 Found 676 hits with Last Name = 'sadowski' and Initial = 's'
Found 676 hits with Last Name = 'sadowski' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85790
(Salmon MCH)Show SMILES CSCCC(NC(=O)C(NC(=O)C(N)CC(O)=O)C(C)O)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(NC(=O)C(CCSC)NC1=O)C(C)C)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(O)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,3.32,;17.66,2.55,;18.99,3.32,;18.99,4.86,;20.32,5.63,;20.32,7.17,;21.66,7.94,;22.99,7.17,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;25.66,11.79,;24.33,14.1,;25.66,14.87,;25.66,16.41,;26.99,14.1,;20.32,10.25,;18.99,9.48,;20.32,11.79,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;30.99,2.55,;32.33,3.32,;29.66,3.32,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C89H139N27O24S4/c1-43(2)67-82(135)101-40-64(119)102-53(18-12-30-97-87(91)92)74(127)113-68(44(3)4)83(136)109-59(36-47-22-24-49(118)25-23-47)77(130)107-58(20-14-32-99-89(95)96)85(138)116-33-15-21-63(116)81(134)111-62(80(133)108-60(37-48-39-100-52-17-11-10-16-50(48)52)78(131)104-55(26-27-65(120)121)75(128)114-69(45(5)6)86(139)140)42-144-143-41-61(79(132)105-57(29-35-142-9)76(129)112-67)110-72(125)54(19-13-31-98-88(93)94)103-73(126)56(28-34-141-8)106-84(137)70(46(7)117)115-71(124)51(90)38-66(122)123/h10-11,16-17,22-25,39,43-46,51,53-63,67-70,100,117-118H,12-15,18-21,26-38,40-42,90H2,1-9H3,(H,101,135)(H,102,119)(H,103,126)(H,104,131)(H,105,132)(H,106,137)(H,107,130)(H,108,133)(H,109,136)(H,110,125)(H,111,134)(H,112,129)(H,113,127)(H,114,128)(H,115,124)(H,120,121)(H,122,123)(H,139,140)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM85789
([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85789

([Phe13,Tyr19]MCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccccc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(Cc1ccc(O)cc1)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;5.24,.24,;3.91,1.01,;2.57,.24,;1.24,1.01,;2.57,-1.3,;3.91,-2.07,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C109H160N30O26S4/c1-57(2)45-74-90(148)122-54-85(142)123-68(27-17-39-118-107(112)113)95(153)138-88(59(5)6)104(162)134-77(48-61-23-13-10-14-24-61)97(155)128-73(29-19-41-120-109(116)117)105(163)139-42-20-30-83(139)103(161)137-82(102(160)132-78(50-63-53-121-67-26-16-15-25-65(63)67)99(157)125-70(35-36-84(111)141)92(150)135-80(106(164)165)49-62-31-33-64(140)34-32-62)56-169-168-55-81(101(159)127-72(38-44-167-8)93(151)130-74)136-91(149)69(28-18-40-119-108(114)115)124-96(154)75(46-58(3)4)131-94(152)71(37-43-166-7)126-100(158)79(52-87(145)146)133-98(156)76(47-60-21-11-9-12-22-60)129-89(147)66(110)51-86(143)144/h9-16,21-26,31-34,53,57-59,66,68-83,88,121,140H,17-20,27-30,35-52,54-56,110H2,1-8H3,(H2,111,141)(H,122,148)(H,123,142)(H,124,154)(H,125,157)(H,126,158)(H,127,159)(H,128,155)(H,129,147)(H,130,151)(H,131,152)(H,132,160)(H,133,156)(H,134,162)(H,135,150)(H,136,149)(H,137,161)(H,138,153)(H,143,144)(H,145,146)(H,164,165)(H4,112,113,118)(H4,114,115,119)(H4,116,117,120) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85788
(MCH | hMCH)Show SMILES CSCCC(NC(=O)C(CC(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)C(N)CC(O)=O)C(=O)NC(CC(C)C)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(CC(C)C)NC(=O)C(CCSC)NC1=O)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(N)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,9.48,;17.66,10.25,;18.99,9.48,;20.32,10.25,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;24.33,14.1,;23.24,15.19,;23.63,16.68,;21.75,14.79,;25.66,11.79,;26.99,12.56,;26.99,14.1,;28.33,11.79,;28.33,10.25,;29.66,9.48,;29.66,7.94,;30.99,7.17,;32.33,7.94,;32.33,9.48,;30.99,10.25,;29.66,12.56,;31.15,12.16,;31.55,10.67,;32.48,12.93,;33.81,12.16,;32.48,14.47,;33.81,15.24,;33.81,16.78,;35.3,14.84,;21.66,7.94,;22.99,7.17,;20.32,7.17,;20.32,5.63,;18.99,4.86,;18.99,3.32,;17.66,2.55,;20.32,2.55,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;30.99,2.55,;32.33,3.32,;32.33,4.86,;33.66,2.55,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C105H160N30O26S4/c1-53(2)42-70-86(144)118-50-80(138)119-64(24-16-36-114-103(108)109)90(148)133-83(55(5)6)100(158)130-73(45-58-28-30-60(136)31-29-58)93(151)124-69(26-18-38-116-105(112)113)101(159)135-39-19-27-78(135)99(157)132-77(98(156)128-74(46-59-49-117-63-23-15-14-22-61(59)63)95(153)121-66(32-33-79(107)137)91(149)134-84(56(7)8)102(160)161)52-165-164-51-76(97(155)123-68(35-41-163-10)88(146)126-70)131-87(145)65(25-17-37-115-104(110)111)120-92(150)71(43-54(3)4)127-89(147)67(34-40-162-9)122-96(154)75(48-82(141)142)129-94(152)72(44-57-20-12-11-13-21-57)125-85(143)62(106)47-81(139)140/h11-15,20-23,28-31,49,53-56,62,64-78,83-84,117,136H,16-19,24-27,32-48,50-52,106H2,1-10H3,(H2,107,137)(H,118,144)(H,119,138)(H,120,150)(H,121,153)(H,122,154)(H,123,155)(H,124,151)(H,125,143)(H,126,146)(H,127,147)(H,128,156)(H,129,152)(H,130,158)(H,131,145)(H,132,157)(H,133,148)(H,134,149)(H,139,140)(H,141,142)(H,160,161)(H4,108,109,114)(H4,110,111,115)(H4,112,113,116) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50117522
((5S,6R,9S)-5-(4-Fluoro-phenyl)-9-(2-trifluoromethy...)Show SMILES Fc1ccc(cc1)[C@@H]1NCCOC11CC[C@H](CO1)c1ccccc1C(F)(F)F Show InChI InChI=1S/C21H21F4NO2/c22-16-7-5-14(6-8-16)19-20(27-12-11-26-19)10-9-15(13-28-20)17-3-1-2-4-18(17)21(23,24)25/h1-8,15,19,26H,9-13H2/t15-,19+,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound to human Tachykinin receptor 1 |
Bioorg Med Chem Lett 12: 2515-8 (2002)
BindingDB Entry DOI: 10.7270/Q27P8XQ9 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50117508

