

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50299467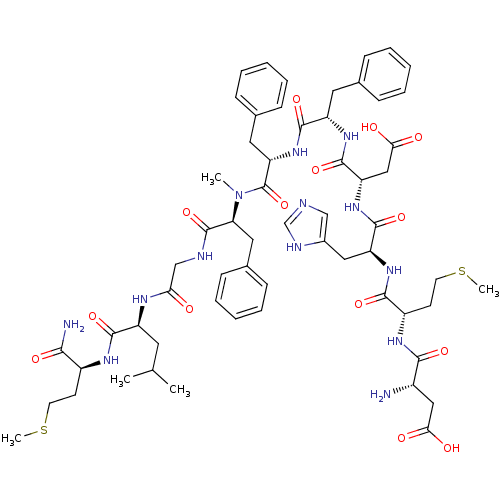 ((5S,8S,14S,17S,20S,23S,26S,29S,32S)-26-((1H-imidaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to human NK3 receptor | J Med Chem 52: 7103-12 (2009) Article DOI: 10.1021/jm900948q BindingDB Entry DOI: 10.7270/Q21G0MBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233239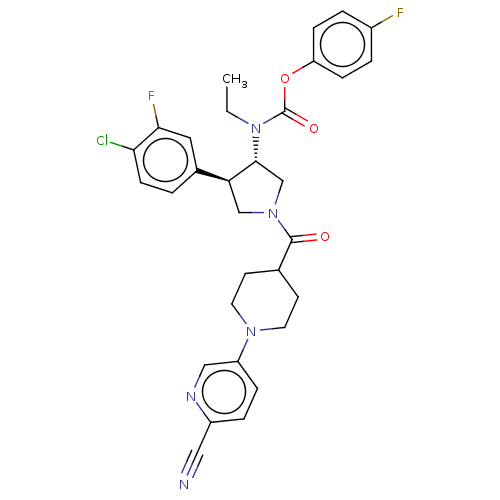 (US9346786, 43) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | -50.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077223 (CHEMBL3416881) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cell membranes after 1 hr by scintillation proximity assay | J Med Chem 58: 2275-89 (2015) Article DOI: 10.1021/jm501745f BindingDB Entry DOI: 10.7270/Q2JW8GK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233309 (US9346786, 113) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50291261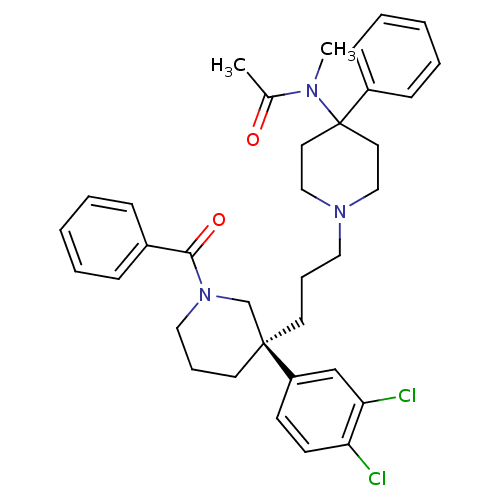 ((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]osanetant from human recombinant NK3 receptor expressed in HEK293 cells | J Med Chem 52: 7103-12 (2009) Article DOI: 10.1021/jm900948q BindingDB Entry DOI: 10.7270/Q21G0MBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233255 (US9346786, 59) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233312 (US9346786, 116) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233237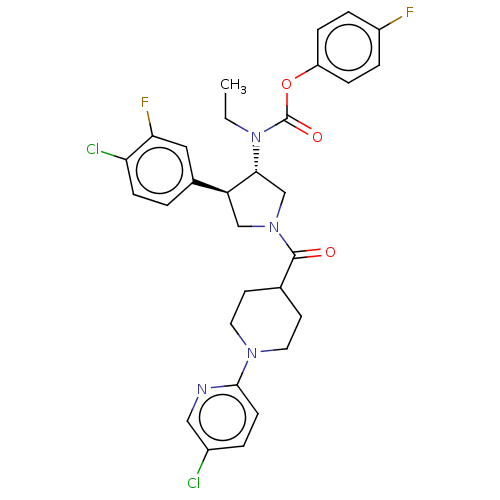 (US9346786, 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -48.