 Found 73 hits with Last Name = 'sides' and Initial = 'k'
Found 73 hits with Last Name = 'sides' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156461
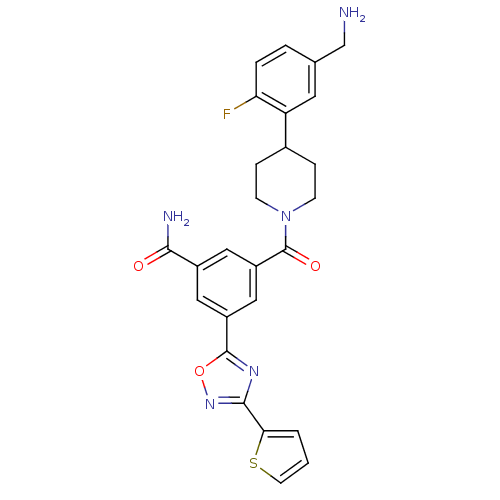
(3-[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidine-1-...)Show SMILES NCc1ccc(F)c(c1)C1CCN(CC1)C(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(N)=O Show InChI InChI=1S/C26H24FN5O3S/c27-21-4-3-15(14-28)10-20(21)16-5-7-32(8-6-16)26(34)19-12-17(23(29)33)11-18(13-19)25-30-24(31-35-25)22-2-1-9-36-22/h1-4,9-13,16H,5-8,14,28H2,(H2,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156460
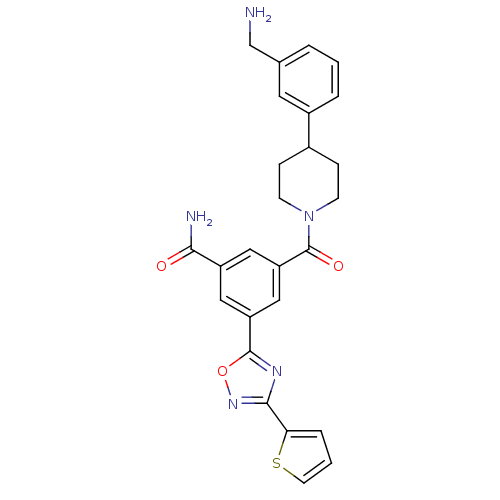
(3-[4-(3-Aminomethyl-phenyl)-piperidine-1-carbonyl]...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(N)=O Show InChI InChI=1S/C26H25N5O3S/c27-15-16-3-1-4-18(11-16)17-6-8-31(9-7-17)26(33)21-13-19(23(28)32)12-20(14-21)25-29-24(30-34-25)22-5-2-10-35-22/h1-5,10-14,17H,6-9,15,27H2,(H2,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156457
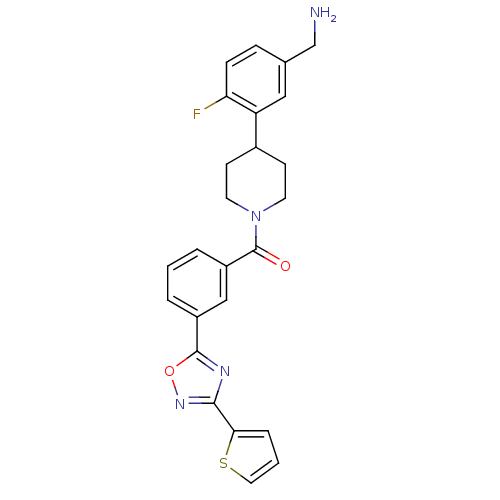
(CHEMBL186730 | [4-(5-Aminomethyl-2-fluoro-phenyl)-...)Show SMILES NCc1ccc(F)c(c1)C1CCN(CC1)C(=O)c1cccc(c1)-c1nc(no1)-c1cccs1 Show InChI InChI=1S/C25H23FN4O2S/c26-21-7-6-16(15-27)13-20(21)17-8-10-30(11-9-17)25(31)19-4-1-3-18(14-19)24-28-23(29-32-24)22-5-2-12-33-22/h1-7,12-14,17H,8-11,15,27H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167517
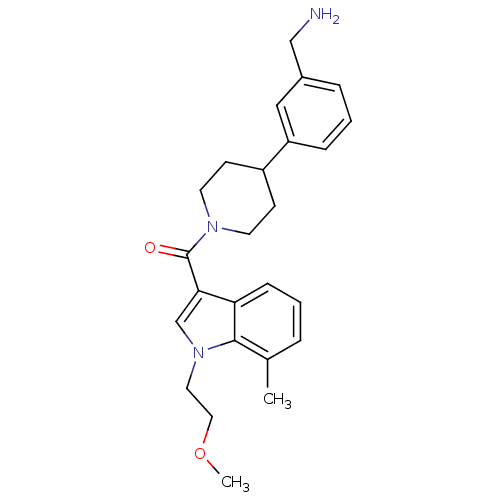
(CHEMBL191546 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES COCCn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2cccc(C)c12 Show InChI InChI=1S/C25H31N3O2/c1-18-5-3-8-22-23(17-28(24(18)22)13-14-30-2)25(29)27-11-9-20(10-12-27)21-7-4-6-19(15-21)16-26/h3-8,15,17,20H,9-14,16,26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167534
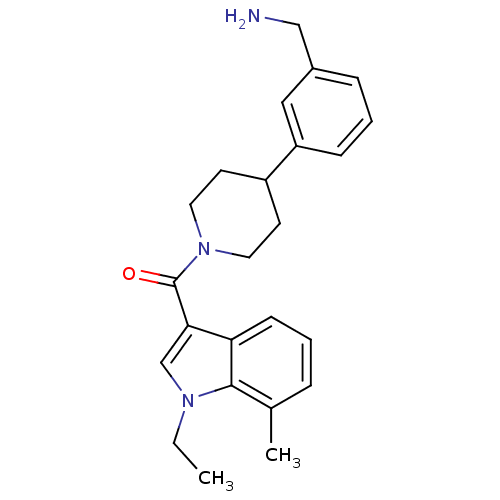
(CHEMBL370284 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES CCn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2cccc(C)c12 Show InChI InChI=1S/C24H29N3O/c1-3-26-16-22(21-9-4-6-17(2)23(21)26)24(28)27-12-10-19(11-13-27)20-8-5-7-18(14-20)15-25/h4-9,14,16,19H,3,10-13,15,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167531
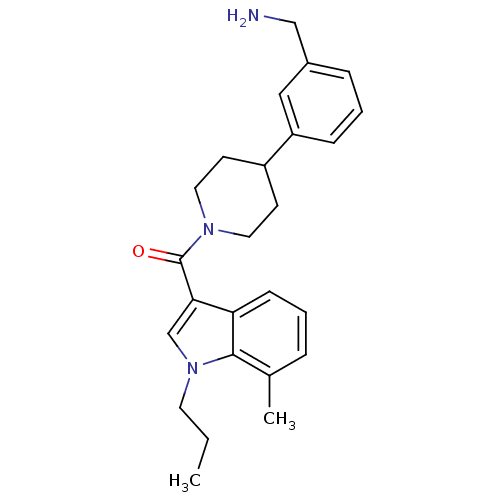
(CHEMBL365527 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES CCCn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2cccc(C)c12 Show InChI InChI=1S/C25H31N3O/c1-3-12-28-17-23(22-9-4-6-18(2)24(22)28)25(29)27-13-10-20(11-14-27)21-8-5-7-19(15-21)16-26/h4-9,15,17,20H,3,10-14,16,26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167524
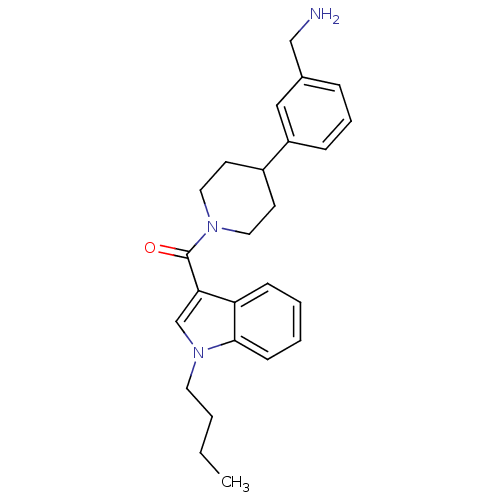