((5S,6R,9R)-5-(4-Fluoro-phenyl)-9-(2-trifluoromethy...)Show SMILES Fc1ccc(cc1)[C@@H]1NCCOC11CC[C@@H](CO1)c1ccccc1C(F)(F)F Show InChI InChI=1S/C21H21F4NO2/c22-16-7-5-14(6-8-16)19-20(27-12-11-26-19)10-9-15(13-28-20)17-3-1-2-4-18(17)21(23,24)25/h1-8,15,19,26H,9-13H2/t15-,19-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound to human Tachykinin receptor 1 |
Bioorg Med Chem Lett 12: 2515-8 (2002)
BindingDB Entry DOI: 10.7270/Q27P8XQ9 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 2
(Homo sapiens (Human)) | BDBM85790

(Salmon MCH)Show SMILES CSCCC(NC(=O)C(NC(=O)C(N)CC(O)=O)C(C)O)C(=O)NC(CCCN=C(N)N)C(=O)NC1CSSCC(NC(=O)C2CCCN2C(=O)C(CCCN=C(N)N)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(NC(=O)C(CCCN=C(N)N)NC(=O)CNC(=O)C(NC(=O)C(CCSC)NC1=O)C(C)C)C(C)C)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)NC(CCC(O)=O)C(=O)NC(C(C)C)C(O)=O |(16.32,3.32,;17.66,2.55,;18.99,3.32,;18.99,4.86,;20.32,5.63,;20.32,7.17,;21.66,7.94,;22.99,7.17,;21.66,9.48,;22.99,10.25,;22.99,11.79,;21.66,12.56,;24.33,12.56,;25.66,11.79,;24.33,14.1,;25.66,14.87,;25.66,16.41,;26.99,14.1,;20.32,10.25,;18.99,9.48,;20.32,11.79,;21.66,4.86,;22.99,5.63,;21.66,3.32,;22.99,2.55,;24.33,3.32,;24.33,4.86,;25.66,5.63,;25.66,7.17,;26.99,7.94,;26.99,9.48,;28.33,7.17,;22.99,1.01,;21.66,.24,;24.33,.24,;24.33,-1.3,;22.99,-2.07,;21.66,-1.3,;20.32,-2.07,;20.32,-3.61,;18.99,-4.38,;18.99,-5.92,;17.66,-6.69,;16.12,-6.69,;17.66,-8.23,;16.52,-9.26,;17.15,-10.66,;18.67,-10.5,;18.99,-9,;20.32,-8.23,;21.09,-6.9,;21.66,-9,;21.66,-10.54,;20.32,-11.31,;20.32,-12.85,;18.99,-13.62,;18.99,-15.16,;17.66,-15.93,;20.32,-15.93,;22.99,-8.23,;24.33,-9,;24.33,-10.54,;25.66,-8.23,;26.99,-9,;26.99,-10.54,;25.66,-11.31,;25.66,-12.85,;26.99,-13.62,;26.99,-15.16,;28.33,-12.85,;28.33,-11.31,;25.66,-6.69,;26.99,-5.92,;26.99,-4.38,;28.33,-6.69,;29.66,-5.92,;30.99,-6.69,;30.99,-8.23,;32.33,-5.92,;33.66,-6.69,;33.66,-8.23,;34.99,-9,;34.99,-10.54,;36.33,-11.31,;36.33,-12.85,;37.66,-10.54,;32.33,-4.38,;33.66,-3.61,;34.99,-4.38,;33.66,-2.07,;32.33,-1.3,;32.33,.24,;33.66,1.01,;30.99,1.01,;29.66,.24,;28.33,1.01,;28.33,2.55,;26.99,.24,;25.81,1.23,;26.08,2.75,;27.53,3.27,;27.8,4.79,;26.99,-1.3,;25.66,-2.07,;25.66,-3.61,;30.99,2.55,;32.33,3.32,;29.66,3.32,;28.33,-8.23,;29.66,-9,;28.33,-9.77,;17.66,-3.61,;17.66,-2.07,;16.32,-4.38,;14.99,-3.61,;14.99,-2.07,;13.66,-1.3,;12.26,-1.93,;11.23,-.79,;11.99,.54,;11.51,2,;12.54,3.15,;14.05,2.83,;14.53,1.37,;13.5,.22,;13.66,-4.38,;13.66,-5.92,;12.12,-3.61,;10.78,-4.38,;10.78,-5.92,;9.45,-6.69,;9.45,-8.23,;8.11,-9,;10.78,-9,;9.45,-3.61,;9.45,-2.07,;7.91,-4.38,;6.57,-3.61,;6.57,-2.07,;5.24,-1.3,;7.91,-1.3,;5.24,-4.38,;3.91,-3.61,;5.24,-5.92,)| Show InChI InChI=1S/C89H139N27O24S4/c1-43(2)67-82(135)101-40-64(119)102-53(18-12-30-97-87(91)92)74(127)113-68(44(3)4)83(136)109-59(36-47-22-24-49(118)25-23-47)77(130)107-58(20-14-32-99-89(95)96)85(138)116-33-15-21-63(116)81(134)111-62(80(133)108-60(37-48-39-100-52-17-11-10-16-50(48)52)78(131)104-55(26-27-65(120)121)75(128)114-69(45(5)6)86(139)140)42-144-143-41-61(79(132)105-57(29-35-142-9)76(129)112-67)110-72(125)54(19-13-31-98-88(93)94)103-73(126)56(28-34-141-8)106-84(137)70(46(7)117)115-71(124)51(90)38-66(122)123/h10-11,16-17,22-25,39,43-46,51,53-63,67-70,100,117-118H,12-15,18-21,26-38,40-42,90H2,1-9H3,(H,101,135)(H,102,119)(H,103,126)(H,104,131)(H,105,132)(H,106,137)(H,107,130)(H,108,133)(H,109,136)(H,110,125)(H,111,134)(H,112,129)(H,113,127)(H,114,128)(H,115,124)(H,120,121)(H,122,123)(H,139,140)(H4,91,92,97)(H4,93,94,98)(H4,95,96,99) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 437 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 98: 7564-9 (2001)
Article DOI: 10.1073/pnas.121170598
BindingDB Entry DOI: 10.7270/Q2RJ4H14 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50052278
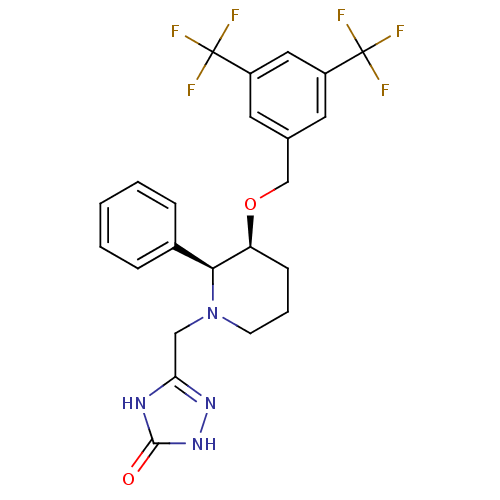
(5-[(2S,3S)-3-(3,5-Bis-trifluoromethyl-benzyloxy)-2...)Show SMILES FC(F)(F)c1cc(CO[C@H]2CCCN(Cc3n[nH]c(=O)[nH]3)[C@H]2c2ccccc2)cc(c1)C(F)(F)F Show InChI InChI=1S/C23H22F6N4O2/c24-22(25,26)16-9-14(10-17(11-16)23(27,28)29)13-35-18-7-4-8-33(12-19-30-21(34)32-31-19)20(18)15-5-2-1-3-6-15/h1-3,5-6,9-11,18,20H,4,7-8,12-13H2,(H2,30,31,32,34)/t18-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-Tyr8 SP from the cloned human Tachykinin receptor 1 expressed in CHO cells |
J Med Chem 39: 2907-14 (1996)
Article DOI: 10.1021/jm9506534
BindingDB Entry DOI: 10.7270/Q2D21WP4 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50067933
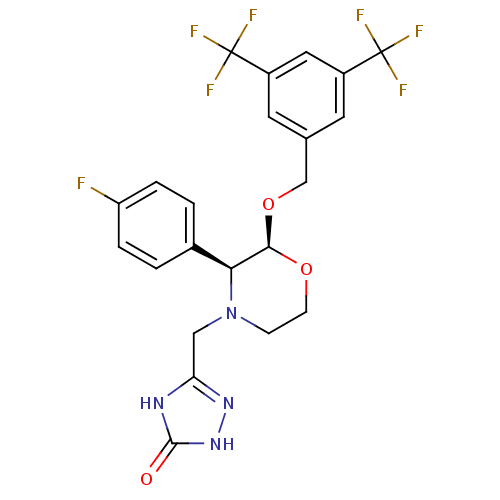
(5-(((2S,3S)-2-(3,5-bis(trifluoromethyl)benzyloxy)-...)Show SMILES Fc1ccc(cc1)[C@H]1[C@@H](OCc2cc(cc(c2)C(F)(F)F)C(F)(F)F)OCCN1Cc1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C22H19F7N4O3/c23-16-3-1-13(2-4-16)18-19(35-6-5-33(18)10-17-30-20(34)32-31-17)36-11-12-7-14(21(24,25)26)9-15(8-12)22(27,28)29/h1-4,7-9,18-19H,5-6,10-11H2,(H2,30,31,32,34)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells |
J Med Chem 41: 4607-14 (1998)
Article DOI: 10.1021/jm980299k
BindingDB Entry DOI: 10.7270/Q2XW4HZ7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50408664
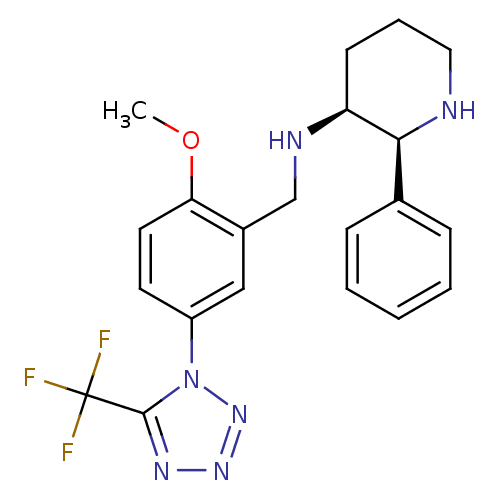
(GR-205171 | VOFOPITANT)Show SMILES COc1ccc(cc1CN[C@H]1CCCN[C@H]1c1ccccc1)-n1nnnc1C(F)(F)F Show InChI InChI=1S/C21H23F3N6O/c1-31-18-10-9-16(30-20(21(22,23)24)27-28-29-30)12-15(18)13-26-17-8-5-11-25-19(17)14-6-3-2-4-7-14/h2-4,6-7,9-10,12,17,19,25-26H,5,8,11,13H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells |
J Med Chem 41: 4607-14 (1998)
Article DOI: 10.1021/jm980299k
BindingDB Entry DOI: 10.7270/Q2XW4HZ7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220136
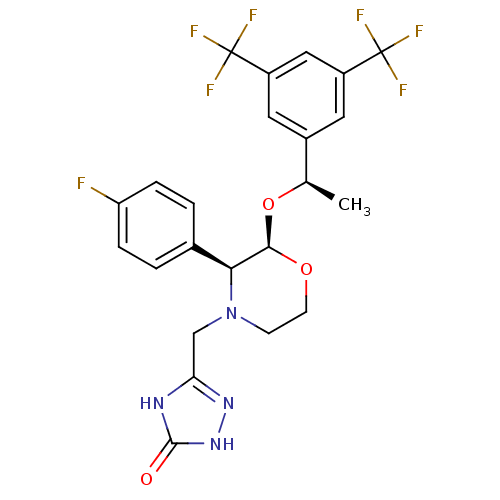
(3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4504-11 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.044
BindingDB Entry DOI: 10.7270/Q20P0ZM5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220136

