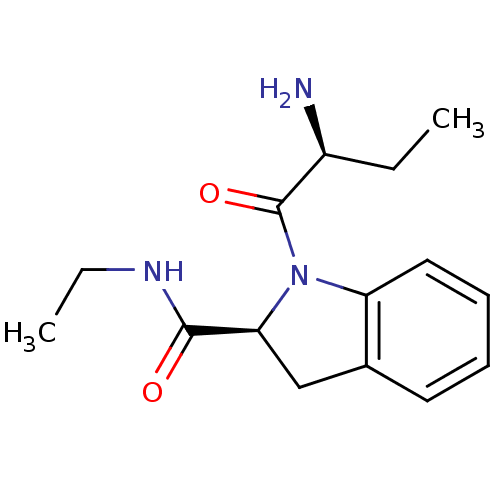
((2S)-aminobutyryl-(2S)-indoline carboxylic acid et...)Show SMILES CCNC(=O)[C@@H]1Cc2ccccc2N1C(=O)[C@@H](N)CC |r| Show InChI InChI=1S/C15H21N3O2/c1-3-11(16)15(20)18-12-8-6-5-7-10(12)9-13(18)14(19)17-4-2/h5-8,11,13H,3-4,9,16H2,1-2H3,(H,17,19)/t11-,13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176934

((2S)-aminobutyryl-L-proline n-butylamide | CHEMBL2...)Show InChI InChI=1S/C13H25N3O2/c1-3-5-8-15-12(17)11-7-6-9-16(11)13(18)10(14)4-2/h10-11H,3-9,14H2,1-2H3,(H,15,17)/t10-,11-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176913
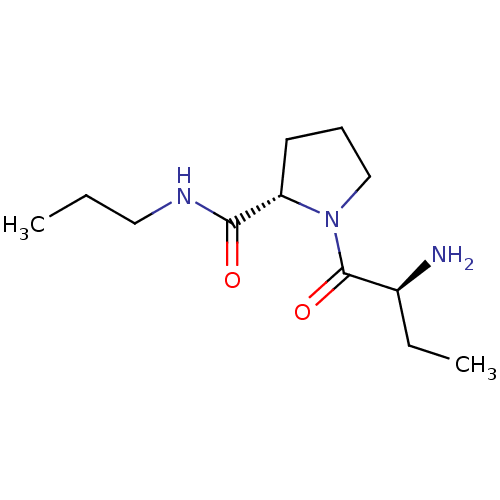
((2S)-aminobutyryl-L-proline n-propylamide | CHEMBL...)Show InChI InChI=1S/C12H23N3O2/c1-3-7-14-11(16)10-6-5-8-15(10)12(17)9(13)4-2/h9-10H,3-8,13H2,1-2H3,(H,14,16)/t9-,10-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176939
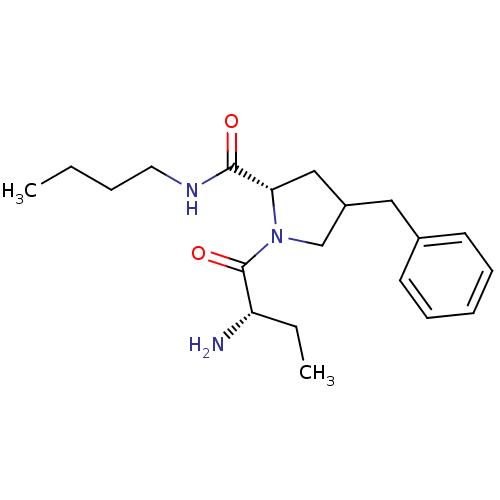
((2S)-1-((S)-2-aminobutanoyl)-4-benzyl-N-butylpyrro...)Show SMILES CCCCNC(=O)[C@@H]1CC(Cc2ccccc2)CN1C(=O)[C@@H](N)CC |r| Show InChI InChI=1S/C20H31N3O2/c1-3-5-11-22-19(24)18-13-16(12-15-9-7-6-8-10-15)14-23(18)20(25)17(21)4-2/h6-10,16-18H,3-5,11-14,21H2,1-2H3,(H,22,24)/t16?,17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176936
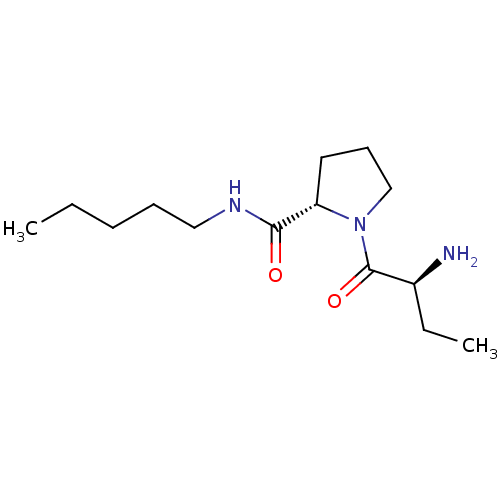
((2S)-aminobutyryl-L-proline n-pentylamide | CHEMBL...)Show SMILES CCCCCNC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC |r| Show InChI InChI=1S/C14H27N3O2/c1-3-5-6-9-16-13(18)12-8-7-10-17(12)14(19)11(15)4-2/h11-12H,3-10,15H2,1-2H3,(H,16,18)/t11-,12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176927