(CHEMBL192963 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES CCCCn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2ccccc12 Show InChI InChI=1S/C25H31N3O/c1-2-3-13-28-18-23(22-9-4-5-10-24(22)28)25(29)27-14-11-20(12-15-27)21-8-6-7-19(16-21)17-26/h4-10,16,18,20H,2-3,11-15,17,26H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167525
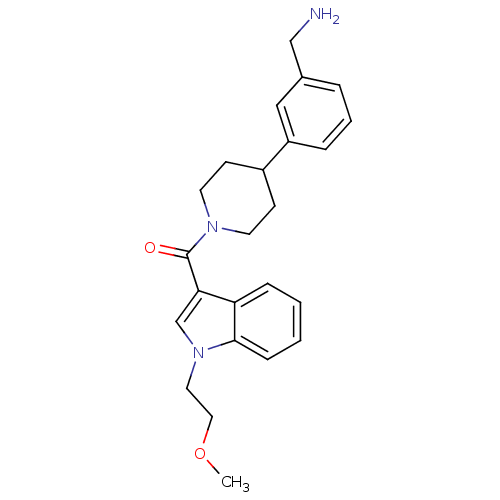
(CHEMBL193514 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES COCCn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2ccccc12 Show InChI InChI=1S/C24H29N3O2/c1-29-14-13-27-17-22(21-7-2-3-8-23(21)27)24(28)26-11-9-19(10-12-26)20-6-4-5-18(15-20)16-25/h2-8,15,17,19H,9-14,16,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156459
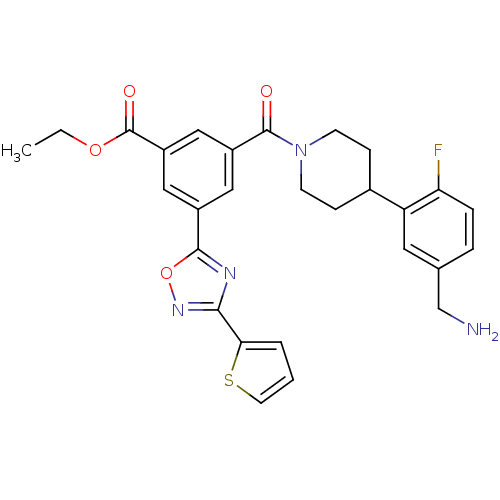
(3-[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidine-1-...)Show SMILES CCOC(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(=O)N1CCC(CC1)c1cc(CN)ccc1F Show InChI InChI=1S/C28H27FN4O4S/c1-2-36-28(35)21-14-19(26-31-25(32-37-26)24-4-3-11-38-24)13-20(15-21)27(34)33-9-7-18(8-10-33)22-12-17(16-30)5-6-23(22)29/h3-6,11-15,18H,2,7-10,16,30H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167530
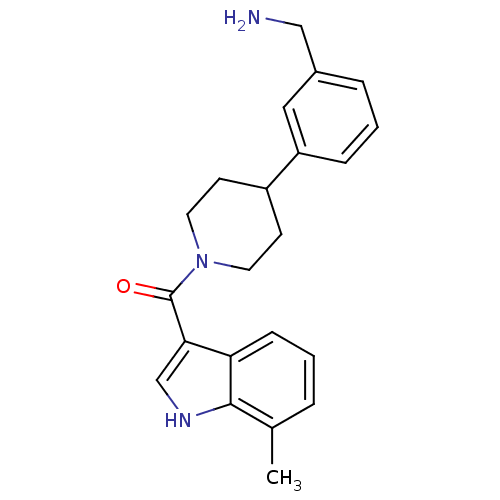
(CHEMBL189101 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES Cc1cccc2c(c[nH]c12)C(=O)N1CCC(CC1)c1cccc(CN)c1 Show InChI InChI=1S/C22H25N3O/c1-15-4-2-7-19-20(14-24-21(15)19)22(26)25-10-8-17(9-11-25)18-6-3-5-16(12-18)13-23/h2-7,12,14,17,24H,8-11,13,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167514
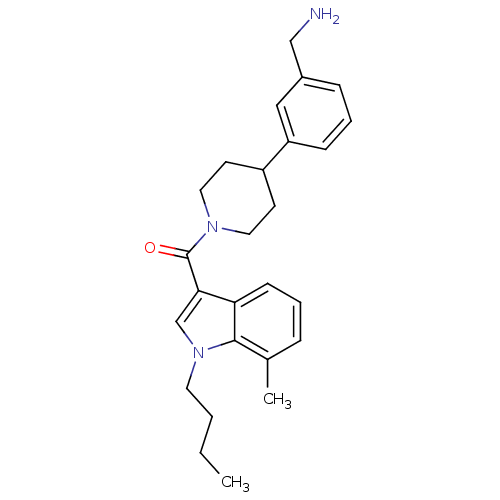
(CHEMBL370463 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES CCCCn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2cccc(C)c12 Show InChI InChI=1S/C26H33N3O/c1-3-4-13-29-18-24(23-10-5-7-19(2)25(23)29)26(30)28-14-11-21(12-15-28)22-9-6-8-20(16-22)17-27/h5-10,16,18,21H,3-4,11-15,17,27H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity of compound towards Factor Xa |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50156458
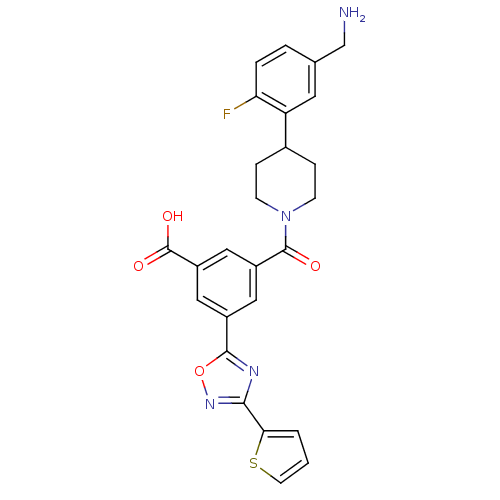
(3-[4-(5-Aminomethyl-2-fluoro-phenyl)-piperidine-1-...)Show SMILES NCc1ccc(F)c(c1)C1CCN(CC1)C(=O)c1cc(cc(c1)-c1nc(no1)-c1cccs1)C(O)=O Show InChI InChI=1S/C26H23FN4O4S/c27-21-4-3-15(14-28)10-20(21)16-5-7-31(8-6-16)25(32)18-11-17(12-19(13-18)26(33)34)24-29-23(30-35-24)22-2-1-9-36-22/h1-4,9-13,16H,5-8,14,28H2,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human mast cell tryptase beta |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50152197
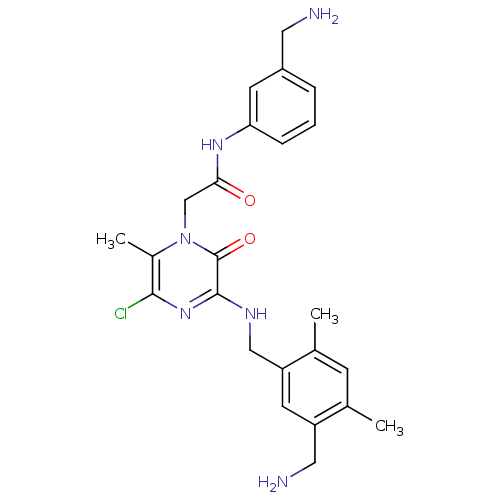