(3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
compounds were evaluated for inhibitory activity against human Tachykinin receptor 1 |
J Med Chem 43: 1234-41 (2000)
BindingDB Entry DOI: 10.7270/Q2G73CZM |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049469
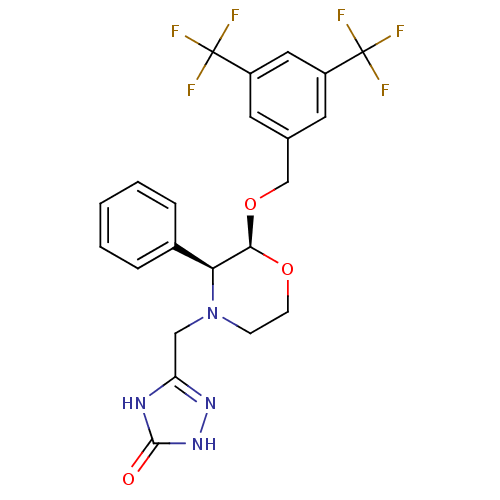
(5-(((2S,3S)-2-(3,5-bis(trifluoromethyl)benzyloxy)-...)Show SMILES FC(F)(F)c1cc(CO[C@H]2OCCN(Cc3n[nH]c(=O)[nH]3)[C@H]2c2ccccc2)cc(c1)C(F)(F)F |r| Show InChI InChI=1S/C22H20F6N4O3/c23-21(24,25)15-8-13(9-16(10-15)22(26,27)28)12-35-19-18(14-4-2-1-3-5-14)32(6-7-34-19)11-17-29-20(33)31-30-17/h1-5,8-10,18-19H,6-7,11-12H2,(H2,29,30,31,33)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4497-503 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.035
BindingDB Entry DOI: 10.7270/Q21J99DZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220136

(3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4497-503 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.035
BindingDB Entry DOI: 10.7270/Q21J99DZ |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50106711

(5-[2-[1-(3,5-Bis-trifluoromethyl-phenyl)-ethoxy]-3...)Show SMILES C[C@@H](OC1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
In vitro inhibition of binding of [125I]-substance P to tachykinin receptor 1 |
J Med Chem 44: 4296-9 (2001)
BindingDB Entry DOI: 10.7270/Q2PN96BR |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50290861

(5-{3-[1-(3,5-Dichloro-phenyl)-ethoxy]-2-phenyl-pip...)Show SMILES C[C@@H](O[C@H]1CCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccccc1)c1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C22H24Cl2N4O2/c1-14(16-10-17(23)12-18(24)11-16)30-19-8-5-9-28(13-20-25-22(29)27-26-20)21(19)15-6-3-2-4-7-15/h2-4,6-7,10-12,14,19,21H,5,8-9,13H2,1H3,(H2,25,26,27,29)/t14-,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonism of NK1 receptor in rat liver microsomes. |
Bioorg Med Chem Lett 7: 2959-2962 (1997)
Article DOI: 10.1016/S0960-894X(97)10118-4
BindingDB Entry DOI: 10.7270/Q2W66KRX |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049469