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50329161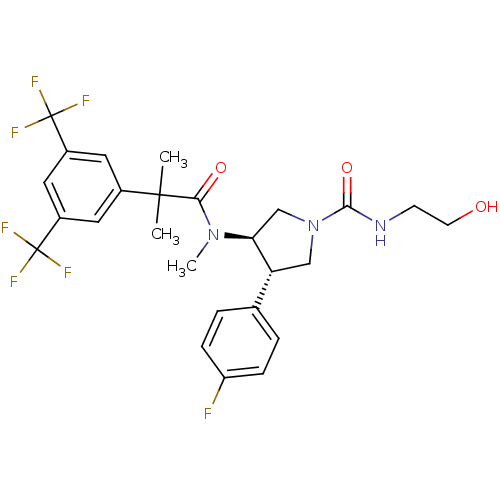 ((3R,4S)-3-(2-(3,5-bis(trifluoromethyl)phenyl)-N,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human neurokinin NK1 receptor expressed in CHO cell | Bioorg Med Chem Lett 20: 6735-8 (2010) Article DOI: 10.1016/j.bmcl.2010.08.138 BindingDB Entry DOI: 10.7270/Q22Z15SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50291261 ((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]osanetant from guinea pig recombinant NK3 receptor expressed in HEK293 cells | J Med Chem 52: 7103-12 (2009) Article DOI: 10.1021/jm900948q BindingDB Entry DOI: 10.7270/Q21G0MBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233236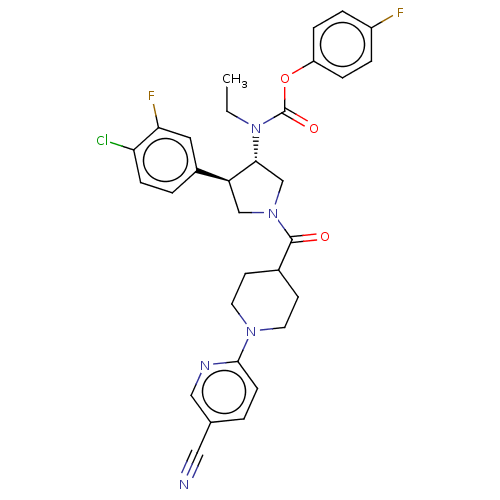 (US9346786, 40) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233253 (US9346786, 57) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233259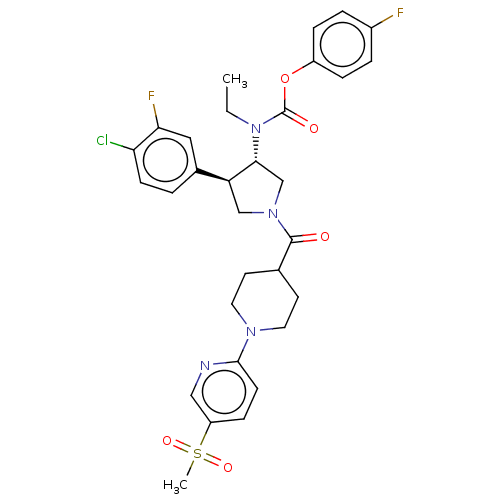 (US9346786, 63) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233308 (US9346786, 112) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50299468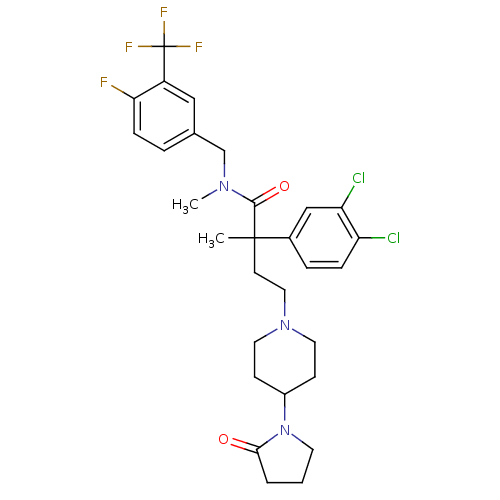 (2-(3,4-dichlorophenyl)-N-(4-fluoro-3-trifluorometh...