(2-[3-(5-Aminomethyl-2,4-dimethyl-benzylamino)-5-ch...)Show SMILES Cc1cc(C)c(CNc2nc(Cl)c(C)n(CC(=O)Nc3cccc(CN)c3)c2=O)cc1CN Show InChI InChI=1S/C24H29ClN6O2/c1-14-7-15(2)19(9-18(14)11-27)12-28-23-24(33)31(16(3)22(25)30-23)13-21(32)29-20-6-4-5-17(8-20)10-26/h4-9H,10-13,26-27H2,1-3H3,(H,28,30)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167520
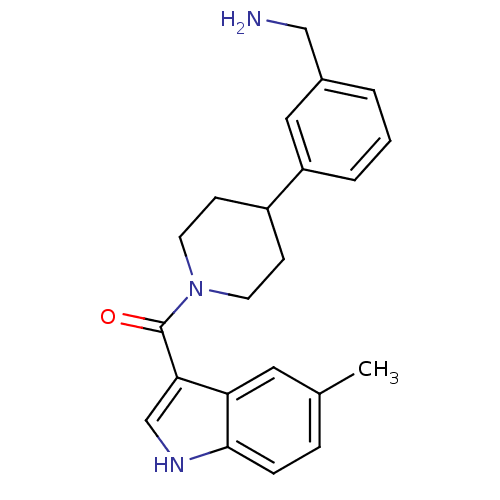
(CHEMBL365148 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES Cc1ccc2[nH]cc(C(=O)N3CCC(CC3)c3cccc(CN)c3)c2c1 Show InChI InChI=1S/C22H25N3O/c1-15-5-6-21-19(11-15)20(14-24-21)22(26)25-9-7-17(8-10-25)18-4-2-3-16(12-18)13-23/h2-6,11-12,14,17,24H,7-10,13,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167526
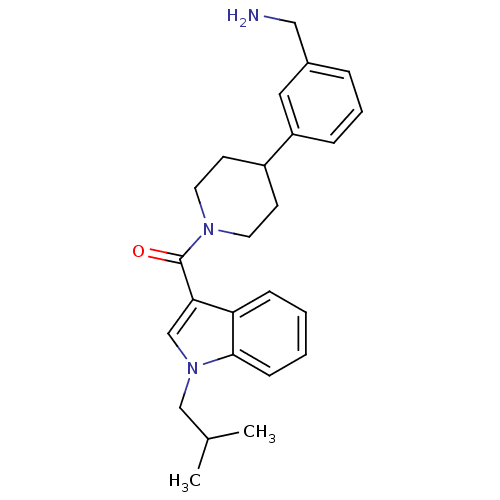
(CHEMBL365189 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES CC(C)Cn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2ccccc12 Show InChI InChI=1S/C25H31N3O/c1-18(2)16-28-17-23(22-8-3-4-9-24(22)28)25(29)27-12-10-20(11-13-27)21-7-5-6-19(14-21)15-26/h3-9,14,17-18,20H,10-13,15-16,26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167522
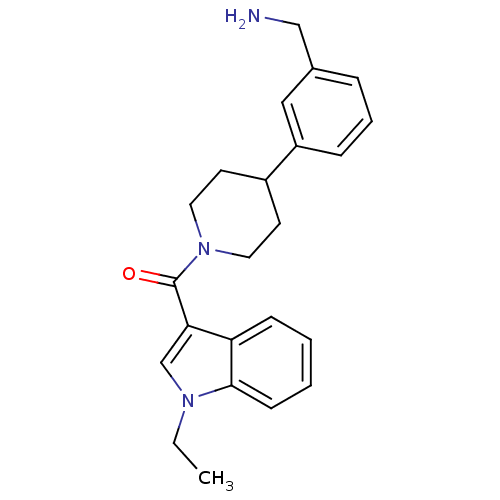
(CHEMBL372028 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES CCn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2ccccc12 Show InChI InChI=1S/C23H27N3O/c1-2-25-16-21(20-8-3-4-9-22(20)25)23(27)26-12-10-18(11-13-26)19-7-5-6-17(14-19)15-24/h3-9,14,16,18H,2,10-13,15,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167521
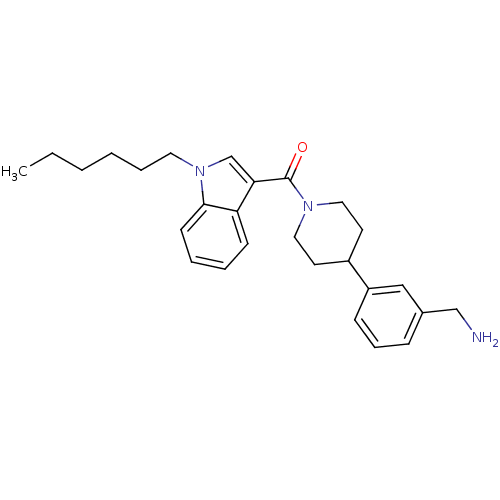
(CHEMBL364661 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES CCCCCCn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2ccccc12 Show InChI InChI=1S/C27H35N3O/c1-2-3-4-7-15-30-20-25(24-11-5-6-12-26(24)30)27(31)29-16-13-22(14-17-29)23-10-8-9-21(18-23)19-28/h5-6,8-12,18,20,22H,2-4,7,13-17,19,28H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167511
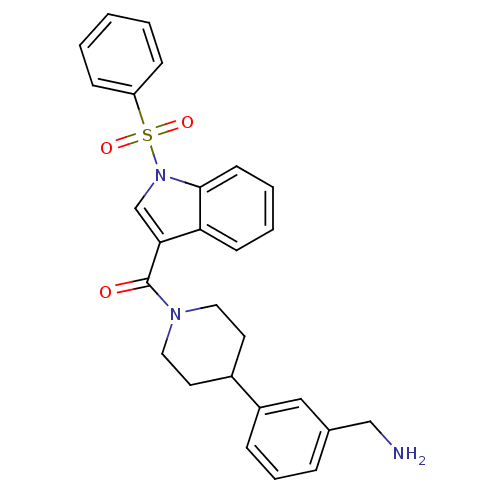
(CHEMBL189531 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1cn(c2ccccc12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C27H27N3O3S/c28-18-20-7-6-8-22(17-20)21-13-15-29(16-14-21)27(31)25-19-30(26-12-5-4-11-24(25)26)34(32,33)23-9-2-1-3-10-23/h1-12,17,19,21H,13-16,18,28H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50152198
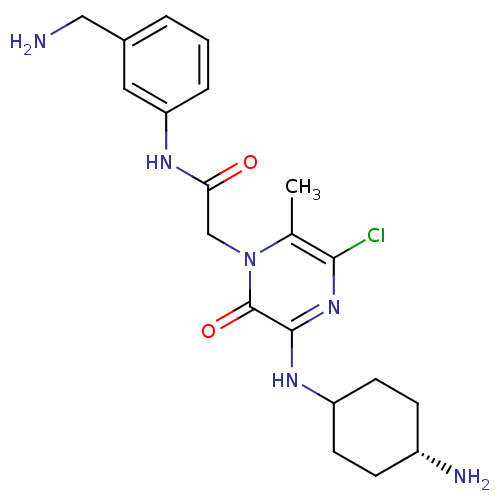
(2-[3-(4-Amino-cyclohexylamino)-5-chloro-6-methyl-2...)Show SMILES Cc1c(Cl)nc(NC2CC[C@H](N)CC2)c(=O)n1CC(=O)Nc1cccc(CN)c1 |wU:10.10,(.97,.46,;-.37,-.31,;-1.7,.46,;-1.72,2,;-3.03,-.31,;-3.03,-1.86,;-4.39,-2.63,;-5.72,-1.86,;-5.7,-.31,;-7.03,.46,;-8.36,-.31,;-9.72,.46,;-8.39,-1.86,;-7.05,-2.63,;-1.7,-2.63,;-1.7,-4.17,;-.37,-1.86,;.97,-2.63,;2.3,-1.86,;2.3,-.31,;3.66,-2.63,;4.99,-1.86,;4.96,-.31,;6.3,.46,;7.65,-.31,;7.65,-1.86,;8.99,-2.63,;10.32,-1.86,;6.32,-2.63,)| Show InChI InChI=1S/C20H27ClN6O2/c1-12-18(21)26-19(25-15-7-5-14(23)6-8-15)20(29)27(12)11-17(28)24-16-4-2-3-13(9-16)10-22/h2-4,9,14-15H,5-8,10-11,22-23H2,1H3,(H,24,28)(H,25,26)/t14-,15? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM16127