(5-(((2S,3S)-2-(3,5-bis(trifluoromethyl)benzyloxy)-...)Show SMILES FC(F)(F)c1cc(CO[C@H]2OCCN(Cc3n[nH]c(=O)[nH]3)[C@H]2c2ccccc2)cc(c1)C(F)(F)F |r| Show InChI InChI=1S/C22H20F6N4O3/c23-21(24,25)15-8-13(9-16(10-15)22(26,27)28)12-35-19-18(14-4-2-1-3-5-14)32(6-7-34-19)11-17-29-20(33)31-30-17/h1-5,8-10,18-19H,6-7,11-12H2,(H2,29,30,31,33)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled SP from human Tachykinin receptor 1 expressed in CHO cells |
J Med Chem 39: 1760-2 (1996)
Article DOI: 10.1021/jm950654w
BindingDB Entry DOI: 10.7270/Q2GB2346 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50067940

(5-{(2R,3S)-2-[(S)-1-(3,5-Bis-trifluoromethyl-pheny...)Show SMILES C[C@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H22F6N4O3/c1-13(15-9-16(22(24,25)26)11-17(10-15)23(27,28)29)36-20-19(14-5-3-2-4-6-14)33(7-8-35-20)12-18-30-21(34)32-31-18/h2-6,9-11,13,19-20H,7-8,12H2,1H3,(H2,30,31,32,34)/t13-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells |
J Med Chem 41: 4607-14 (1998)
Article DOI: 10.1021/jm980299k
BindingDB Entry DOI: 10.7270/Q2XW4HZ7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049469

(5-(((2S,3S)-2-(3,5-bis(trifluoromethyl)benzyloxy)-...)Show SMILES FC(F)(F)c1cc(CO[C@H]2OCCN(Cc3n[nH]c(=O)[nH]3)[C@H]2c2ccccc2)cc(c1)C(F)(F)F |r| Show InChI InChI=1S/C22H20F6N4O3/c23-21(24,25)15-8-13(9-16(10-15)22(26,27)28)12-35-19-18(14-4-2-1-3-5-14)32(6-7-34-19)11-17-29-20(33)31-30-17/h1-5,8-10,18-19H,6-7,11-12H2,(H2,29,30,31,33)/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells |
J Med Chem 41: 4607-14 (1998)
Article DOI: 10.1021/jm980299k
BindingDB Entry DOI: 10.7270/Q2XW4HZ7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50220136

(3-[2-{1-[3,5-di(trifluoromethyl)phenyl]ethoxy}-3-(...)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F |r| Show InChI InChI=1S/C23H21F7N4O3/c1-12(14-8-15(22(25,26)27)10-16(9-14)23(28,29)30)37-20-19(13-2-4-17(24)5-3-13)34(6-7-36-20)11-18-31-21(35)33-32-18/h2-5,8-10,12,19-20H,6-7,11H2,1H3,(H2,31,32,33,35)/t12-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells |
J Med Chem 41: 4607-14 (1998)
Article DOI: 10.1021/jm980299k
BindingDB Entry DOI: 10.7270/Q2XW4HZ7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50067939
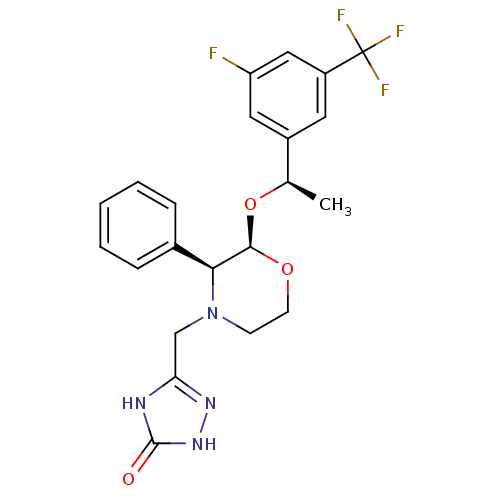
(5-{(2R,3S)-2-[(R)-1-(3-Fluoro-5-trifluoromethyl-ph...)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccccc1)c1cc(F)cc(c1)C(F)(F)F Show InChI InChI=1S/C22H22F4N4O3/c1-13(15-9-16(22(24,25)26)11-17(23)10-15)33-20-19(14-5-3-2-4-6-14)30(7-8-32-20)12-18-27-21(31)29-28-18/h2-6,9-11,13,19-20H,7-8,12H2,1H3,(H2,27,28,29,31)/t13-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells |
J Med Chem 41: 4607-14 (1998)
Article DOI: 10.1021/jm980299k
BindingDB Entry DOI: 10.7270/Q2XW4HZ7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50117509

((3S,5S,10S)-5-(4-Fluoro-phenyl)-10-[2-methoxy-5-(5...)Show SMILES COc1ccc(cc1[C@H]1COCC2(C1)OCCN[C@H]2c1ccc(F)cc1)-n1nnnc1C(F)(F)F Show InChI InChI=1S/C23H23F4N5O3/c1-33-19-7-6-17(32-21(23(25,26)27)29-30-31-32)10-18(19)15-11-22(13-34-12-15)20(28-8-9-35-22)14-2-4-16(24)5-3-14/h2-7,10,15,20,28H,8-9,11-13H2,1H3/t15-,20+,22?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 12: 2515-8 (2002)
BindingDB Entry DOI: 10.7270/Q27P8XQ9 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50117523

(5-{(5S,10S)-5-(4-Fluoro-phenyl)-10-[2-methoxy-5-(5...)Show SMILES COc1ccc(cc1[C@H]1COCC2(C1)OCCN(Cc1n[nH]c(=O)[nH]1)[C@H]2c1ccc(F)cc1)-n1nnnc1C(F)(F)F Show InChI InChI=1S/C26H26F4N8O4/c1-40-20-7-6-18(38-23(26(28,29)30)33-35-36-38)10-19(20)16-11-25(14-41-13-16)22(15-2-4-17(27)5-3-15)37(8-9-42-25)12-21-31-24(39)34-32-21/h2-7,10,16,22H,8-9,11-14H2,1H3,(H2,31,32,34,39)/t16-,22+,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled substance P from human Tachykinin receptor 1 expressed in CHO cells |
Bioorg Med Chem Lett 12: 2515-8 (2002)
BindingDB Entry DOI: 10.7270/Q27P8XQ9 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50071484
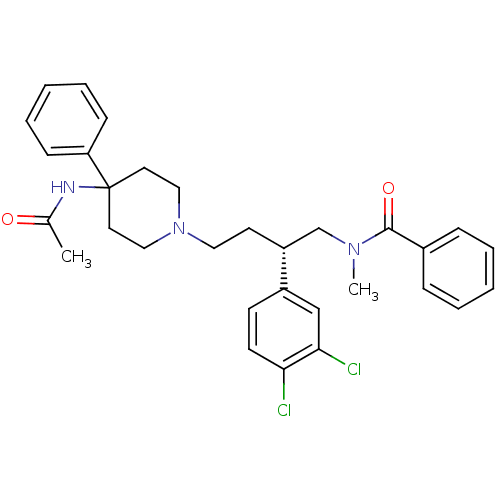
(CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...)Show SMILES CN(C[C@@H](CCN1CCC(CC1)(NC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H35Cl2N3O2/c1-23(37)34-31(27-11-7-4-8-12-27)16-19-36(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-35(2)30(38)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3,(H,34,37)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of cloned human NK2 (Neurokinin 2) receptor, stably expressed in chinese hamster ovary (CHO) cells was determined |
Bioorg Med Chem Lett 8: 2259-62 (1999)
BindingDB Entry DOI: 10.7270/Q2Z31XTN |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50049467
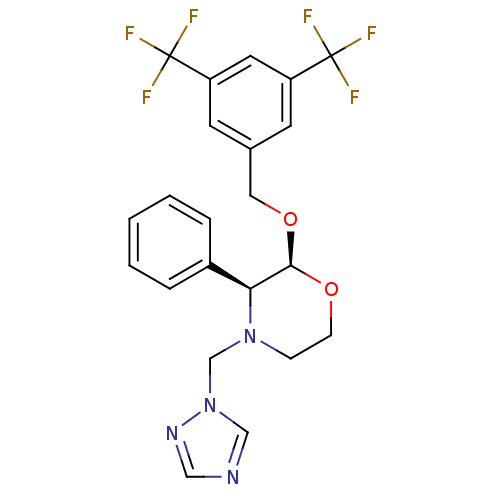
((2S,3S)-2-(3,5-Bis-trifluoromethyl-benzyloxy)-3-ph...)Show SMILES FC(F)(F)c1cc(CO[C@H]2OCCN(Cn3cncn3)[C@H]2c2ccccc2)cc(c1)C(F)(F)F Show InChI InChI=1S/C22H20F6N4O2/c23-21(24,25)17-8-15(9-18(10-17)22(26,27)28)11-34-20-19(16-4-2-1-3-5-16)31(6-7-33-20)14-32-13-29-12-30-32/h1-5,8-10,12-13,19-20H,6-7,11,14H2/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled SP from human Tachykinin receptor 1 expressed in CHO cells |
J Med Chem 39: 1760-2 (1996)
Article DOI: 10.1021/jm950654w
BindingDB Entry DOI: 10.7270/Q2GB2346 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50191091

