

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14044 (Aminofurazanyl-azabenzimidazole 6k | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14042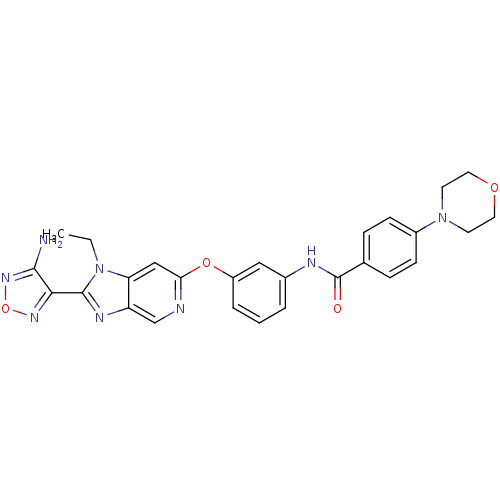 (Aminofurazanyl-azabenzimidazole 6i | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14043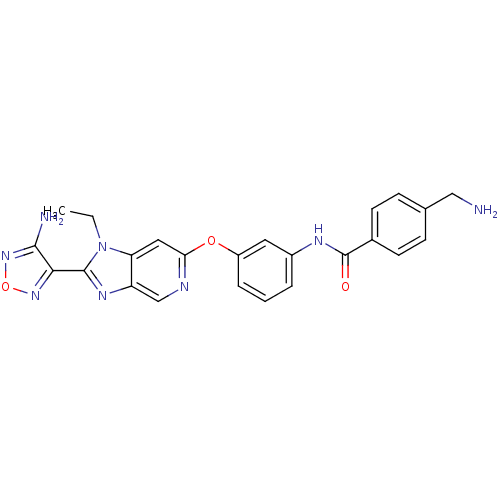 (Aminofurazanyl-azabenzimidazole 6j | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14045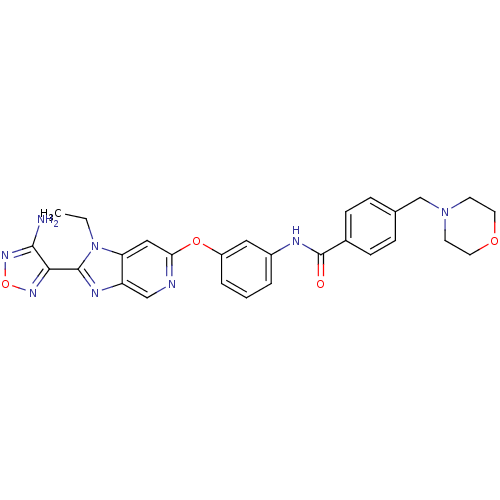 (Aminofurazanyl-azabenzimidazole 6l | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14046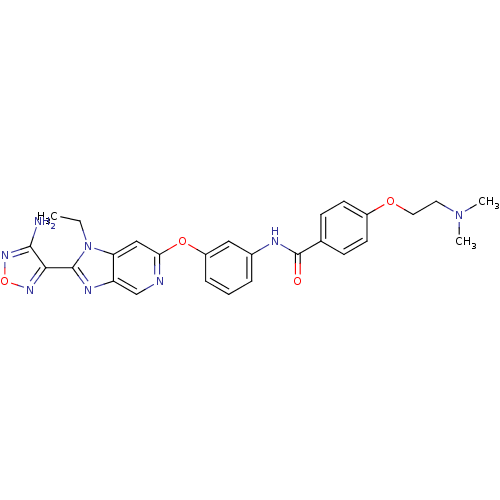 (Aminofurazanyl-azabenzimidazole 6m | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14035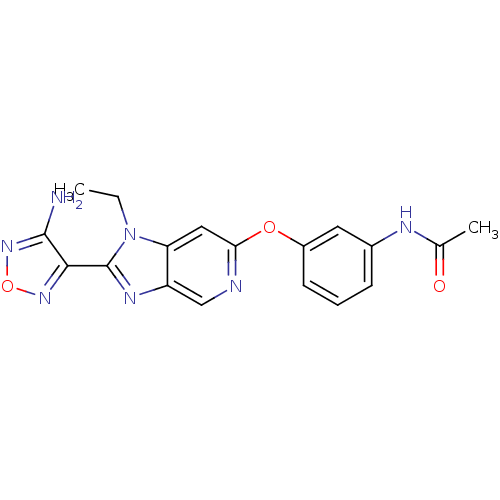 (Aminofurazanyl-azabenzimidazole 6c | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14047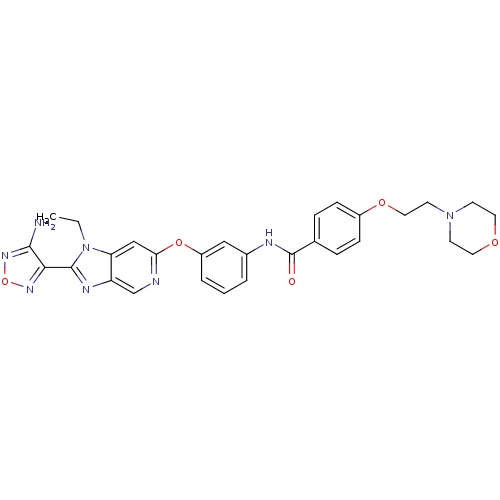 (Aminofurazanyl-azabenzimidazole 6n | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14041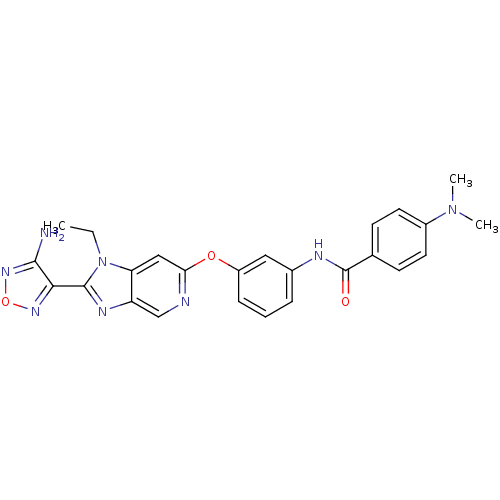 (Aminofurazanyl-azabenzimidazole 6h | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14040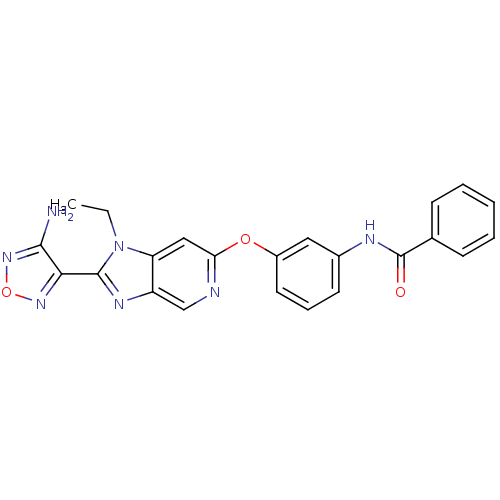 (Aminofurazanyl-azabenzimidazole 6g | N-(3-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14033 (4-{1-ethyl-6-methoxy-1H-imidazo[4,5-c]pyridin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14050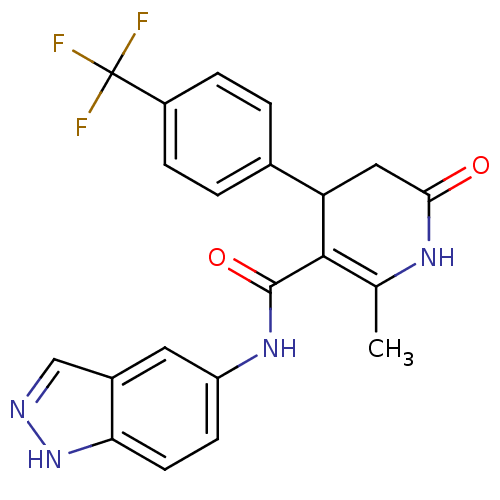 (N-(1H-indazol-5-yl)-2-methyl-6-oxo-4-[4-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 6-9 (2007) Article DOI: 10.1021/jm0609014 BindingDB Entry DOI: 10.7270/Q298858V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14056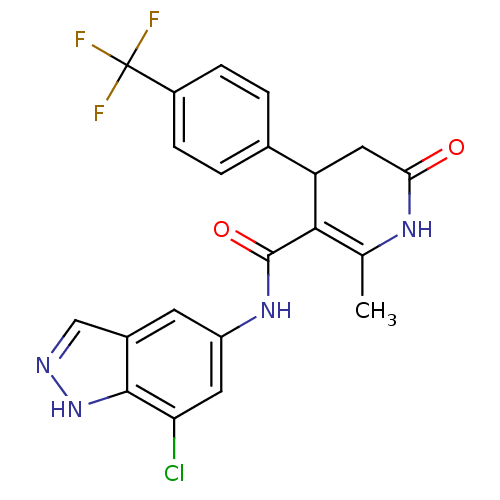 (N-(7-chloro-1H-indazol-5-yl)-2-methyl-6-oxo-4-[4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 6-9 (2007) Article DOI: 10.1021/jm0609014 BindingDB Entry DOI: 10.7270/Q298858V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14051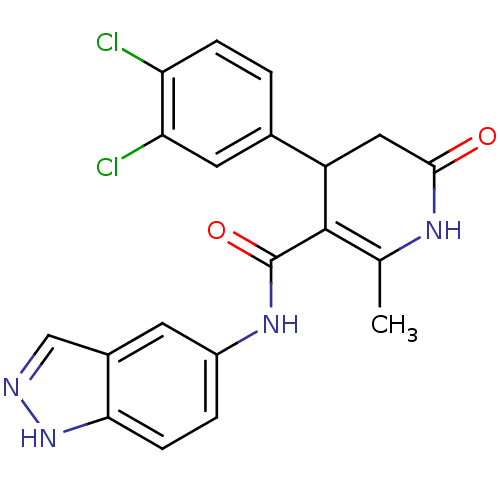 (4-(3,4-Dichlorophenyl)-N-1H-indazol-5-yl-2-methyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 