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]osanetant from human recombinant NK3 receptor expressed in HEK293 cells | J Med Chem 52: 7103-12 (2009) Article DOI: 10.1021/jm900948q BindingDB Entry DOI: 10.7270/Q21G0MBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50291261 ((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]osanetant from guinea pig recombinant NK3 receptor A1142.58T expressed in HEK293 cells | J Med Chem 52: 7103-12 (2009) Article DOI: 10.1021/jm900948q BindingDB Entry DOI: 10.7270/Q21G0MBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233257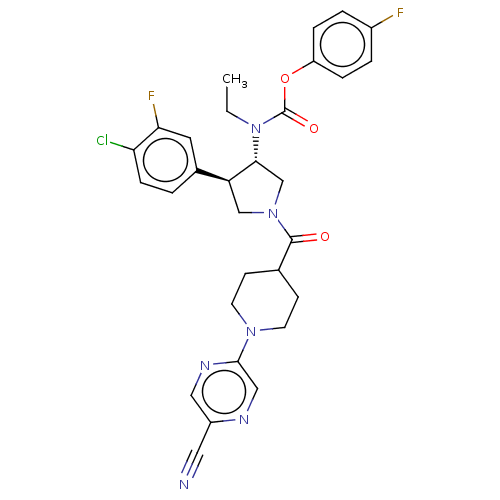 (US9346786, 61) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50329160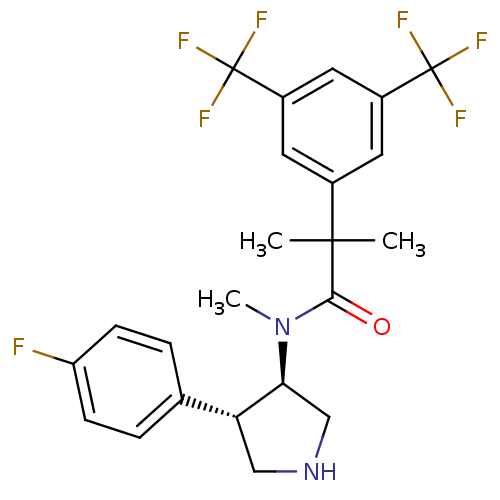 (2-(3,5-bis(trifluoromethyl)phenyl)-N-((3R,4S)-4-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human neurokinin NK1 receptor expressed in CHO cell | Bioorg Med Chem Lett 20: 6735-8 (2010) Article DOI: 10.1016/j.bmcl.2010.08.138 BindingDB Entry DOI: 10.7270/Q22Z15SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233311 (US9346786, 115) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | -48.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233242 (US9346786, 46) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077452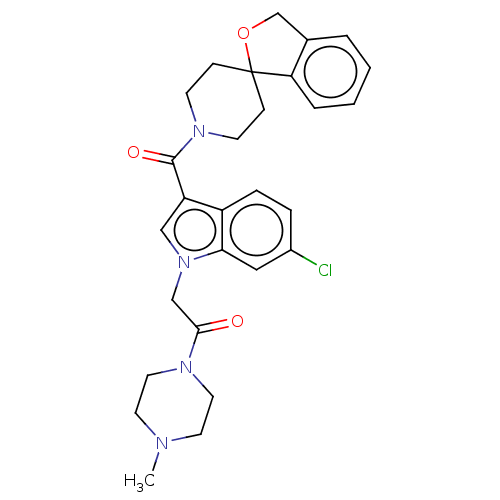 (CHEMBL3416868) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cell membranes after 1 hr