((S)-5-((((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@H]([C@@H]1c1ccc(F)cc1)N(C)C[C@@H]1CCC(=O)N1C)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C28H31F7N2O2/c1-16(18-12-19(27(30,31)32)14-20(13-18)28(33,34)35)39-24-10-9-23(26(24)17-4-6-21(29)7-5-17)36(2)15-22-8-11-25(38)37(22)3/h4-7,12-14,16,22-24,26H,8-11,15H2,1-3H3/t16-,22+,23-,24+,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4504-11 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.044
BindingDB Entry DOI: 10.7270/Q20P0ZM5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50067935
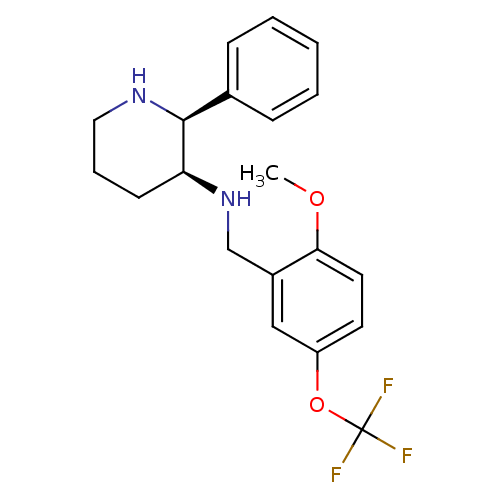
((2-Methoxy-5-trifluoromethoxy-benzyl)-((2S,3S)-2-p...)Show SMILES COc1ccc(OC(F)(F)F)cc1CN[C@H]1CCCN[C@H]1c1ccccc1 |r| Show InChI InChI=1S/C20H23F3N2O2/c1-26-18-10-9-16(27-20(21,22)23)12-15(18)13-25-17-8-5-11-24-19(17)14-6-3-2-4-7-14/h2-4,6-7,9-10,12,17,19,24-25H,5,8,11,13H2,1H3/t17-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]-labeled SP from the human Tachykinin receptor 1 expressed in CHO cells |
J Med Chem 41: 4607-14 (1998)
Article DOI: 10.1021/jm980299k
BindingDB Entry DOI: 10.7270/Q2XW4HZ7 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50290865
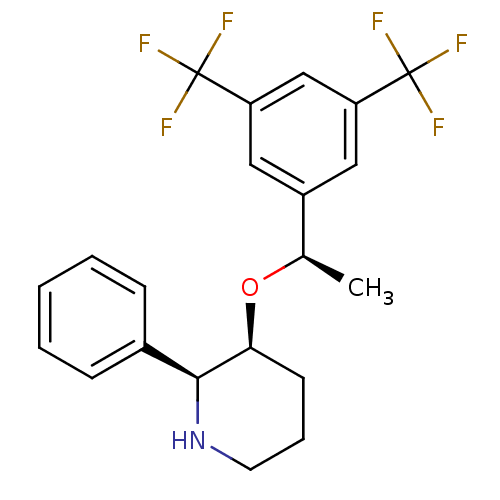
(3-[1-(3,5-Bis-trifluoromethyl-phenyl)-ethoxy]-2-ph...)Show SMILES C[C@@H](O[C@H]1CCCN[C@H]1c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C21H21F6NO/c1-13(15-10-16(20(22,23)24)12-17(11-15)21(25,26)27)29-18-8-5-9-28-19(18)14-6-3-2-4-7-14/h2-4,6-7,10-13,18-19,28H,5,8-9H2,1H3/t13-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonism of NK1 receptor in rat liver microsomes. |
Bioorg Med Chem Lett 7: 2959-2962 (1997)
Article DOI: 10.1016/S0960-894X(97)10118-4
BindingDB Entry DOI: 10.7270/Q2W66KRX |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50290856
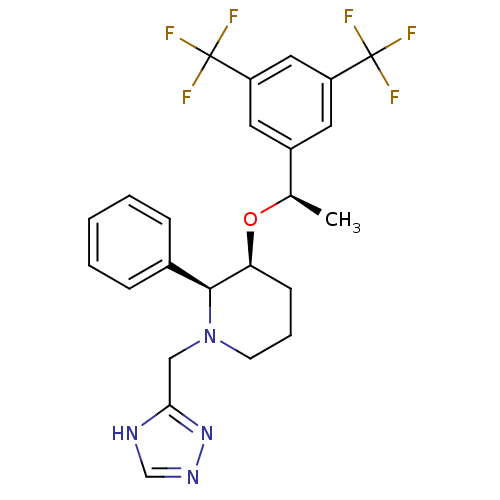
(3-[1-(3,5-Bis-trifluoromethyl-phenyl)-ethoxy]-2-ph...)Show SMILES C[C@@H](O[C@H]1CCCN(Cc2nnc[nH]2)[C@H]1c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H24F6N4O/c1-15(17-10-18(23(25,26)27)12-19(11-17)24(28,29)30)35-20-8-5-9-34(13-21-31-14-32-33-21)22(20)16-6-3-2-4-7-16/h2-4,6-7,10-12,14-15,20,22H,5,8-9,13H2,1H3,(H,31,32,33)/t15-,20+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonism of NK1 receptor in rat liver microsomes. |
Bioorg Med Chem Lett 7: 2959-2962 (1997)
Article DOI: 10.1016/S0960-894X(97)10118-4
BindingDB Entry DOI: 10.7270/Q2W66KRX |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50290859
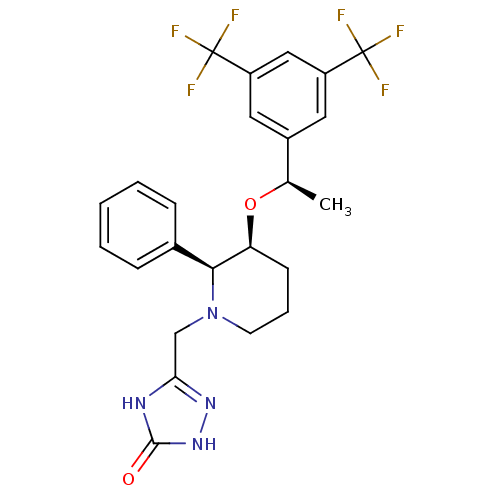
(5-{3-[1-(3,5-Bis-trifluoromethyl-phenyl)-ethoxy]-2...)Show SMILES C[C@@H](O[C@H]1CCCN(Cc2n[nH]c(=O)[nH]2)[C@H]1c1ccccc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H24F6N4O2/c1-14(16-10-17(23(25,26)27)12-18(11-16)24(28,29)30)36-19-8-5-9-34(13-20-31-22(35)33-32-20)21(19)15-6-3-2-4-7-15/h2-4,6-7,10-12,14,19,21H,5,8-9,13H2,1H3,(H2,31,32,33,35)/t14-,19+,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonism of NK1 receptor in rat liver microsomes. |
Bioorg Med Chem Lett 7: 2959-2962 (1997)
Article DOI: 10.1016/S0960-894X(97)10118-4
BindingDB Entry DOI: 10.7270/Q2W66KRX |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50191087