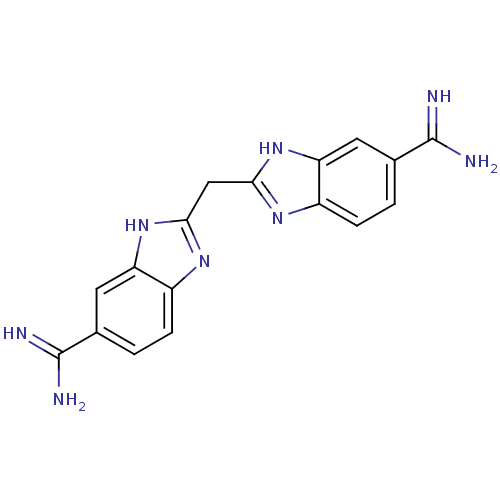
(2,2 -methanediylbis(1H-benzimidazole-6-carboximida...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4ccc(cc4[nH]3)C(N)=N)[nH]c2c1 Show InChI InChI=1S/C17H16N8/c18-16(19)8-1-3-10-12(5-8)24-14(22-10)7-15-23-11-4-2-9(17(20)21)6-13(11)25-15/h1-6H,7H2,(H3,18,19)(H3,20,21)(H,22,24)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM16127

(2,2 -methanediylbis(1H-benzimidazole-6-carboximida...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4ccc(cc4[nH]3)C(N)=N)[nH]c2c1 Show InChI InChI=1S/C17H16N8/c18-16(19)8-1-3-10-12(5-8)24-14(22-10)7-15-23-11-4-2-9(17(20)21)6-13(11)25-15/h1-6H,7H2,(H3,18,19)(H3,20,21)(H,22,24)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167523
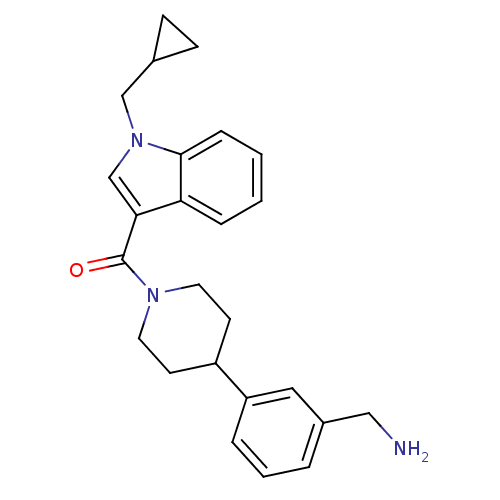
(CHEMBL190103 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1cn(CC2CC2)c2ccccc12 Show InChI InChI=1S/C25H29N3O/c26-15-19-4-3-5-21(14-19)20-10-12-27(13-11-20)25(29)23-17-28(16-18-8-9-18)24-7-2-1-6-22(23)24/h1-7,14,17-18,20H,8-13,15-16,26H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167535

(CHEMBL191007 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES Cn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2ccccc12 Show InChI InChI=1S/C22H25N3O/c1-24-15-20(19-7-2-3-8-21(19)24)22(26)25-11-9-17(10-12-25)18-6-4-5-16(13-18)14-23/h2-8,13,15,17H,9-12,14,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 155 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167533
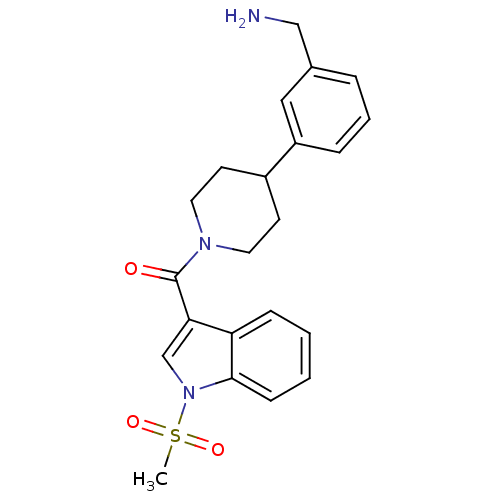
(CHEMBL435694 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES CS(=O)(=O)n1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2ccccc12 Show InChI InChI=1S/C22H25N3O3S/c1-29(27,28)25-15-20(19-7-2-3-8-21(19)25)22(26)24-11-9-17(10-12-24)18-6-4-5-16(13-18)14-23/h2-8,13,15,17H,9-12,14,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167510
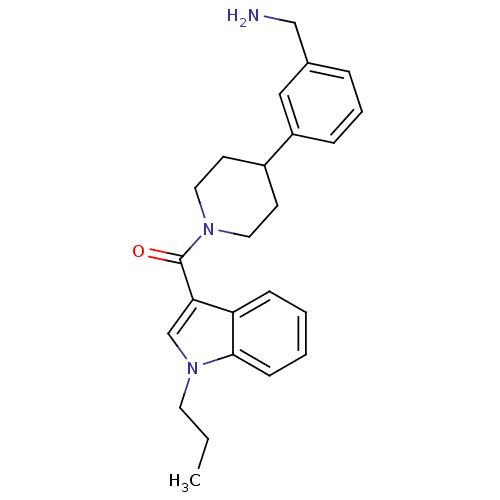
(CHEMBL361040 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES CCCn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2ccccc12 Show InChI InChI=1S/C24H29N3O/c1-2-12-27-17-22(21-8-3-4-9-23(21)27)24(28)26-13-10-19(11-14-26)20-7-5-6-18(15-20)16-25/h3-9,15,17,19H,2,10-14,16,25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 185 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167529