by scintillation proximity assay | J Med Chem 58: 2275-89 (2015) Article DOI: 10.1021/jm501745f BindingDB Entry DOI: 10.7270/Q2JW8GK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077221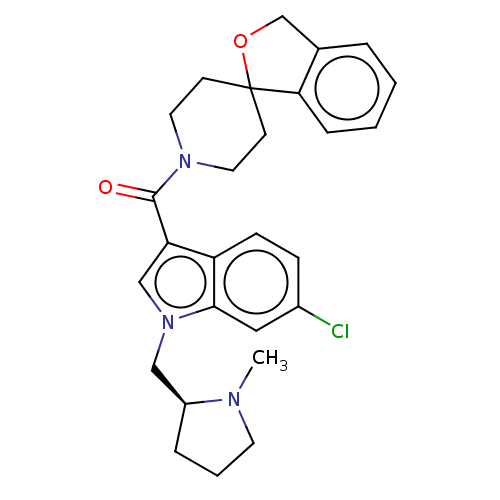 (CHEMBL3416883) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cell membranes after 1 hr by scintillation proximity assay | J Med Chem 58: 2275-89 (2015) Article DOI: 10.1021/jm501745f BindingDB Entry DOI: 10.7270/Q2JW8GK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233262 (US9346786, 66) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233197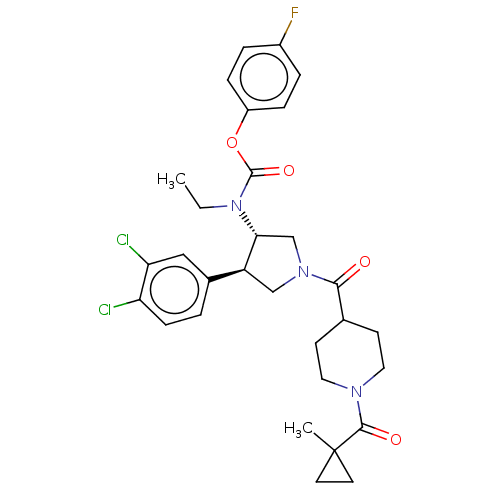 (US9346786, 1 | US9346786, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077217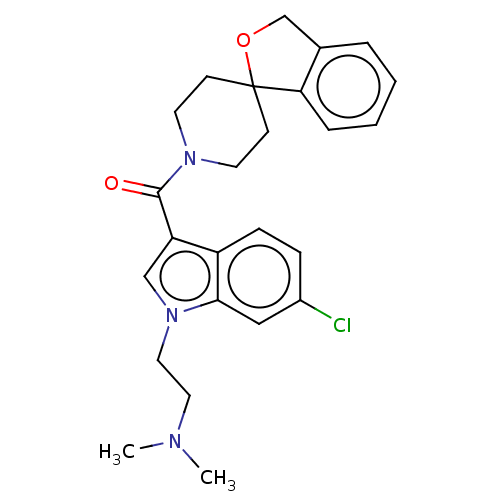 (CHEMBL3416885) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cell membranes after 1 hr by scintillation proximity assay | J Med Chem 58: 2275-89 (2015) Article DOI: 10.1021/jm501745f BindingDB Entry DOI: 10.7270/Q2JW8GK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50329162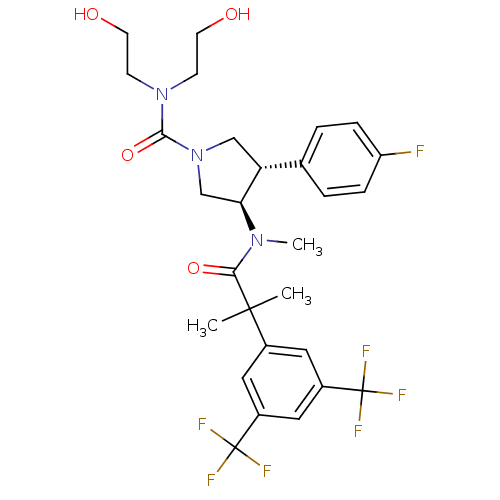 ((3R,4S)-3-(2-(3,5-bis(trifluoromethyl)phenyl)-N,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human neurokinin NK1 receptor expressed in CHO cell | Bioorg Med Chem Lett 20: 6735-8 (2010) Article DOI: 10.1016/j.bmcl.2010.08.138 BindingDB Entry DOI: 