((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)pheny...)Show SMILES CCN(Cc1nnc[nH]1)[C@@H]1CC[C@H](O[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)[C@H]1c1ccc(F)cc1 Show InChI InChI=1S/C26H27F7N4O/c1-3-37(13-23-34-14-35-36-23)21-8-9-22(24(21)16-4-6-20(27)7-5-16)38-15(2)17-10-18(25(28,29)30)12-19(11-17)26(31,32)33/h4-7,10-12,14-15,21-22,24H,3,8-9,13H2,1-2H3,(H,34,35,36)/t15-,21-,22+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4504-11 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.044
BindingDB Entry DOI: 10.7270/Q20P0ZM5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50191092

((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)pheny...)Show SMILES C[C@@H](O[C@H]1CC[C@H]([C@@H]1c1ccc(F)cc1)N(C)Cc1nnc[nH]1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C25H25F7N4O/c1-14(16-9-17(24(27,28)29)11-18(10-16)25(30,31)32)37-21-8-7-20(36(2)12-22-33-13-34-35-22)23(21)15-3-5-19(26)6-4-15/h3-6,9-11,13-14,20-21,23H,7-8,12H2,1-2H3,(H,33,34,35)/t14-,20-,21+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4504-11 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.044
BindingDB Entry DOI: 10.7270/Q20P0ZM5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50191079

(2-(((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)p...)Show SMILES COCCN(CC(N)=O)[C@@H]1CC[C@H](O[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)[C@H]1c1ccc(F)cc1 Show InChI InChI=1S/C26H29F7N2O3/c1-15(17-11-18(25(28,29)30)13-19(12-17)26(31,32)33)38-22-8-7-21(35(9-10-37-2)14-23(34)36)24(22)16-3-5-20(27)6-4-16/h3-6,11-13,15,21-22,24H,7-10,14H2,1-2H3,(H2,34,36)/t15-,21-,22+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4504-11 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.044
BindingDB Entry DOI: 10.7270/Q20P0ZM5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030435

(CHEMBL420543 | N-[(S)-4-(3,5-Bis-trifluoromethyl-p...)Show SMILES FC(F)(F)c1cc(CCC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)CCCN2CCOCC2)cc(c1)C(F)(F)F Show InChI InChI=1S/C29H31F6N3O3/c30-28(31,32)21-14-19(15-22(17-21)29(33,34)35)7-8-26(39)25(16-20-18-36-24-5-2-1-4-23(20)24)37-27(40)6-3-9-38-10-12-41-13-11-38/h1-2,4-5,14-15,17-18,25,36H,3,6-13,16H2,(H,37,40)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against human Tachykinin receptor 1 expressed in CHO cells was measured by its ability to displace [125I]- Tyr-8 substance P. |
J Med Chem 38: 934-41 (1995)
BindingDB Entry DOI: 10.7270/Q2R78D8F |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Rattus norvegicus (rat)) | BDBM50052280

((2S,3S)-3-(3,5-Bis-trifluoromethyl-benzyloxy)-2-ph...)Show SMILES FC(F)(F)c1cc(CO[C@H]2CCCN(Cc3nnc[nH]3)[C@H]2c2ccccc2)cc(c1)C(F)(F)F Show InChI InChI=1S/C23H22F6N4O/c24-22(25,26)17-9-15(10-18(11-17)23(27,28)29)13-34-19-7-4-8-33(12-20-30-14-31-32-20)21(19)16-5-2-1-3-6-16/h1-3,5-6,9-11,14,19,21H,4,7-8,12-13H2,(H,30,31,32)/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Antagonism of NK1 receptor in rat liver microsomes. |
Bioorg Med Chem Lett 7: 2959-2962 (1997)
Article DOI: 10.1016/S0960-894X(97)10118-4
BindingDB Entry DOI: 10.7270/Q2W66KRX |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50106712

(4-(5-Azetidin-1-ylmethyl-1H-[1,2,3]triazol-4-ylmet...)Show SMILES C[C@@H](OC1OCCN(Cc2[nH]nnc2CN2CCC2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H28F7N5O2/c1-16(18-11-19(26(29,30)31)13-20(12-18)27(32,33)34)41-25-24(17-3-5-21(28)6-4-17)39(9-10-40-25)15-23-22(35-37-36-23)14-38-7-2-8-38/h3-6,11-13,16,24-25H,2,7-10,14-15H2,1H3,(H,35,36,37)/t16-,24+,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
In vitro inhibition of binding of [125I]-substance P to tachykinin receptor 1 |
J Med Chem 44: 4296-9 (2001)
BindingDB Entry DOI: 10.7270/Q2PN96BR |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50191107

(2-(((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)p...)Show SMILES CNC(=O)CN(C)[C@@H]1CC[C@H](O[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)[C@H]1c1ccc(F)cc1 Show InChI InChI=1S/C25H27F7N2O2/c1-14(16-10-17(24(27,28)29)12-18(11-16)25(30,31)32)36-21-9-8-20(34(3)13-22(35)33-2)23(21)15-4-6-19(26)7-5-15/h4-7,10-12,14,20-21,23H,8-9,13H2,1-3H3,(H,33,35)/t14-,20-,21+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4504-11 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.044
BindingDB Entry DOI: 10.7270/Q20P0ZM5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50191095

(2-(((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)p...)Show SMILES C[C@@H](O[C@H]1CC[C@H]([C@@H]1c1ccc(F)cc1)N(C)CC(N)=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H25F7N2O2/c1-13(15-9-16(23(26,27)28)11-17(10-15)24(29,30)31)35-20-8-7-19(33(2)12-21(32)34)22(20)14-3-5-18(25)6-4-14/h3-6,9-11,13,19-20,22H,7-8,12H2,1-2H3,(H2,32,34)/t13-,19-,20+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4504-11 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.044
BindingDB Entry DOI: 10.7270/Q20P0ZM5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50106717