6-9 (2007) Article DOI: 10.1021/jm0609014 BindingDB Entry DOI: 10.7270/Q298858V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14053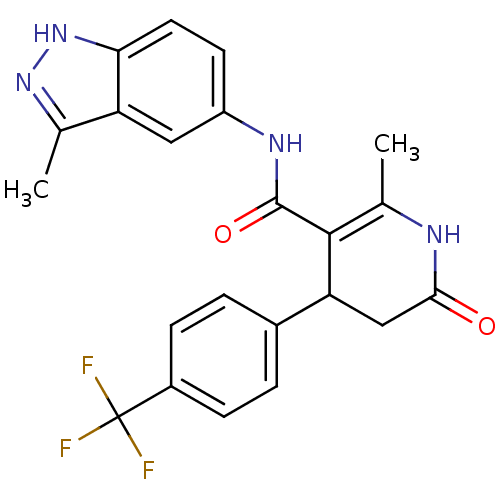 (2-methyl-N-(3-methyl-1H-indazol-5-yl)-6-oxo-4-[4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 6-9 (2007) Article DOI: 10.1021/jm0609014 BindingDB Entry DOI: 10.7270/Q298858V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14052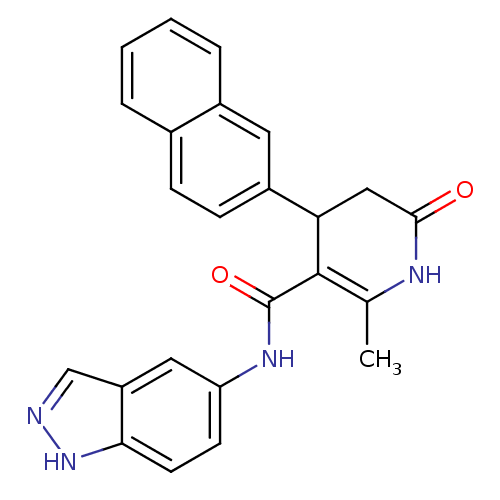 (N-(1H-indazol-5-yl)-2-methyl-4-(naphthalen-2-yl)-6...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 6-9 (2007) Article DOI: 10.1021/jm0609014 BindingDB Entry DOI: 10.7270/Q298858V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14037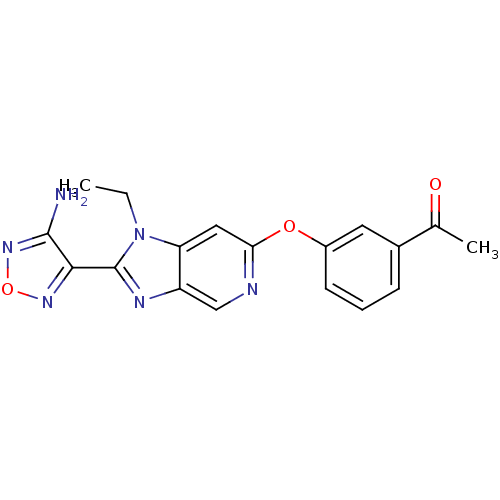 (1-(3-{[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-1H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14048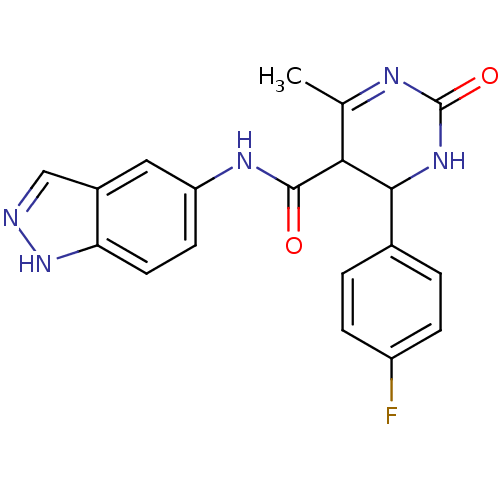 (4-(4-fluorophenyl)-N-(1H-indazol-5-yl)-6-methyl-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 6-9 (2007) Article