((2S)-aminobutyryl-L-proline 3-methylthiopropylamid...)Show InChI InChI=1S/C13H25N3O2S/c1-3-10(14)13(18)16-8-4-6-11(16)12(17)15-7-5-9-19-2/h10-11H,3-9,14H2,1-2H3,(H,15,17)/t10-,11-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176935
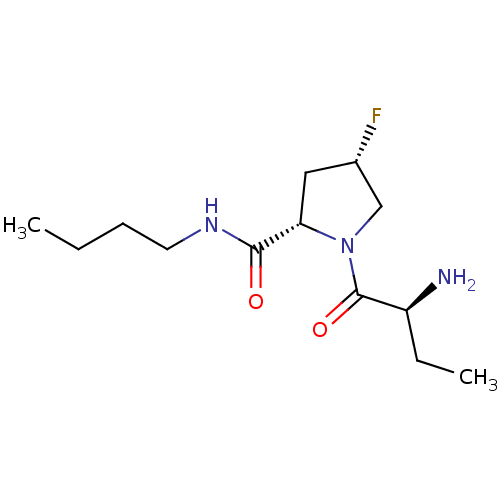
((2S,4S)-1-((S)-2-aminobutanoyl)-N-butyl-4-fluoropy...)Show SMILES CCCCNC(=O)[C@@H]1C[C@H](F)CN1C(=O)[C@@H](N)CC |r| Show InChI InChI=1S/C13H24FN3O2/c1-3-5-6-16-12(18)11-7-9(14)8-17(11)13(19)10(15)4-2/h9-11H,3-8,15H2,1-2H3,(H,16,18)/t9-,10-,11-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176945
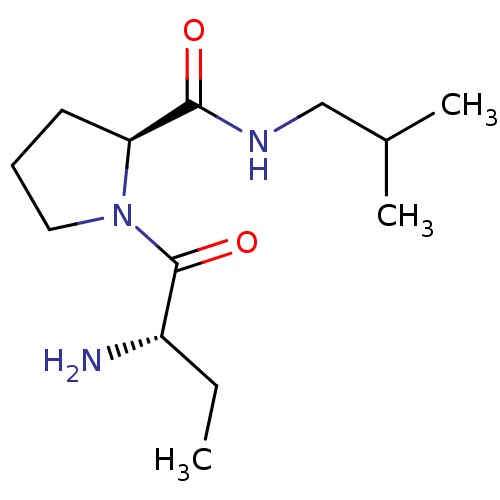
((2S)-aminobutyryl-L-proline isobutylamide | CHEMBL...)Show InChI InChI=1S/C13H25N3O2/c1-4-10(14)13(18)16-7-5-6-11(16)12(17)15-8-9(2)3/h9-11H,4-8,14H2,1-3H3,(H,15,17)/t10-,11-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176922
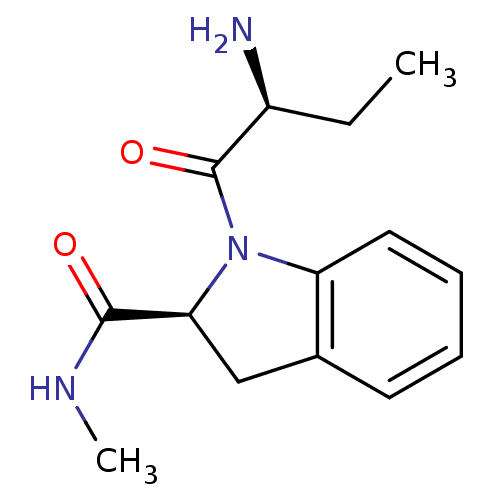
((2S)-aminobutyryl-(2S)-indoline carboxylic acid me...)Show SMILES CC[C@H](N)C(=O)N1[C@@H](Cc2ccccc12)C(=O)NC |r| Show InChI InChI=1S/C14H19N3O2/c1-3-10(15)14(19)17-11-7-5-4-6-9(11)8-12(17)13(18)16-2/h4-7,10,12H,3,8,15H2,1-2H3,(H,16,18)/t10-,12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176914
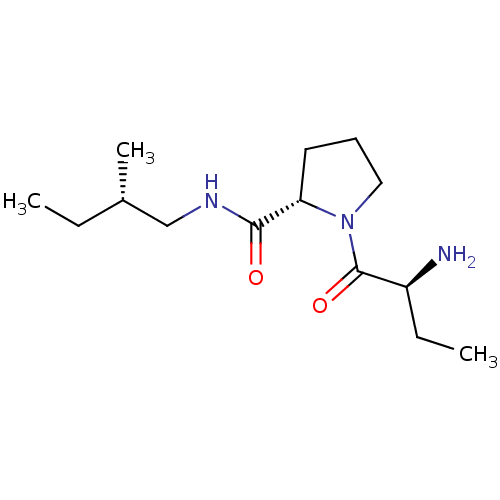
((2S)-aminobutyryl-L-proline-(2S)-methylbutylamide ...)Show SMILES CC[C@H](C)CNC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC |r| Show InChI InChI=1S/C14H27N3O2/c1-4-10(3)9-16-13(18)12-7-6-8-17(12)14(19)11(15)5-2/h10-12H,4-9,15H2,1-3H3,(H,16,18)/t10-,11-,12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176920
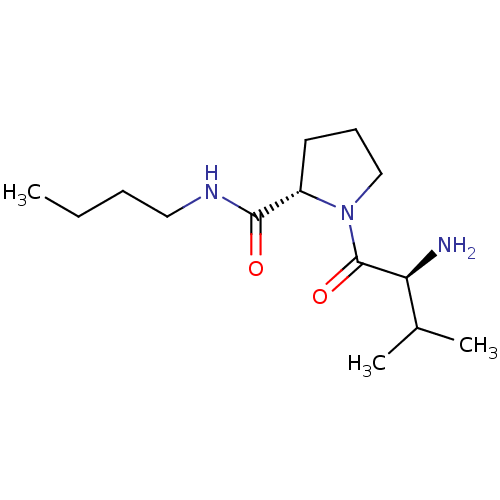
((S)-1-((S)-2-amino-3-methylbutanoyl)-N-butylpyrrol...)Show SMILES CCCCNC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C(C)C |r| Show InChI InChI=1S/C14H27N3O2/c1-4-5-8-16-13(18)11-7-6-9-17(11)14(19)12(15)10(2)3/h10-12H,4-9,15H2,1-3H3,(H,16,18)/t11-,12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176932
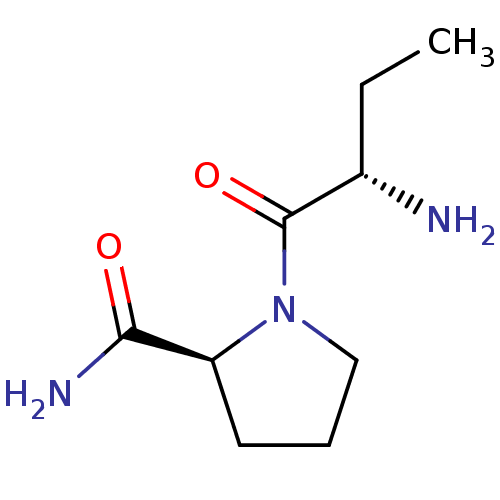
((2S)-aminobutyryl-L-prolinamide | CHEMBL224063)Show InChI InChI=1S/C9H17N3O2/c1-2-6(10)9(14)12-5-3-4-7(12)8(11)13/h6-7H,2-5,10H2,1H3,(H2,11,13)/t6-,7-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176928
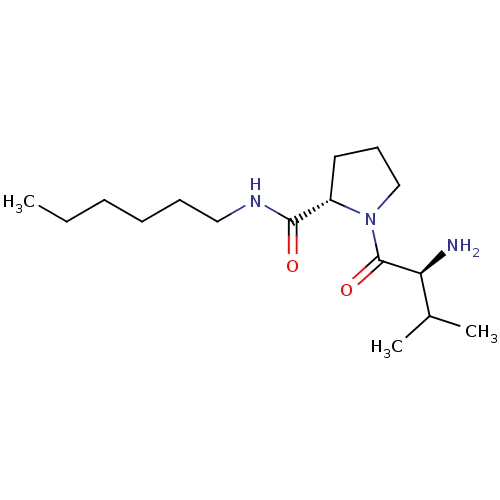
(CHEMBL375490 | L-valyl-L-proline hexylamide)Show SMILES CCCCCCNC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)C(C)C |r| Show InChI InChI=1S/C16H31N3O2/c1-4-5-6-7-10-18-15(20)13-9-8-11-19(13)16(21)14(17)12(2)3/h12-14H,4-11,17H2,1-3H3,(H,18,20)/t13-,14-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176926