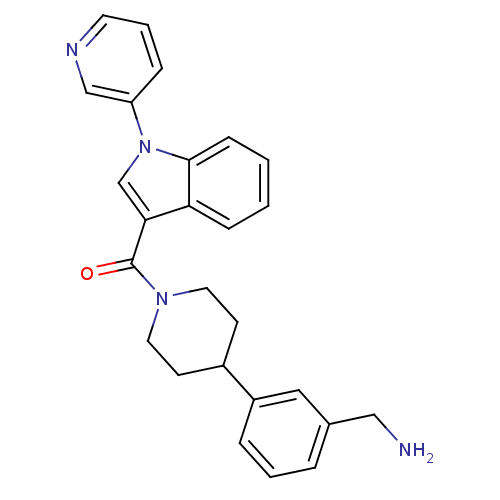
(CHEMBL372912 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1cn(-c2cccnc2)c2ccccc12 Show InChI InChI=1S/C26H26N4O/c27-16-19-5-3-6-21(15-19)20-10-13-29(14-11-20)26(31)24-18-30(22-7-4-12-28-17-22)25-9-2-1-8-23(24)25/h1-9,12,15,17-18,20H,10-11,13-14,16,27H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 197 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167515
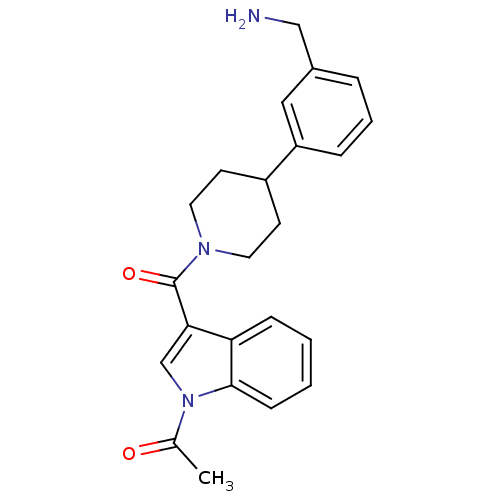
(1-{3-[4-(3-Aminomethyl-phenyl)-piperidine-1-carbon...)Show SMILES CC(=O)n1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2ccccc12 Show InChI InChI=1S/C23H25N3O2/c1-16(27)26-15-21(20-7-2-3-8-22(20)26)23(28)25-11-9-18(10-12-25)19-6-4-5-17(13-19)14-24/h2-8,13,15,18H,9-12,14,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50152202
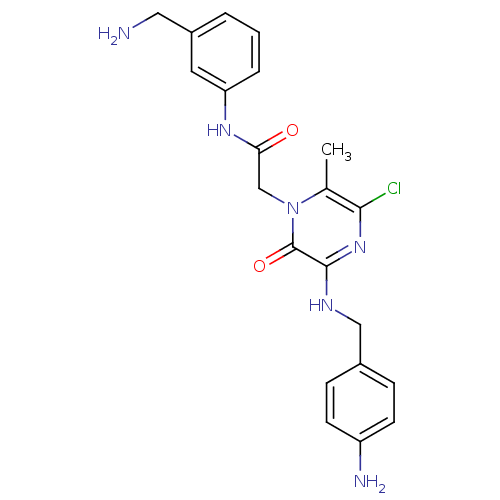
(2-[3-(4-Amino-benzylamino)-5-chloro-6-methyl-2-oxo...)Show SMILES Cc1c(Cl)nc(NCc2ccc(N)cc2)c(=O)n1CC(=O)Nc1cccc(CN)c1 Show InChI InChI=1S/C21H23ClN6O2/c1-13-19(22)27-20(25-11-14-5-7-16(24)8-6-14)21(30)28(13)12-18(29)26-17-4-2-3-15(9-17)10-23/h2-9H,10-12,23-24H2,1H3,(H,25,27)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 245 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167513

(CHEMBL192214 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1cn(C(=O)c2cccs2)c2ccccc12 Show InChI InChI=1S/C26H25N3O2S/c27-16-18-5-3-6-20(15-18)19-10-12-28(13-11-19)25(30)22-17-29(23-8-2-1-7-21(22)23)26(31)24-9-4-14-32-24/h1-9,14-15,17,19H,10-13,16,27H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50152200
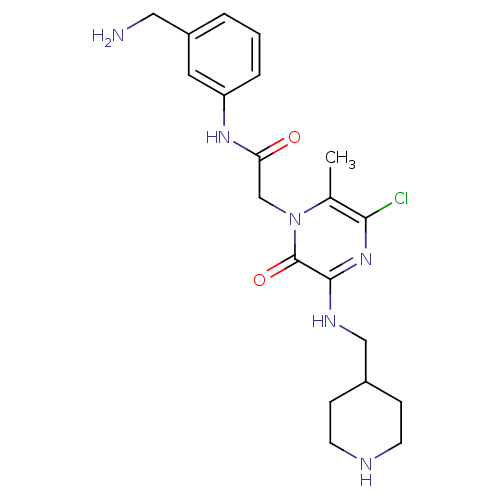
(CHEMBL183528 | N-(3-Aminomethyl-phenyl)-2-{5-chlor...)Show SMILES Cc1c(Cl)nc(NCC2CCNCC2)c(=O)n1CC(=O)Nc1cccc(CN)c1 Show InChI InChI=1S/C20H27ClN6O2/c1-13-18(21)26-19(24-11-14-5-7-23-8-6-14)20(29)27(13)12-17(28)25-16-4-2-3-15(9-16)10-22/h2-4,9,14,23H,5-8,10-12,22H2,1H3,(H,24,26)(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167528
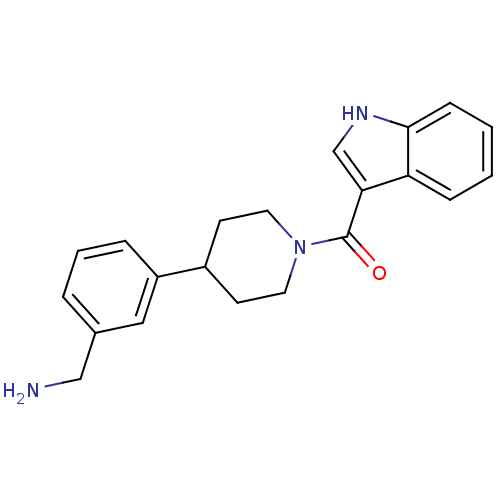
(CHEMBL436537 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1c[nH]c2ccccc12 Show InChI InChI=1S/C21H23N3O/c22-13-15-4-3-5-17(12-15)16-8-10-24(11-9-16)21(25)19-14-23-20-7-2-1-6-18(19)20/h1-7,12,14,16,23H,8-11,13,22H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167519

(CHEMBL190135 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES CC(C)n1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2ccccc12 Show InChI InChI=1S/C24H29N3O/c1-17(2)27-16-22(21-8-3-4-9-23(21)27)24(28)26-12-10-19(11-13-26)20-7-5-6-18(14-20)15-25/h3-9,14,16-17,19H,10-13,15,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167527