(CHEMBL553778 | {5-[2-[1-(3,5-Bis-trifluoromethyl-p...)Show SMILES C[C@@H](OC1OCCN(Cc2[nH]nnc2CN(C)C)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C26H28F7N5O2/c1-15(17-10-18(25(28,29)30)12-19(11-17)26(31,32)33)40-24-23(16-4-6-20(27)7-5-16)38(8-9-39-24)14-22-21(13-37(2)3)34-36-35-22/h4-7,10-12,15,23-24H,8-9,13-14H2,1-3H3,(H,34,35,36)/t15-,23+,24?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
In vitro inhibition of binding of [125I]-substance P to tachykinin receptor 1 |
J Med Chem 44: 4296-9 (2001)
BindingDB Entry DOI: 10.7270/Q2PN96BR |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50191081
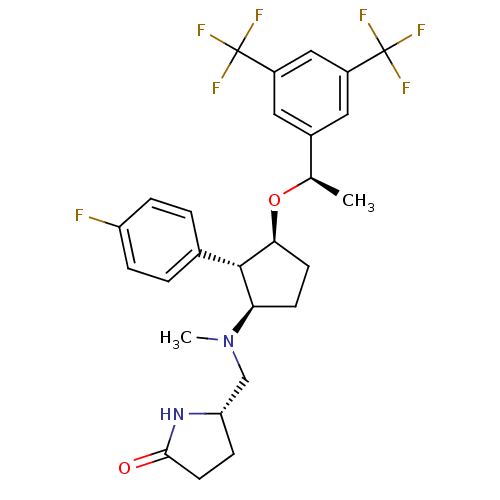
((S)-5-((((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@H]([C@@H]1c1ccc(F)cc1)N(C)C[C@@H]1CCC(=O)N1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H29F7N2O2/c1-15(17-11-18(26(29,30)31)13-19(12-17)27(32,33)34)38-23-9-8-22(25(23)16-3-5-20(28)6-4-16)36(2)14-21-7-10-24(37)35-21/h3-6,11-13,15,21-23,25H,7-10,14H2,1-2H3,(H,35,37)/t15-,21+,22-,23+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4504-11 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.044
BindingDB Entry DOI: 10.7270/Q20P0ZM5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50000040

(((2S,3S)-2-Benzhydryl-1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1ccccc1CN[C@H]1C2CCN(CC2)[C@H]1C(c1ccccc1)c1ccccc1 |wD:10.10,17.20,(11.79,-2.71,;10.31,-3.13,;9.22,-2.04,;10.55,-1.27,;10.57,.27,;9.22,1.04,;7.89,.27,;7.89,-1.26,;6.56,-2.01,;6.56,-3.55,;5.21,-4.32,;3.92,-3.55,;3.16,-4.88,;4.65,-5.3,;3.88,-6.63,;2.57,-5.86,;2.57,-4.32,;5.21,-5.86,;6.56,-6.63,;6.54,-8.17,;5.21,-8.94,;5.21,-10.48,;6.54,-11.25,;7.89,-10.48,;7.87,-8.94,;7.89,-5.86,;9.22,-6.63,;10.55,-5.88,;10.55,-4.34,;9.22,-3.55,;7.89,-4.34,)| Show InChI InChI=1S/C28H32N2O/c1-31-25-15-9-8-14-24(25)20-29-27-23-16-18-30(19-17-23)28(27)26(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-15,23,26-29H,16-20H2,1H3/t27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Tested against cloned human NK1 receptor by displacement of 125 I -labeled substance P expressed in CHO cells |
J Med Chem 36: 2044-5 (1993)
BindingDB Entry DOI: 10.7270/Q2959GMD |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50409466

(CHEMBL2112743)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2[nH]nnc2CN2CCCC2)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C28H30F7N5O2/c1-17(19-12-20(27(30,31)32)14-21(13-19)28(33,34)35)42-26-25(18-4-6-22(29)7-5-18)40(10-11-41-26)16-24-23(36-38-37-24)15-39-8-2-3-9-39/h4-7,12-14,17,25-26H,2-3,8-11,15-16H2,1H3,(H,36,37,38)/t17-,25+,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
In vitro inhibition of binding of [125I]-substance P to tachykinin receptor 1 |
J Med Chem 44: 4296-9 (2001)
BindingDB Entry DOI: 10.7270/Q2PN96BR |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50052280

((2S,3S)-3-(3,5-Bis-trifluoromethyl-benzyloxy)-2-ph...)Show SMILES FC(F)(F)c1cc(CO[C@H]2CCCN(Cc3nnc[nH]3)[C@H]2c2ccccc2)cc(c1)C(F)(F)F Show InChI InChI=1S/C23H22F6N4O/c24-22(25,26)17-9-15(10-18(11-17)23(27,28)29)13-34-19-7-4-8-33(12-20-30-14-31-32-20)21(19)16-5-2-1-3-6-16/h1-3,5-6,9-11,14,19,21H,4,7-8,12-13H2,(H,30,31,32)/t19-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125 I]-Tyr8 SP from the cloned human Tachykinin receptor 1 expressed in CHO cells |
J Med Chem 39: 2907-14 (1996)
Article DOI: 10.1021/jm9506534
BindingDB Entry DOI: 10.7270/Q2D21WP4 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50191082
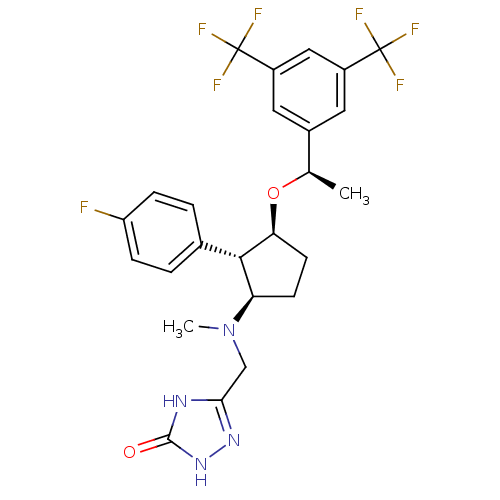
(5-((((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)...)Show SMILES C[C@@H](O[C@H]1CC[C@H]([C@@H]1c1ccc(F)cc1)N(C)Cc1n[nH]c(=O)[nH]1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C25H25F7N4O2/c1-13(15-9-16(24(27,28)29)11-17(10-15)25(30,31)32)38-20-8-7-19(22(20)14-3-5-18(26)6-4-14)36(2)12-21-33-23(37)35-34-21/h3-6,9-11,13,19-20,22H,7-8,12H2,1-2H3,(H2,33,34,35,37)/t13-,19-,20+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4504-11 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.044
BindingDB Entry DOI: 10.7270/Q20P0ZM5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50067961
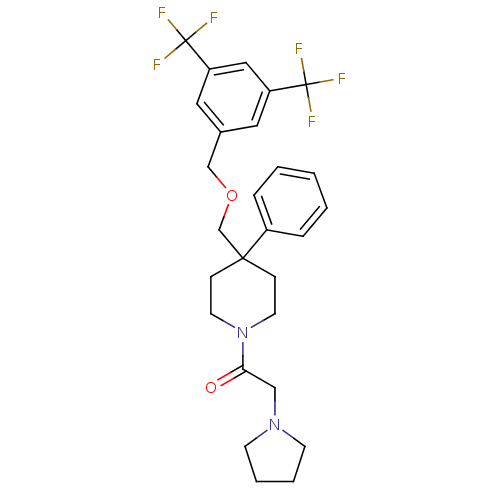
(1-[4-(3,5-Bis-trifluoromethyl-benzyloxymethyl)-4-p...)Show SMILES FC(F)(F)c1cc(COCC2(CCN(CC2)C(=O)CN2CCCC2)c2ccccc2)cc(c1)C(F)(F)F Show InChI InChI=1S/C27H30F6N2O2/c28-26(29,30)22-14-20(15-23(16-22)27(31,32)33)18-37-19-25(21-6-2-1-3-7-21)8-12-35(13-9-25)24(36)17-34-10-4-5-11-34/h1-3,6-7,14-16H,4-5,8-13,17-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp and Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity at human tachykinin receptor 1 by measuring its ability to displace [125I]-labeled substance P from the cloned receptor expressed in... |
J Med Chem 41: 4623-35 (1998)
Article DOI: 10.1021/jm980376b
BindingDB Entry DOI: 10.7270/Q2T43S77 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50191099
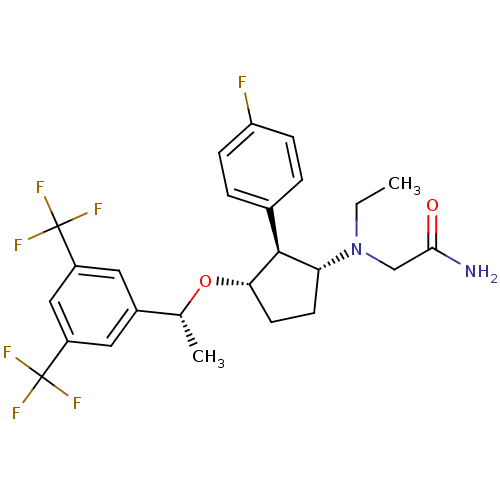
(2-(((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)p...)Show SMILES CCN(CC(N)=O)[C@@H]1CC[C@H](O[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)[C@H]1c1ccc(F)cc1 Show InChI InChI=1S/C25H27F7N2O2/c1-3-34(13-22(33)35)20-8-9-21(23(20)15-4-6-19(26)7-5-15)36-14(2)16-10-17(24(27,28)29)12-18(11-16)25(30,31)32/h4-7,10-12,14,20-21,23H,3,8-9,13H2,1-2H3,(H2,33,35)/t14-,20-,21+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4504-11 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.044
BindingDB Entry DOI: 10.7270/Q20P0ZM5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50030435