(CHEMBL224628 | L-norvalyl-L-prolinamide)Show InChI InChI=1S/C10H19N3O2/c1-2-4-7(11)10(15)13-6-3-5-8(13)9(12)14/h7-8H,2-6,11H2,1H3,(H2,12,14)/t7-,8-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176919
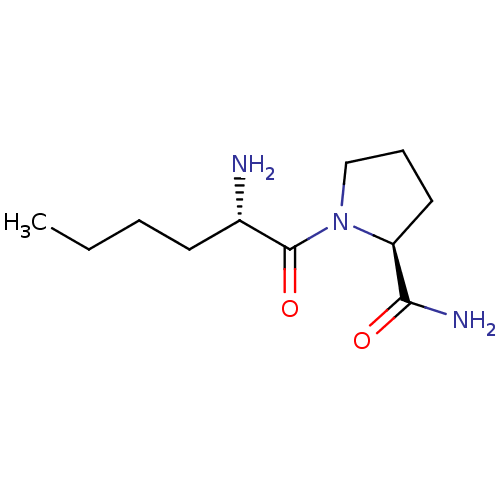
(CHEMBL388843 | L-norleucyl-L-prolinamide)Show InChI InChI=1S/C11H21N3O2/c1-2-3-5-8(12)11(16)14-7-4-6-9(14)10(13)15/h8-9H,2-7,12H2,1H3,(H2,13,15)/t8-,9-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176925
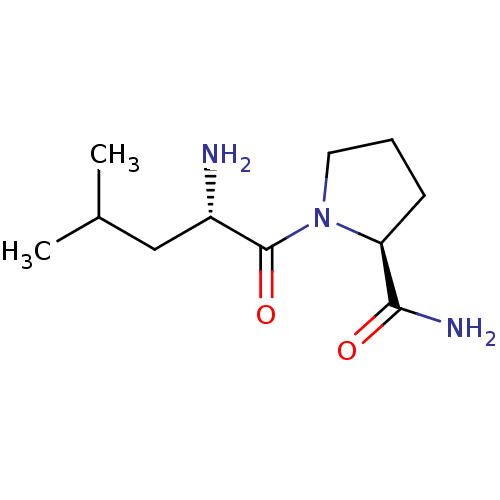
(CHEMBL224340 | L-leucyl-L-prolinamide)Show InChI InChI=1S/C11H21N3O2/c1-7(2)6-8(12)11(16)14-5-3-4-9(14)10(13)15/h7-9H,3-6,12H2,1-2H3,(H2,13,15)/t8-,9-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176943
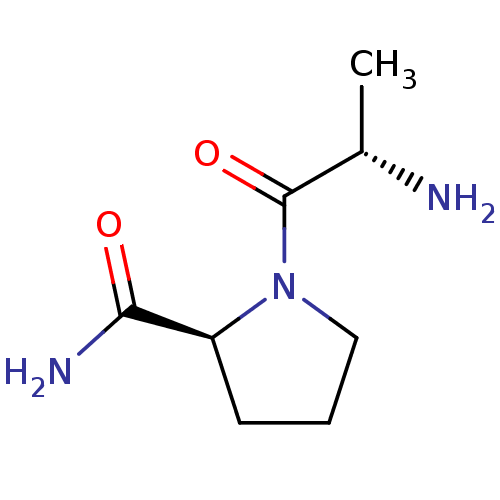
((S)-1-((S)-2-aminopropanoyl)pyrrolidine-2-carboxam...)Show InChI InChI=1S/C8H15N3O2/c1-5(9)8(13)11-4-2-3-6(11)7(10)12/h5-6H,2-4,9H2,1H3,(H2,10,12)/t5-,6-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176931