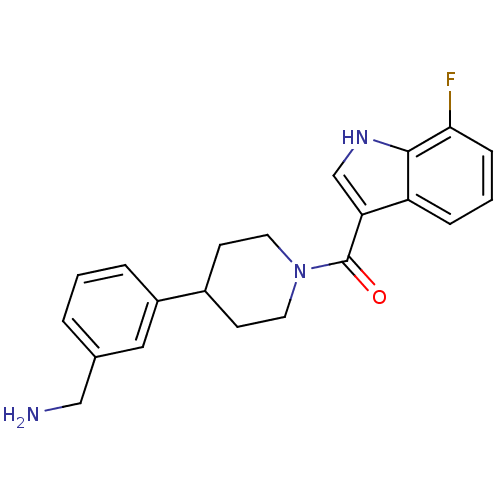
(CHEMBL189983 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1c[nH]c2c(F)cccc12 Show InChI InChI=1S/C21H22FN3O/c22-19-6-2-5-17-18(13-24-20(17)19)21(26)25-9-7-15(8-10-25)16-4-1-3-14(11-16)12-23/h1-6,11,13,15,24H,7-10,12,23H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 368 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167518
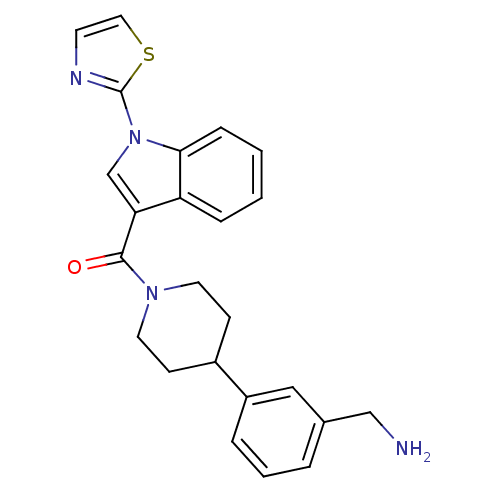
(CHEMBL189372 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1cn(-c2nccs2)c2ccccc12 Show InChI InChI=1S/C24H24N4OS/c25-15-17-4-3-5-19(14-17)18-8-11-27(12-9-18)23(29)21-16-28(24-26-10-13-30-24)22-7-2-1-6-20(21)22/h1-7,10,13-14,16,18H,8-9,11-12,15,25H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 373 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
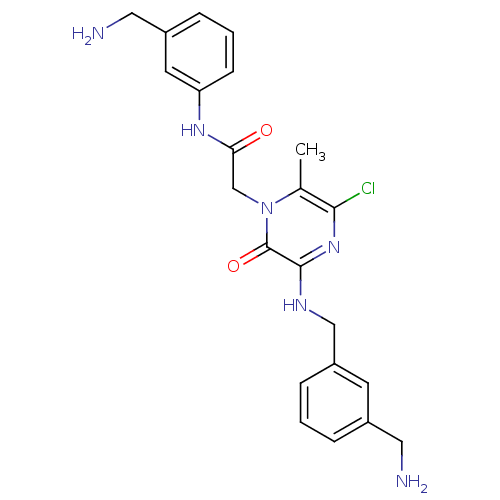
(Homo sapiens (Human)) | BDBM50152205
(2-[3-(3-Aminomethyl-benzylamino)-5-chloro-6-methyl...)Show SMILES Cc1c(Cl)nc(NCc2cccc(CN)c2)c(=O)n1CC(=O)Nc1cccc(CN)c1 Show InChI InChI=1S/C22H25ClN6O2/c1-14-20(23)28-21(26-12-17-6-2-4-15(8-17)10-24)22(31)29(14)13-19(30)27-18-7-3-5-16(9-18)11-25/h2-9H,10-13,24-25H2,1H3,(H,26,28)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50152206
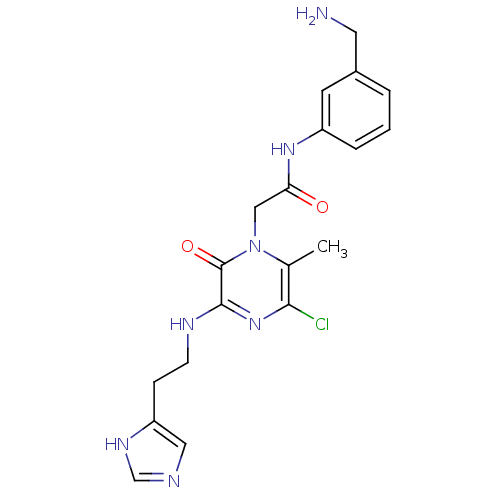
(CHEMBL187587 | N-(3-Aminomethyl-phenyl)-2-{5-chlor...)Show SMILES Cc1c(Cl)nc(NCCc2cnc[nH]2)c(=O)n1CC(=O)Nc1cccc(CN)c1 Show InChI InChI=1S/C19H22ClN7O2/c1-12-17(20)26-18(23-6-5-15-9-22-11-24-15)19(29)27(12)10-16(28)25-14-4-2-3-13(7-14)8-21/h2-4,7,9,11H,5-6,8,10,21H2,1H3,(H,22,24)(H,23,26)(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167516
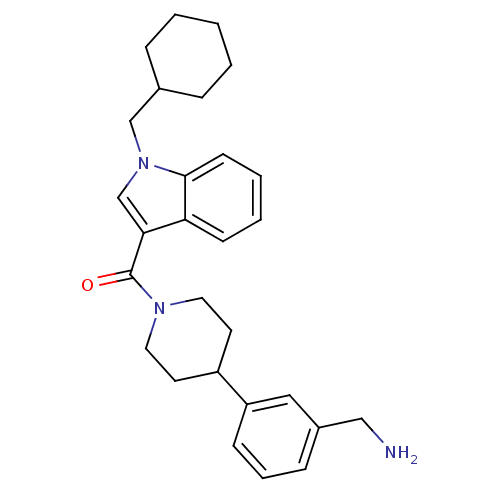
(CHEMBL191731 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES NCc1cccc(c1)C1CCN(CC1)C(=O)c1cn(CC2CCCCC2)c2ccccc12 Show InChI InChI=1S/C28H35N3O/c29-18-22-9-6-10-24(17-22)23-13-15-30(16-14-23)28(32)26-20-31(19-21-7-2-1-3-8-21)27-12-5-4-11-25(26)27/h4-6,9-12,17,20-21,23H,1-3,7-8,13-16,18-19,29H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 497 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50152196
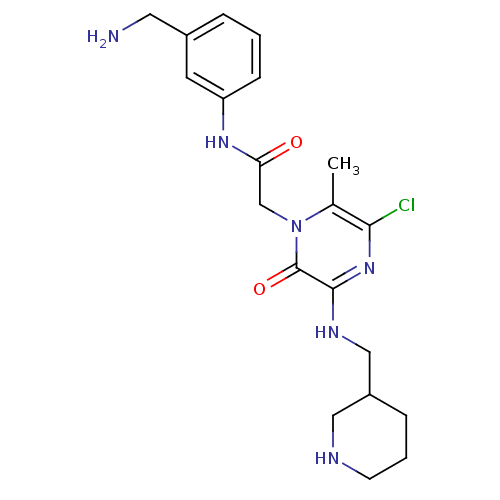
(CHEMBL184815 | N-(3-Aminomethyl-phenyl)-2-{5-chlor...)Show SMILES Cc1c(Cl)nc(NCC2CCCNC2)c(=O)n1CC(=O)Nc1cccc(CN)c1 Show InChI InChI=1S/C20H27ClN6O2/c1-13-18(21)26-19(24-11-15-5-3-7-23-10-15)20(29)27(13)12-17(28)25-16-6-2-4-14(8-16)9-22/h2,4,6,8,15,23H,3,5,7,9-12,22H2,1H3,(H,24,26)(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 518 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50152193
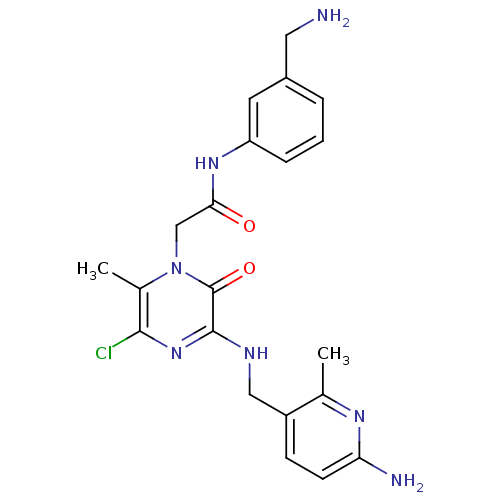