(CHEMBL420543 | N-[(S)-4-(3,5-Bis-trifluoromethyl-p...)Show SMILES FC(F)(F)c1cc(CCC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)CCCN2CCOCC2)cc(c1)C(F)(F)F Show InChI InChI=1S/C29H31F6N3O3/c30-28(31,32)21-14-19(15-22(17-21)29(33,34)35)7-8-26(39)25(16-20-18-36-24-5-2-1-4-23(20)24)37-27(40)6-3-9-38-10-12-41-13-11-38/h1-2,4-5,14-15,17-18,25,36H,3,6-13,16H2,(H,37,40)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck, Sharp & Dohme Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity against wild type human Tachykinin receptor 1 expressed in CHO cells was measured by its ability to displace [125I]- Tyr-8 substance... |
J Med Chem 38: 934-41 (1995)
BindingDB Entry DOI: 10.7270/Q2R78D8F |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50409465
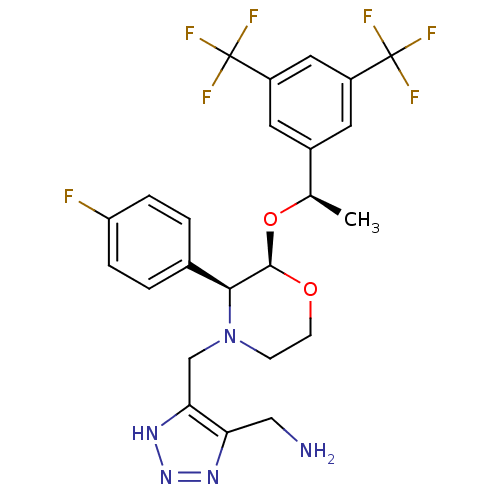
(CHEMBL2112741)Show SMILES C[C@@H](O[C@H]1OCCN(Cc2[nH]nnc2CN)[C@H]1c1ccc(F)cc1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H24F7N5O2/c1-13(15-8-16(23(26,27)28)10-17(9-15)24(29,30)31)38-22-21(14-2-4-18(25)5-3-14)36(6-7-37-22)12-20-19(11-32)33-35-34-20/h2-5,8-10,13,21-22H,6-7,11-12,32H2,1H3,(H,33,34,35)/t13-,21+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
In vitro inhibition of binding of [125I]-substance P to tachykinin receptor 1 |
J Med Chem 44: 4296-9 (2001)
BindingDB Entry DOI: 10.7270/Q2PN96BR |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50191085
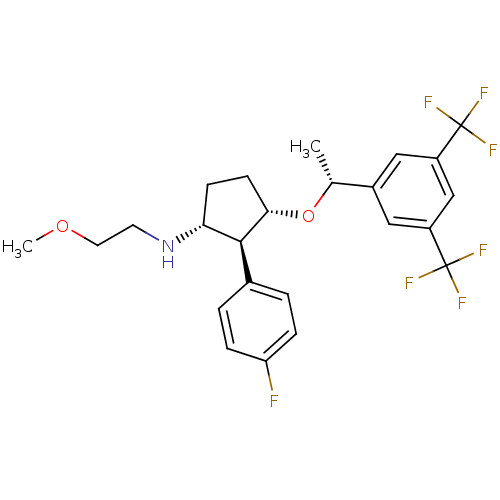
((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromethyl)pheny...)Show SMILES COCCN[C@@H]1CC[C@H](O[C@H](C)c2cc(cc(c2)C(F)(F)F)C(F)(F)F)[C@H]1c1ccc(F)cc1 Show InChI InChI=1S/C24H26F7NO2/c1-14(16-11-17(23(26,27)28)13-18(12-16)24(29,30)31)34-21-8-7-20(32-9-10-33-2)22(21)15-3-5-19(25)6-4-15/h3-6,11-14,20-22,32H,7-10H2,1-2H3/t14-,20-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4504-11 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.044
BindingDB Entry DOI: 10.7270/Q20P0ZM5 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50191106

((R)-5-((((1R,2S,3S)-3-((R)-1-(3,5-bis(trifluoromet...)Show SMILES C[C@@H](O[C@H]1CC[C@H]([C@@H]1c1ccc(F)cc1)N(C)C[C@H]1CCC(=O)N1)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H29F7N2O2/c1-15(17-11-18(26(29,30)31)13-19(12-17)27(32,33)34)38-23-9-8-22(25(23)16-3-5-20(28)6-4-16)36(2)14-21-7-10-24(37)35-21/h3-6,11-13,15,21-23,25H,7-10,14H2,1-2H3,(H,35,37)/t15-,21-,22-,23+,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]SP from human cloned NK1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 16: 4504-11 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.044
BindingDB Entry DOI: 10.7270/Q20P0ZM5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data