(CHEMBL376375 | L-valyl-L-proline benzylamide)Show SMILES CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C17H25N3O2/c1-12(2)15(18)17(22)20-10-6-9-14(20)16(21)19-11-13-7-4-3-5-8-13/h3-5,7-8,12,14-15H,6,9-11,18H2,1-2H3,(H,19,21)/t14-,15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176924
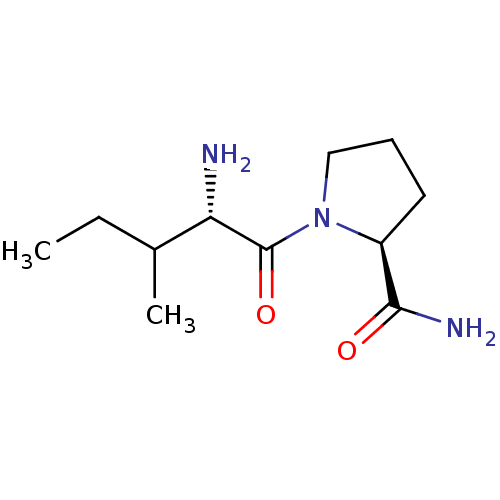
(CHEMBL224341 | L-isoleucyl-L-prolinamide)Show InChI InChI=1S/C11H21N3O2/c1-3-7(2)9(12)11(16)14-6-4-5-8(14)10(13)15/h7-9H,3-6,12H2,1-2H3,(H2,13,15)/t7?,8-,9-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176930
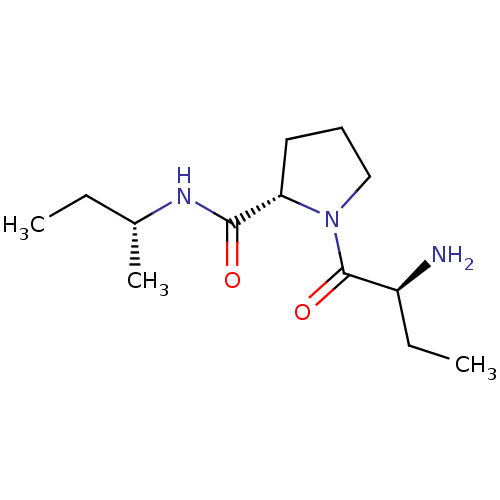
((2S)-aminobutyryl-L-proline (R)-sec-butylamide | C...)Show SMILES CC[C@@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC |r| Show InChI InChI=1S/C13H25N3O2/c1-4-9(3)15-12(17)11-7-6-8-16(11)13(18)10(14)5-2/h9-11H,4-8,14H2,1-3H3,(H,15,17)/t9-,10+,11+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176938
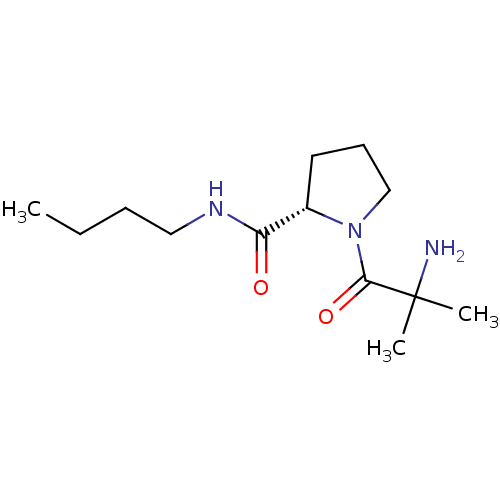
(CHEMBL388062 | alpha-methylalanyl-L-proline butyla...)Show InChI InChI=1S/C13H25N3O2/c1-4-5-8-15-11(17)10-7-6-9-16(10)12(18)13(2,3)14/h10H,4-9,14H2,1-3H3,(H,15,17)/t10-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176929
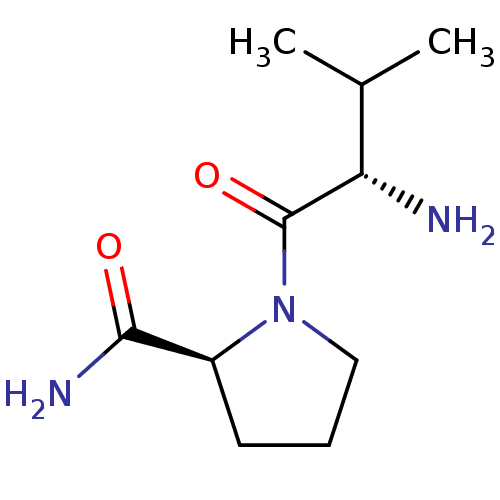
(CHEMBL375933 | L-valyl-L-prolinamide)Show InChI InChI=1S/C10H19N3O2/c1-6(2)8(11)10(15)13-5-3-4-7(13)9(12)14/h6-8H,3-5,11H2,1-2H3,(H2,12,14)/t7-,8-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176944