10.7270/Q22Z15SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077222 (CHEMBL3416882) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cell membranes after 1 hr by scintillation proximity assay | J Med Chem 58: 2275-89 (2015) Article DOI: 10.1021/jm501745f BindingDB Entry DOI: 10.7270/Q2JW8GK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50329163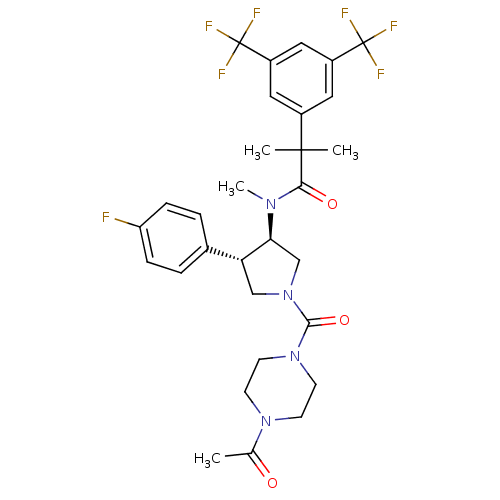 (CHEMBL1269639 | N-((3R,4S)-1-(4-acetylpiperazine-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human neurokinin NK1 receptor expressed in CHO cell | Bioorg Med Chem Lett 20: 6735-8 (2010) Article DOI: 10.1016/j.bmcl.2010.08.138 BindingDB Entry DOI: 10.7270/Q22Z15SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50299468 (2-(3,4-dichlorophenyl)-N-(4-fluoro-3-trifluorometh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]osanetant from guinea pig recombinant NK3 receptor expressed in HEK293 cells | J Med Chem 52: 7103-12 (2009) Article DOI: 10.1021/jm900948q BindingDB Entry DOI: 10.7270/Q21G0MBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233310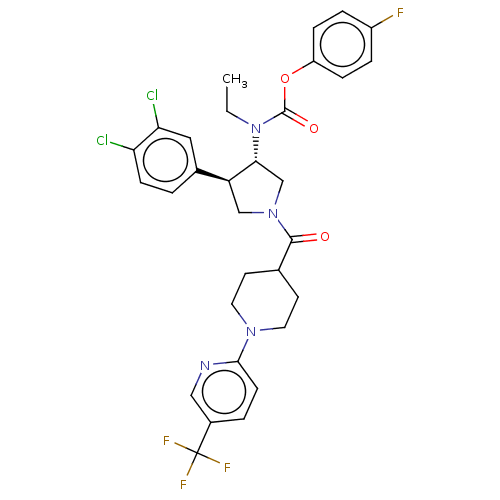 (US9346786, 114) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | -46.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233305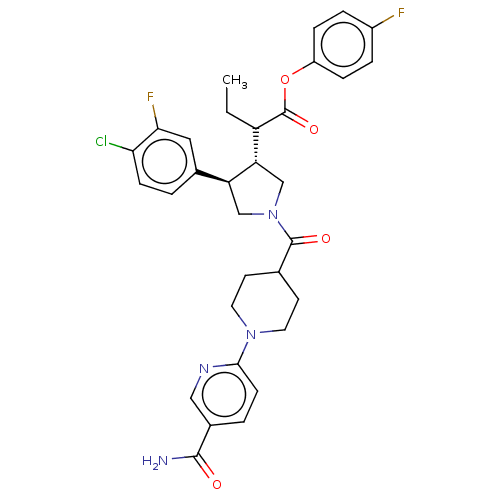 (US9346786, 109) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233316 (US9346786, 120) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.70 