(CHEMBL187649 | N-(3-Aminomethyl-phenyl)-2-{3-[(6-a...)Show SMILES Cc1nc(N)ccc1CNc1nc(Cl)c(C)n(CC(=O)Nc2cccc(CN)c2)c1=O Show InChI InChI=1S/C21H24ClN7O2/c1-12-15(6-7-17(24)26-12)10-25-20-21(31)29(13(2)19(22)28-20)11-18(30)27-16-5-3-4-14(8-16)9-23/h3-8H,9-11,23H2,1-2H3,(H2,24,26)(H,25,28)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50152203
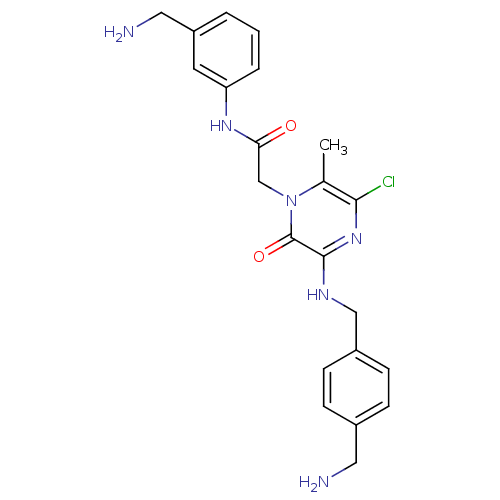
(2-[3-(4-Aminomethyl-benzylamino)-5-chloro-6-methyl...)Show SMILES Cc1c(Cl)nc(NCc2ccc(CN)cc2)c(=O)n1CC(=O)Nc1cccc(CN)c1 Show InChI InChI=1S/C22H25ClN6O2/c1-14-20(23)28-21(26-12-16-7-5-15(10-24)6-8-16)22(31)29(14)13-19(30)27-18-4-2-3-17(9-18)11-25/h2-9H,10-13,24-25H2,1H3,(H,26,28)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 615 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50152194
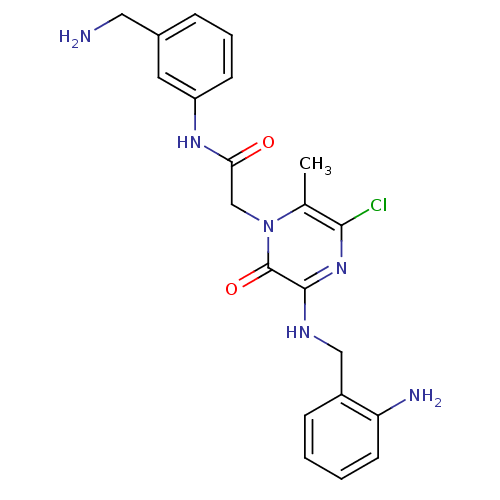
(2-[3-(2-Amino-benzylamino)-5-chloro-6-methyl-2-oxo...)Show SMILES Cc1c(Cl)nc(NCc2ccccc2N)c(=O)n1CC(=O)Nc1cccc(CN)c1 Show InChI InChI=1S/C21H23ClN6O2/c1-13-19(22)27-20(25-11-15-6-2-3-8-17(15)24)21(30)28(13)12-18(29)26-16-7-4-5-14(9-16)10-23/h2-9H,10-12,23-24H2,1H3,(H,25,27)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 875 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50152204

(CHEMBL360210 | N-(3-Aminomethyl-phenyl)-2-[5-chlor...)Show SMILES Cc1c(Cl)nc(NC2Cc3ccccc3C2)c(=O)n1CC(=O)Nc1cccc(CN)c1 Show InChI InChI=1S/C23H24ClN5O2/c1-14-21(24)28-22(27-19-10-16-6-2-3-7-17(16)11-19)23(31)29(14)13-20(30)26-18-8-4-5-15(9-18)12-25/h2-9,19H,10-13,25H2,1H3,(H,26,30)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50152195
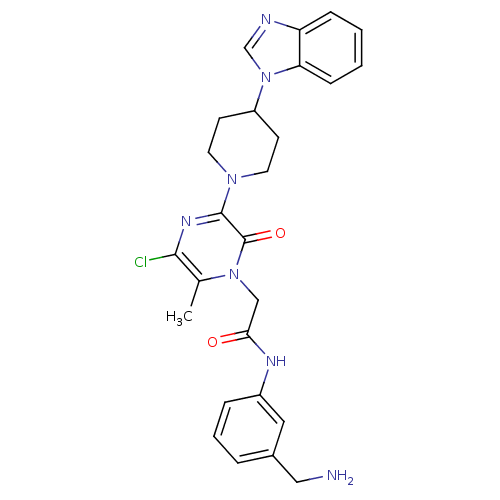
(CHEMBL187168 | N-(3-Aminomethyl-phenyl)-2-[3-(4-be...)Show SMILES Cc1c(Cl)nc(N2CCC(CC2)n2cnc3ccccc23)c(=O)n1CC(=O)Nc1cccc(CN)c1 Show InChI InChI=1S/C26H28ClN7O2/c1-17-24(27)31-25(26(36)33(17)15-23(35)30-19-6-4-5-18(13-19)14-28)32-11-9-20(10-12-32)34-16-29-21-7-2-3-8-22(21)34/h2-8,13,16,20H,9-12,14-15,28H2,1H3,(H,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50152201
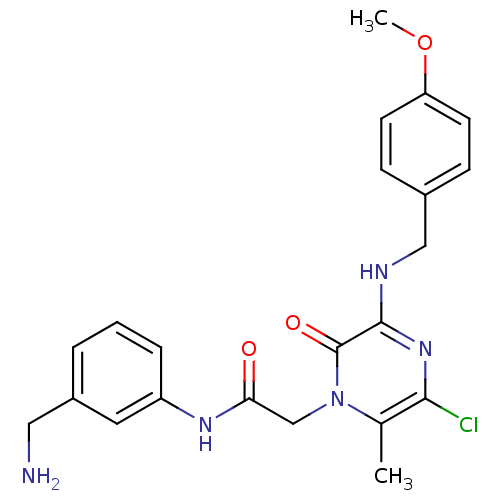
(CHEMBL187651 | N-(3-Aminomethyl-phenyl)-2-[5-chlor...)Show SMILES COc1ccc(CNc2nc(Cl)c(C)n(CC(=O)Nc3cccc(CN)c3)c2=O)cc1 Show InChI InChI=1S/C22H24ClN5O3/c1-14-20(23)27-21(25-12-15-6-8-18(31-2)9-7-15)22(30)28(14)13-19(29)26-17-5-3-4-16(10-17)11-24/h3-10H,11-13,24H2,1-2H3,(H,25,27)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50152199
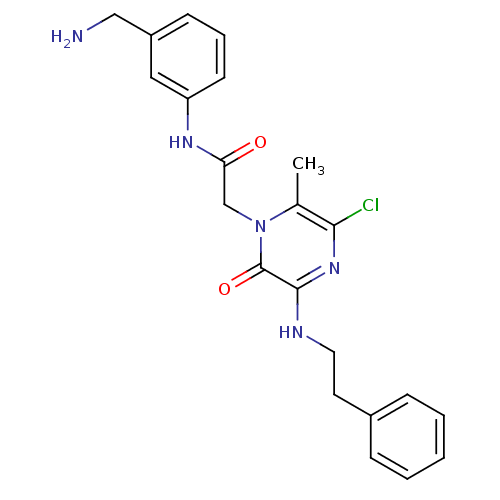
(CHEMBL183942 | N-(3-Aminomethyl-phenyl)-2-(5-chlor...)Show SMILES Cc1c(Cl)nc(NCCc2ccccc2)c(=O)n1CC(=O)Nc1cccc(CN)c1 Show InChI InChI=1S/C22H24ClN5O2/c1-15-20(23)27-21(25-11-10-16-6-3-2-4-7-16)22(30)28(15)14-19(29)26-18-9-5-8-17(12-18)13-24/h2-9,12H,10-11,13-14,24H2,1H3,(H,25,27)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Tryptase beta 2 expressed in yeast cells |