(1-((S)-2-aminobutanoyl)-N-butylazepane-2-carboxami...)Show InChI InChI=1S/C15H29N3O2/c1-3-5-10-17-14(19)13-9-7-6-8-11-18(13)15(20)12(16)4-2/h12-13H,3-11,16H2,1-2H3,(H,17,19)/t12-,13?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176923
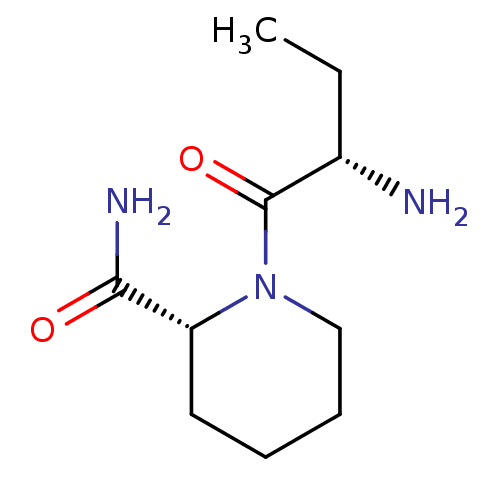
((2S)-aminobutyryl-(R)-pipecolinic acid amide | CHE...)Show InChI InChI=1S/C10H19N3O2/c1-2-7(11)10(15)13-6-4-3-5-8(13)9(12)14/h7-8H,2-6,11H2,1H3,(H2,12,14)/t7-,8+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176921
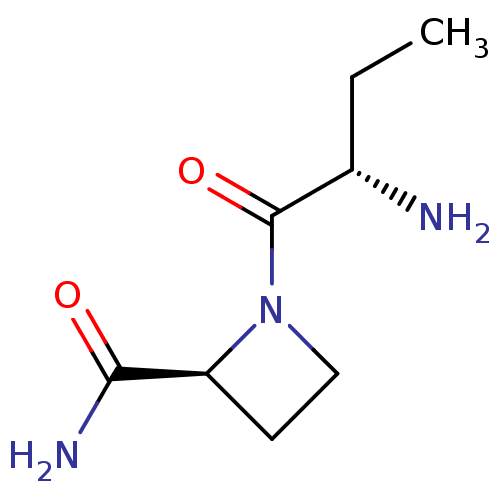
((2S)-aminobutyryl-(2S)-azetidine carboxamide | CHE...)Show InChI InChI=1S/C8H15N3O2/c1-2-5(9)8(13)11-4-3-6(11)7(10)12/h5-6H,2-4,9H2,1H3,(H2,10,12)/t5-,6-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176915
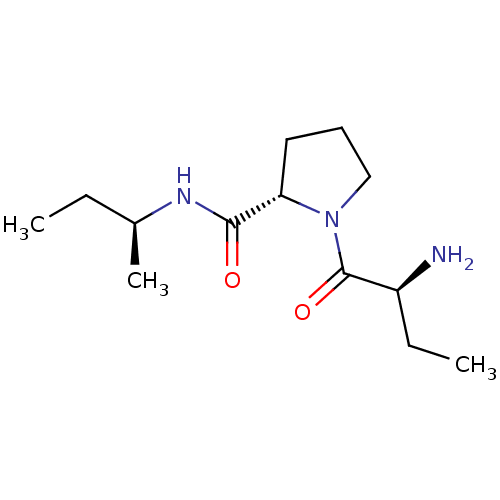
((2S)-aminobutyryl-L-proline (S)-sec-butylamide | C...)Show SMILES CC[C@H](C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC |r| Show InChI InChI=1S/C13H25N3O2/c1-4-9(3)15-12(17)11-7-6-8-16(11)13(18)10(14)5-2/h9-11H,4-8,14H2,1-3H3,(H,15,17)/t9-,10-,11-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176912
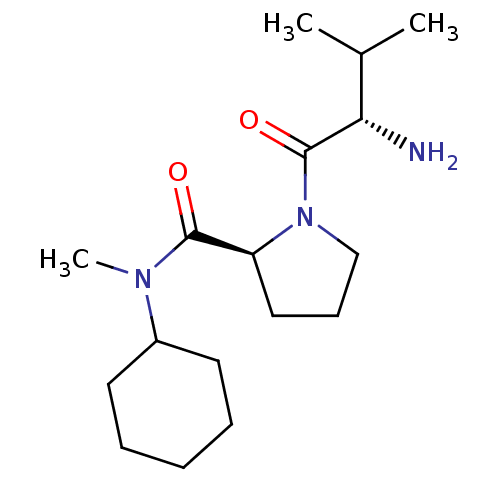
(CHEMBL225596 | L-valyl-L-proline cyclohexylmethyla...)Show SMILES CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N(C)C1CCCCC1 |r| Show InChI InChI=1S/C17H31N3O2/c1-12(2)15(18)17(22)20-11-7-10-14(20)16(21)19(3)13-8-5-4-6-9-13/h12-15H,4-11,18H2,1-3H3/t14-,15-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176911
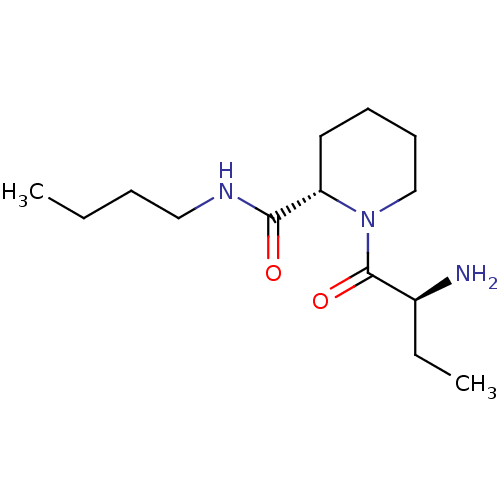
((2S)-aminobutyryl-L-pipecolinic acid n-butylamide ...)Show SMILES CCCCNC(=O)[C@@H]1CCCCN1C(=O)[C@@H](N)CC |r| Show InChI InChI=1S/C14H27N3O2/c1-3-5-9-16-13(18)12-8-6-7-10-17(12)14(19)11(15)4-2/h11-12H,3-10,15H2,1-2H3,(H,16,18)/t11-,12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176916