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233314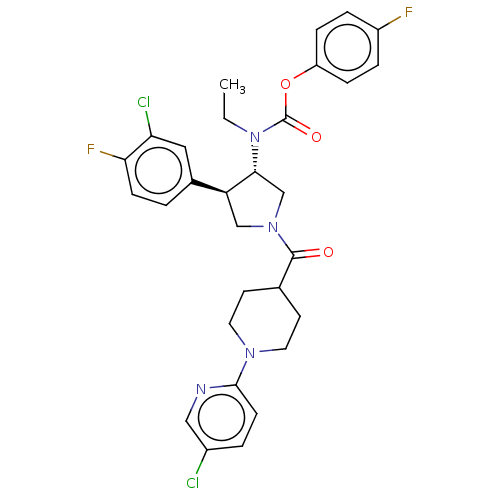 (US9346786, 118) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.80 | -46.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (GUINEA PIG) | BDBM50299468 (2-(3,4-dichlorophenyl)-N-(4-fluoro-3-trifluorometh...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]osanetant from guinea pig recombinant NK3 receptor A1142.58T expressed in HEK293 cells | J Med Chem 52: 7103-12 (2009) Article DOI: 10.1021/jm900948q BindingDB Entry DOI: 10.7270/Q21G0MBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233213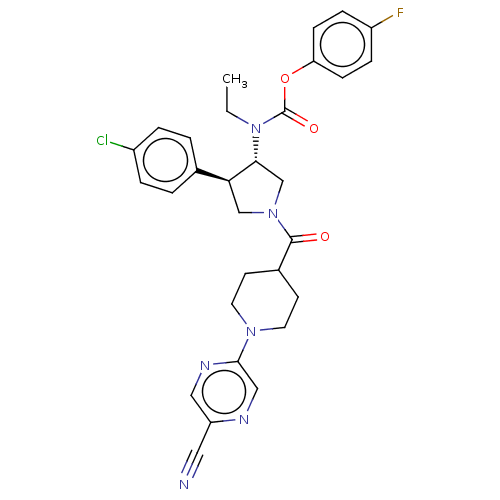 (US9346786, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233214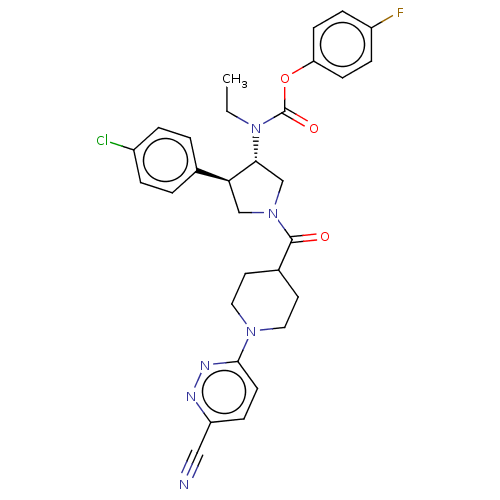 (US9346786, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233263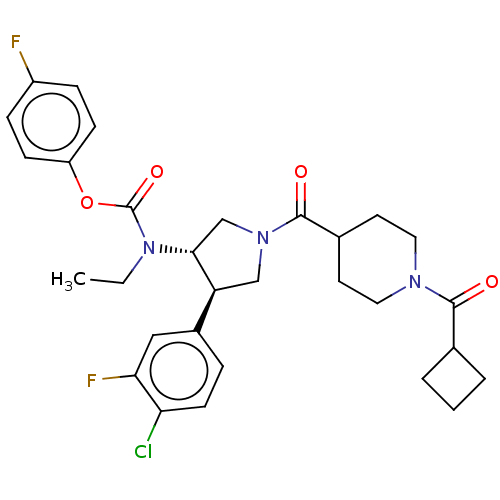 (US9346786, 67) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233240 (US9346786, 44) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233247 (US9346786, 51) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233244 (US9346786, 48) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233243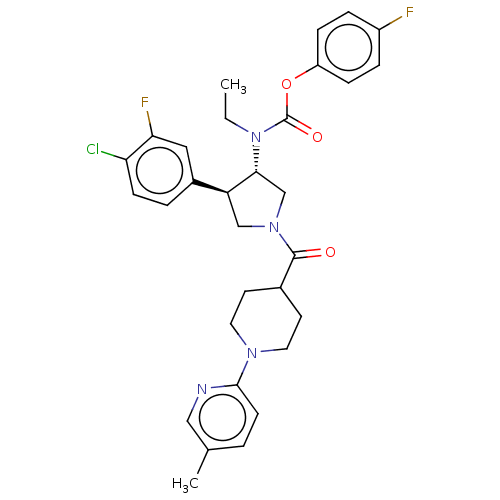 (US9346786, 47) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233276 (US9346786, 80) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233238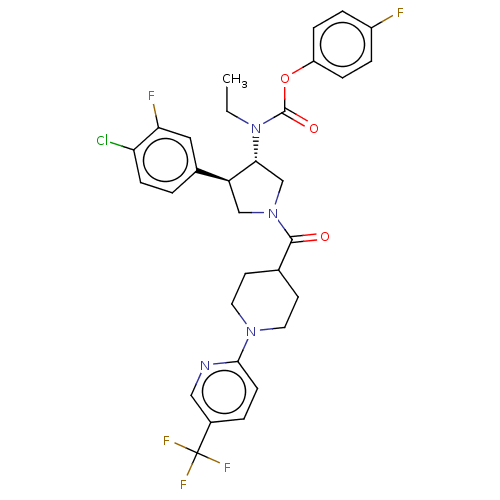 (US9346786, 42) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233268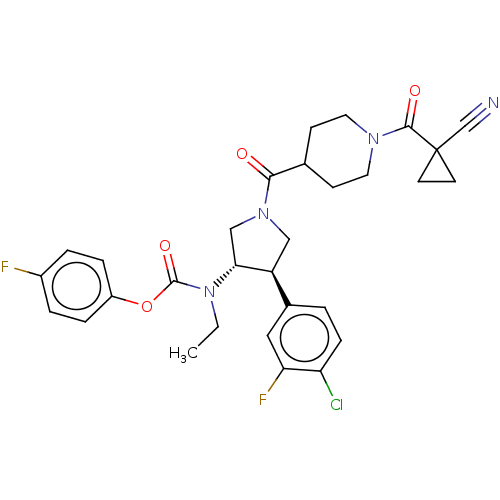 (US9346786, 72) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233265 (US9346786, 69) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233264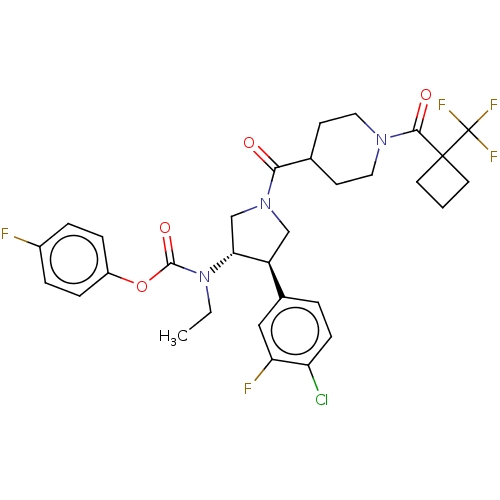 (US9346786, 68) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233197 (US9346786, 1 | US9346786, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233212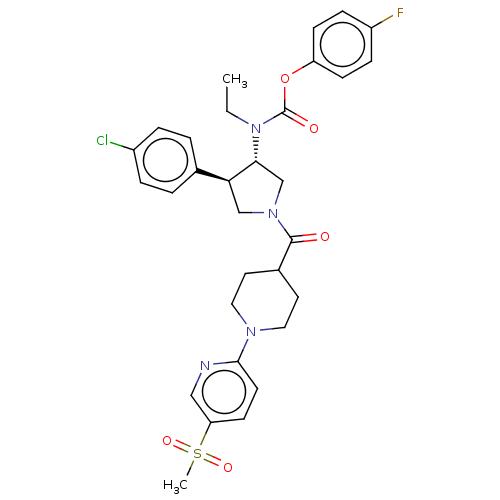 (US9346786, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM233217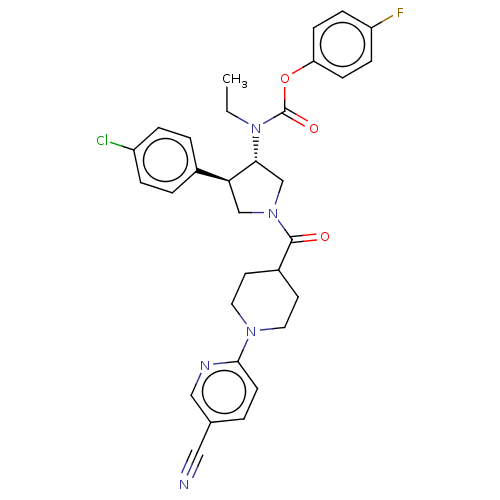 (US9346786, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Hoffmann-La Roche Inc. US Patent | Assay Description hNK3 receptor binding experiment were performed using [3H]SR142801 (Catalog No. TRK1035, specific activity: 74.0 Ci/mmol, Amersham, GE Healthcare UK ... | US Patent US9346786 (2016) BindingDB Entry DOI: 10.7270/Q20V8BPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077310 (CHEMBL3416869) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cell membranes after 1 hr by scintillation proximity assay | J Med Chem 58: 2275-89 (2015) Article DOI: 10.1021/jm501745f BindingDB Entry DOI: 10.7270/Q2JW8GK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 715 total ) | Next | Last >> |