Bioorg Med Chem Lett 14: 4819-23 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.051
BindingDB Entry DOI: 10.7270/Q2H994NZ |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167532
(CHEMBL191072 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES Cc1cccc2[nH]cc(C(=O)N3CCC(CC3)c3cccc(CN)c3)c12 Show InChI InChI=1S/C22H25N3O/c1-15-4-2-7-20-21(15)19(14-24-20)22(26)25-10-8-17(9-11-25)18-6-3-5-16(12-18)13-23/h2-7,12,14,17,24H,8-11,13,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167512
(CHEMBL188104 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES Cc1ccc2c(c[nH]c2c1)C(=O)N1CCC(CC1)c1cccc(CN)c1 Show InChI InChI=1S/C22H25N3O/c1-15-5-6-19-20(14-24-21(19)11-15)22(26)25-9-7-17(8-10-25)18-4-2-3-16(12-18)13-23/h2-6,11-12,14,17,24H,7-10,13,23H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against tryptase |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50167514

(CHEMBL370463 | [4-(3-Aminomethyl-phenyl)-piperidin...)Show SMILES CCCCn1cc(C(=O)N2CCC(CC2)c2cccc(CN)c2)c2cccc(C)c12 Show InChI InChI=1S/C26H33N3O/c1-3-4-13-29-18-24(23-10-5-7-19(2)25(23)29)26(30)28-14-11-21(12-15-28)22-9-6-8-20(16-22)17-27/h5-10,16,18,21H,3-4,11-15,17,27H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Factor Xa |
Bioorg Med Chem Lett 15: 2734-7 (2005)
Article DOI: 10.1016/j.bmcl.2005.04.002
BindingDB Entry DOI: 10.7270/Q2V987K1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50156457

(CHEMBL186730 | [4-(5-Aminomethyl-2-fluoro-phenyl)-...)Show SMILES NCc1ccc(F)c(c1)C1CCN(CC1)C(=O)c1cccc(c1)-c1nc(no1)-c1cccs1 Show InChI InChI=1S/C25H23FN4O2S/c26-21-7-6-16(15-27)13-20(21)17-8-10-30(11-9-17)25(31)19-4-1-3-18(14-19)24-28-23(29-32-24)22-5-2-12-33-22/h1-7,12-14,17H,8-11,15,27H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against cytochrome P450 determined using human liver microsome upon 15 min incubation with probes substrates tolbutamide (2C9) |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50156457

(CHEMBL186730 | [4-(5-Aminomethyl-2-fluoro-phenyl)-...)Show SMILES NCc1ccc(F)c(c1)C1CCN(CC1)C(=O)c1cccc(c1)-c1nc(no1)-c1cccs1 Show InChI InChI=1S/C25H23FN4O2S/c26-21-7-6-16(15-27)13-20(21)17-8-10-30(11-9-17)25(31)19-4-1-3-18(14-19)24-28-23(29-32-24)22-5-2-12-33-22/h1-7,12-14,17H,8-11,15,27H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity against human Potassium channel HERG |
Bioorg Med Chem Lett 14: 6053-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.09.065
BindingDB Entry DOI: 10.7270/Q2KH0MST |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data