((2S)-aminobutyryl-(S)-nipecotamide | CHEMBL223665)Show InChI InChI=1S/C10H19N3O2/c1-2-8(11)10(15)13-5-3-4-7(6-13)9(12)14/h7-8H,2-6,11H2,1H3,(H2,12,14)/t7-,8-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176942
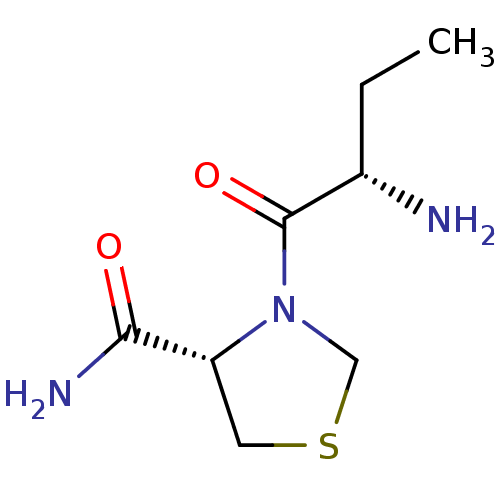
((2S)-aminobutyryl-4-thia-L-prolinamide | CHEMBL375...)Show InChI InChI=1S/C8H15N3O2S/c1-2-5(9)8(13)11-4-14-3-6(11)7(10)12/h5-6H,2-4,9H2,1H3,(H2,10,12)/t5-,6+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176933
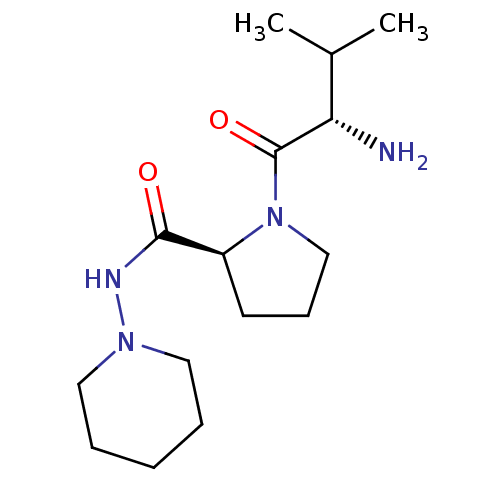
(CHEMBL390771 | L-valyl-L-proline piperidinylamide)Show SMILES CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)NN1CCCCC1 |r| Show InChI InChI=1S/C15H28N4O2/c1-11(2)13(16)15(21)19-10-6-7-12(19)14(20)17-18-8-4-3-5-9-18/h11-13H,3-10,16H2,1-2H3,(H,17,20)/t12-,13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176941
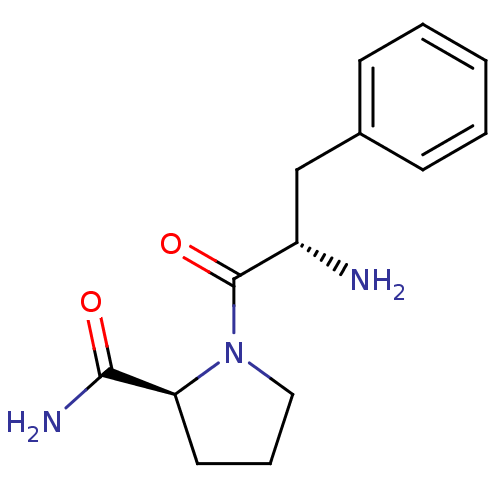
(CHEMBL224610 | L-phenylalanyl-L-prolinamide)Show SMILES N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O |r| Show InChI InChI=1S/C14H19N3O2/c15-11(9-10-5-2-1-3-6-10)14(19)17-8-4-7-12(17)13(16)18/h1-3,5-6,11-12H,4,7-9,15H2,(H2,16,18)/t11-,12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176937
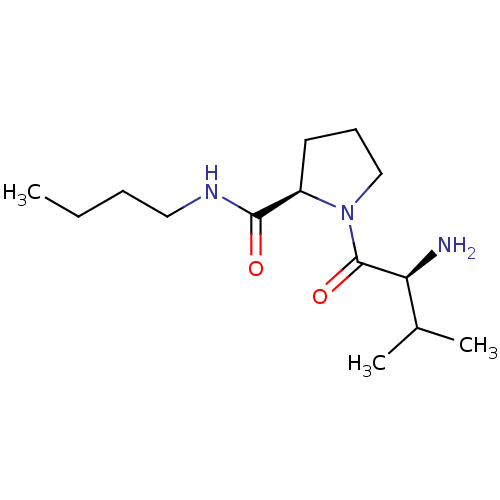
((R)-1-((S)-2-amino-3-methylbutanoyl)-N-butylpyrrol...)Show SMILES CCCCNC(=O)[C@H]1CCCN1C(=O)[C@@H](N)C(C)C |r| Show InChI InChI=1S/C14H27N3O2/c1-4-5-8-16-13(18)11-7-6-9-17(11)14(19)12(15)10(2)3/h10-12H,4-9,15H2,1-3H3,(H,16,18)/t11-,12+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Tripeptidyl-peptidase 2
(Rattus norvegicus) | BDBM50176917
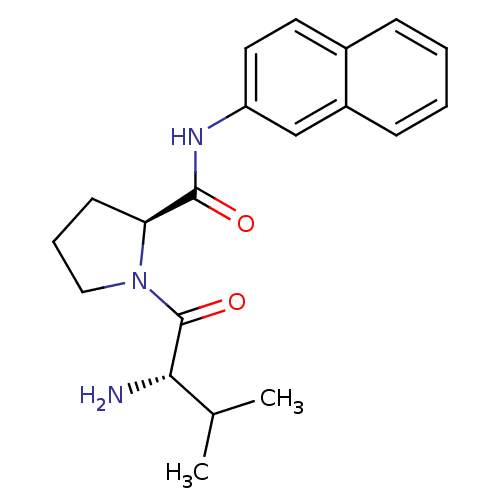
(CHEMBL390838 | L-valyl-L-proline-N-naphthylamide)Show SMILES CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)Nc1ccc2ccccc2c1 |r| Show InChI InChI=1S/C20H25N3O2/c1-13(2)18(21)20(25)23-11-5-8-17(23)19(24)22-16-10-9-14-6-3-4-7-15(14)12-16/h3-4,6-7,9-10,12-13,17-18H,5,8,11,21H2,1-2H3,(H,22,24)/t17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London
Curated by ChEMBL
| Assay Description
Inhibition of TPPII in rat cererbral membrane |
J Med Chem 48: 7333-42 (2005)
Article DOI: 10.1021/jm0500830
BindingDB Entry DOI: 10.7270/Q2KK9CMS |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50380208

(CHEMBL2011408)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4c3ccc(OS(N)(=O)=O)c4[N+]([O-])=O)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C18H22N2O6S/c1-18-9-8-11-10-4-6-15(26-27(19,24)25)17(20(22)23)13(10)3-2-12(11)14(18)5-7-16(18)21/h4,6,11-12,14H,2-3,5,7-9H2,1H3,(H2,19,24,25)/t11-,12-,14+,18+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human MCF7 cells using [3H]E1S as substrate after 20 hrs by scintillation spectrometry |
Bioorg Med Chem 20: 2506-19 (2012)
Article DOI: 10.1016/j.bmc.2012.03.007
BindingDB Entry DOI: 10.7270/Q2XG9S4J |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50380223

(CHEMBL2011422)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)c(cc34)C(F)F)[C@@H]1CCC2=O |r| Show InChI InChI=1S/C19H23F2NO4S/c1-19-7-6-11-12(15(19)4-5-17(19)23)3-2-10-8-16(26-27(22,24)25)14(18(20)21)9-13(10)11/h8-9,11-12,15,18H,2-7H2,1H3,(H2,22,24,25)/t11-,12+,15-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human placental microsomes using [3H]E1S as substrate after 30 mins by scintillation spectrometry |
Bioorg Med Chem 20: 2506-19 (2012)
Article DOI: 10.1016/j.bmc.2012.03.007
BindingDB Entry DOI: 10.7270/Q2XG9S4J |
More data for this
Ligand-Target Pair | |
Steryl-sulfatase
(Homo sapiens (Human)) | BDBM50380216
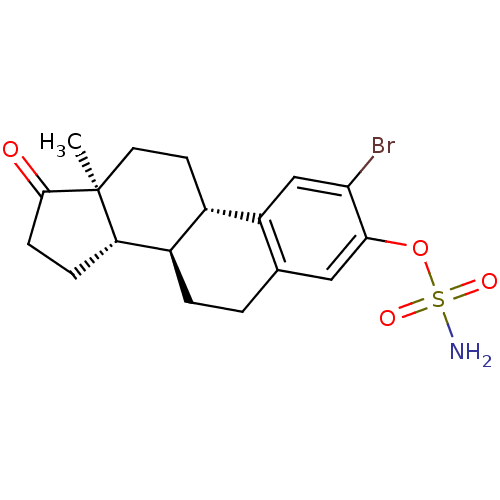
(CHEMBL364332)Show SMILES C[C@]12CC[C@H]3[C@@H](CCc4cc(OS(N)(=O)=O)c(Br)cc34)[C@@H]1CCC2=O Show InChI InChI=1S/C18H22BrNO4S/c1-18-7-6-11-12(14(18)4-5-17(18)21)3-2-10-8-16(24-25(20,22)23)15(19)9-13(10)11/h8-9,11-12,14H,2-7H2,1H3,(H2,20,22,23)/t11-,12+,14-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Inhibition of steroid sulfatase in human MCF7 cells using [3H]E1S as substrate after 20 hrs by scintillation spectrometry |
Bioorg Med Chem 20: 2506-19 (2012)
Article DOI: 10.1016/j.bmc.2012.03.007
BindingDB Entry DOI: 10.7270/Q2XG9S4J |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM125523

(US8772316, 6)Show SMILES CCc1[nH]c(=O)c(Cc2ccc3oc4ccccc4c3c2)c2cc(OC)c(OC)cc12 Show InChI InChI=1S/C26H23NO4/c1-4-21-18-14-25(30-3)24(29-2)13-17(18)20(26(28)27-21)12-15-9-10-23-19(11-15)16-7-5-6-8-22(16)31-23/h5-11,13-14H,4,12H2,1-3H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Allergan, Inc.; Exonhit Therapeutics SA
US Patent
| Assay Description
The PDE assay is based on the homogenous time-resolved fluorescence resonance energy transfer (TR-FRET) technology (LANCE from Perkin Elmer). This co... |
US Patent US8772316 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BR8 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM187126
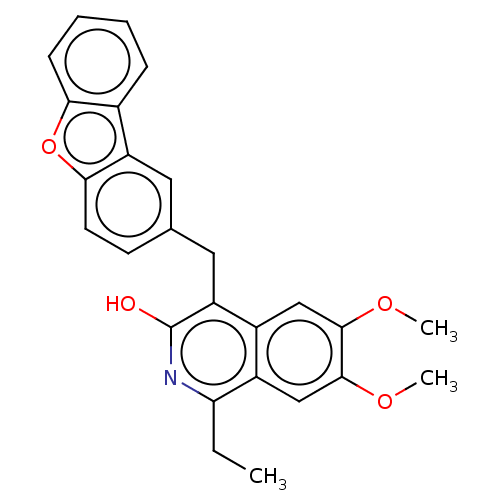
(US9670181, 6 4-(dibenzo[b,d]furan-2-ylmethyl)-1-et...)Show SMILES CCc1nc(O)c(Cc2ccc3oc4ccccc4c3c2)c2cc(OC)c(OC)cc12 Show InChI InChI=1S/C26H23NO4/c1-4-21-18-14-25(30-3)24(29-2)13-17(18)20(26(28)27-21)12-15-9-10-23-19(11-15)16-7-5-6-8-22(16)31-23/h5-11,13-14H,4,12H2,1-3H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | 7.4 | 25 |
ALLERGAN, INC.; EXONHIT THERAPEUTICS SA
US Patent
| Assay Description
The PDE assay is based on the homogenous time-resolved fluorescence resonance energy transfer (TR-FRET) technology (LANCE® from Perkin Elmer). This c... |
US Patent US9670181 (2017)
BindingDB Entry DOI: 10.7270/Q21Z42KJ |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50434358
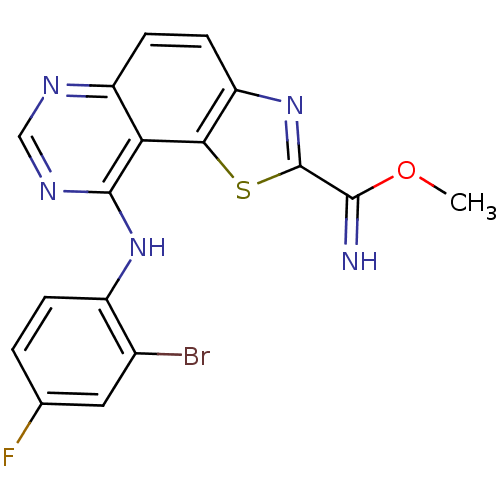
(CHEMBL2386770 | US9446044, 68)Show InChI InChI=1S/C17H11BrFN5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(19)6-9(10)18/h2-7,20H,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 7.5 | 25 |
DIAXONHIT
US Patent
| Assay Description
The DYRK1A and DYRK1B kinase assays to determine IC50 values were performed by Reaction Biology Corporation using HotSpot technology Worldwide websit... |
US Patent US9446044 (2016)
BindingDB Entry DOI: 10.7270/Q2610Z7J |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM194437
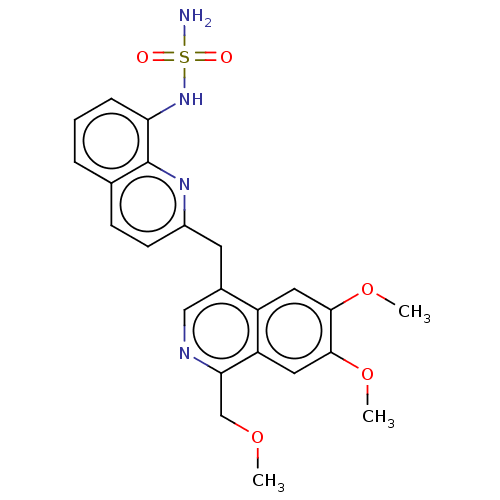
(US9200016, 119 | US9902710, Compound 119)Show SMILES COCc1ncc(Cc2ccc3cccc(NS(N)(=O)=O)c3n2)c2cc(OC)c(OC)cc12 Show InChI InChI=1S/C23H24N4O5S/c1-30-13-20-18-11-22(32-3)21(31-2)10-17(18)15(12-25-20)9-16-8-7-14-5-4-6-19(23(14)26-16)27-33(24,28)29/h4-8,10-12,27H,9,13H2,1-3H3,(H2,24,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Allergan, Inc.; Exonhit Therapeutics, SA
US Patent
| Assay Description
The phosphodiesterase assay was developed using the LANCE cAMP kit
(PerkinElmer). The assay buffer contained HBSS with 5 mM HEPES, 0.1%
BSA, and 1.... |
US Patent US9200016 (2015)
BindingDB Entry DOI: 10.7270/Q2ZC81PH |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM194437

(US9200016, 119 | US9902710, Compound 119)Show SMILES COCc1ncc(Cc2ccc3cccc(NS(N)(=O)=O)c3n2)c2cc(OC)c(OC)cc12 Show InChI InChI=1S/C23H24N4O5S/c1-30-13-20-18-11-22(32-3)21(31-2)10-17(18)15(12-25-20)9-16-8-7-14-5-4-6-19(23(14)26-16)27-33(24,28)29/h4-8,10-12,27H,9,13H2,1-3H3,(H2,24,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Mississippi
| Assay Description
The phosphodiesterase assay was developed using the LANCE® cAMP kit (PerkinElmer). The assay buffer contained HBSS with 5 mM HEPES, 0.1% BSA, and 1.5... |
J Med Chem 48: 2906-15 (2005)
BindingDB Entry DOI: 10.7270/Q2PC34QT |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50434353

(CHEMBL2386747 | US9446044, 72)Show SMILES COC(=N)c1nc2ccc3ncnc(Nc4ccc(Cl)cc4Cl)c3c2s1 Show InChI InChI=1S/C17H11Cl2N5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(18)6-9(10)19/h2-7,20H,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.5 | 25 |
DIAXONHIT
US Patent
| Assay Description
The DYRK1A and DYRK1B kinase assays to determine IC50 values were performed by Reaction Biology Corporation using HotSpot technology Worldwide websit... |
US Patent US9446044 (2016)
BindingDB Entry DOI: 10.7270/Q2610Z7J |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50434353

(CHEMBL2386747 | US9446044, 72)Show SMILES COC(=N)c1nc2ccc3ncnc(Nc4ccc(Cl)cc4Cl)c3c2s1 Show InChI InChI=1S/C17H11Cl2N5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(18)6-9(10)19/h2-7,20H,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of DYRK1A (unknown origin) |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2GH9NMG |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50434353

(CHEMBL2386747 | US9446044, 72)Show SMILES COC(=N)c1nc2ccc3ncnc(Nc4ccc(Cl)cc4Cl)c3c2s1 Show InChI InChI=1S/C17H11Cl2N5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(18)6-9(10)19/h2-7,20H,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human DYRK1A using [33P]-ATP incubated for 120 mins |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2GH9NMG |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50434358

(CHEMBL2386770 | US9446044, 68)Show InChI InChI=1S/C17H11BrFN5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(19)6-9(10)18/h2-7,20H,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.5 | 25 |
DIAXONHIT
US Patent
| Assay Description
The DYRK1A and DYRK1B kinase assays to determine IC50 values were performed by Reaction Biology Corporation using HotSpot technology Worldwide websit... |
US Patent US9446044 (2016)
BindingDB Entry DOI: 10.7270/Q2610Z7J |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50434353

(CHEMBL2386747 | US9446044, 72)Show SMILES COC(=N)c1nc2ccc3ncnc(Nc4ccc(Cl)cc4Cl)c3c2s1 Show InChI InChI=1S/C17H11Cl2N5OS/c1-25-15(20)17-24-12-5-4-11-13(14(12)26-17)16(22-7-21-11)23-10-3-2-8(18)6-9(10)19/h2-7,20H,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of DYRK1B (unknown origin) |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2GH9NMG |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM125567
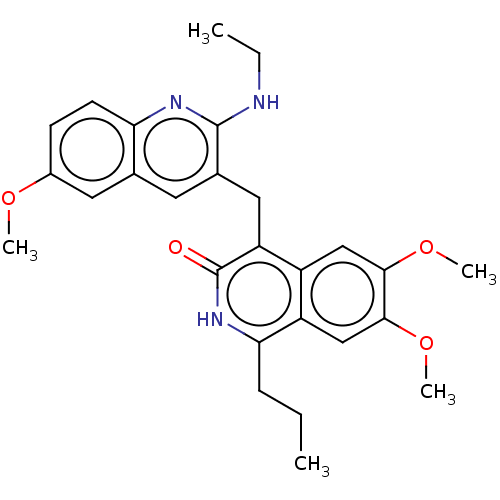
(US8772316, 50)Show SMILES CCCc1[nH]c(=O)c(Cc2cc3cc(OC)ccc3nc2NCC)c2cc(OC)c(OC)cc12 Show InChI InChI=1S/C27H31N3O4/c1-6-8-23-20-15-25(34-5)24(33-4)14-19(20)21(27(31)30-23)13-17-11-16-12-18(32-3)9-10-22(16)29-26(17)28-7-2/h9-12,14-15H,6-8,13H2,1-5H3,(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Allergan, Inc.; Exonhit Therapeutics SA
US Patent
| Assay Description
The PDE assay is based on the homogenous time-resolved fluorescence resonance energy transfer (TR-FRET) technology (LANCE from Perkin Elmer). This co... |
US Patent US8772316 (2014)
BindingDB Entry DOI: 10.7270/Q2Q81BR8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data