DOI: 10.1021/jm0609014 BindingDB Entry DOI: 10.7270/Q298858V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14055 (N-(6-fluoro-1H-indazol-5-yl)-2-methyl-6-oxo-4-[4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 6-9 (2007) Article DOI: 10.1021/jm0609014 BindingDB Entry DOI: 10.7270/Q298858V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14039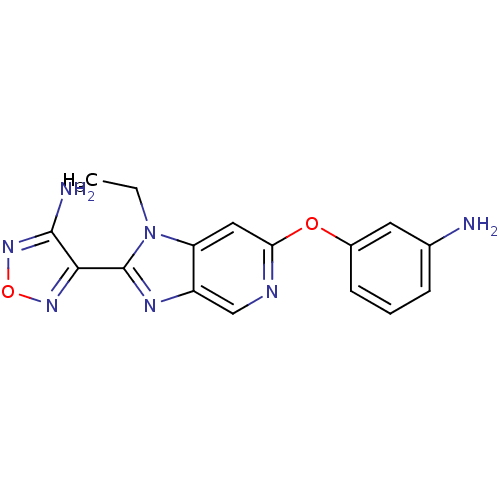 (4-[6-(3-aminophenoxy)-1-ethyl-1H-imidazo[4,5-c]pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14032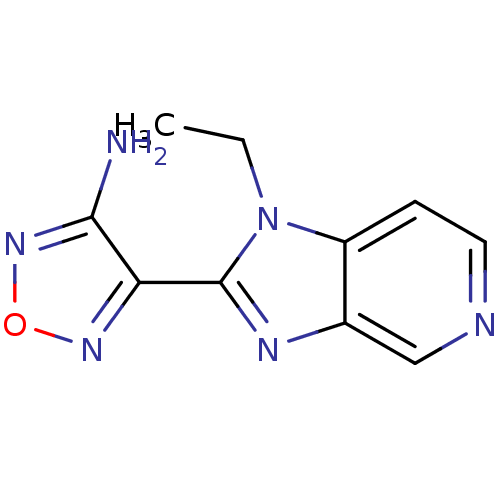 (4-(1-ethyl-1H-imidazo[4,5-c]pyridin-2-yl)-1,2,5-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14034 (4-{1-ethyl-6-phenoxy-1H-imidazo[4,5-c]pyridin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14038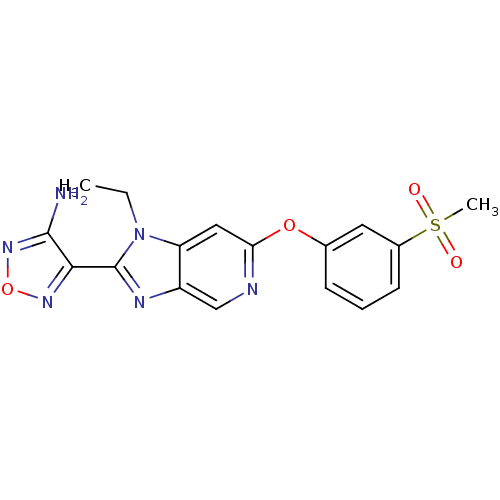 (4-[1-ethyl-6-(3-methanesulfonylphenoxy)-1H-imidazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50510295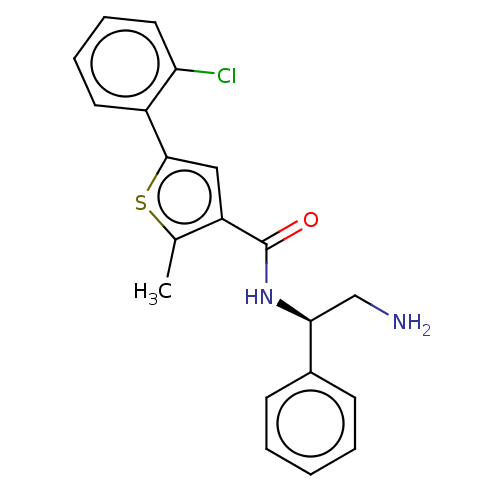 (CHEMBL4443422) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14049 (4-(4-Fluorophenyl)-N-1H-indazol-5-yl-2-methyl-6-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 6-9 (2007) Article DOI: 10.1021/jm0609014 BindingDB Entry DOI: 10.7270/Q298858V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14036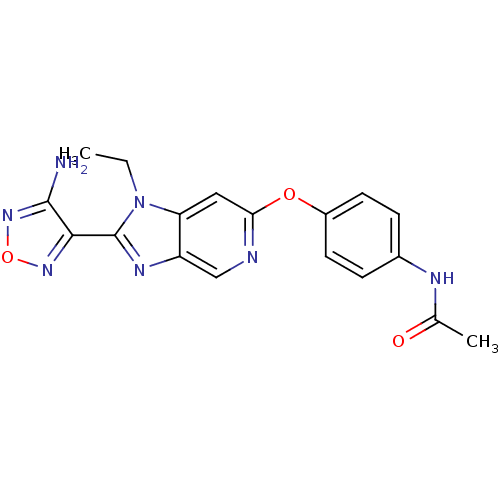 (Aminofurazanyl-azabenzimidazole 6d | N-(4-{[2-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 2-5 (2007) Article DOI: 10.1021/jm060873p BindingDB Entry DOI: 10.7270/Q2F18WZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50510294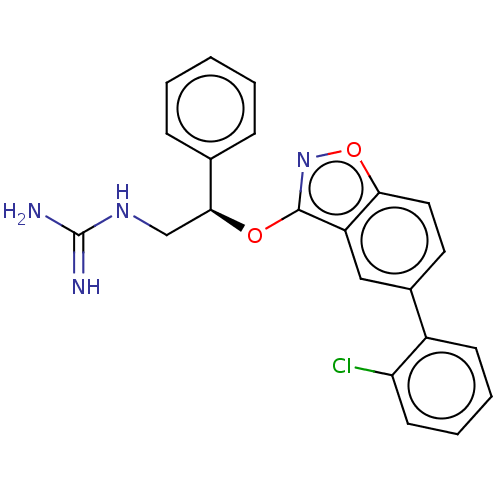 (CHEMBL4592805) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50510288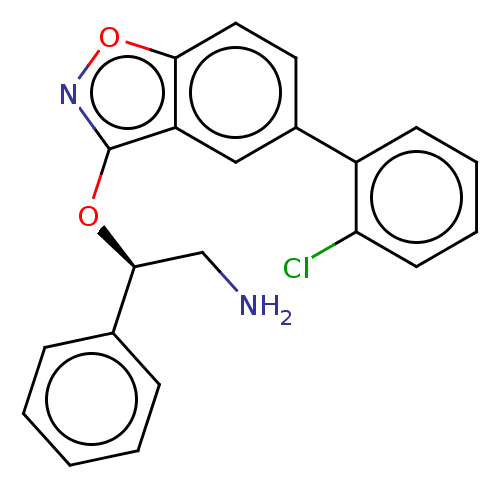 (CHEMBL4445556) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM21690 (1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase A2B2 using relaxed pNO1 as substrate | J Med Chem 61: 3565-3581 (2018) Article DOI: 10.1021/acs.jmedchem.7b01892 BindingDB Entry DOI: 10.7270/Q2765HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50510292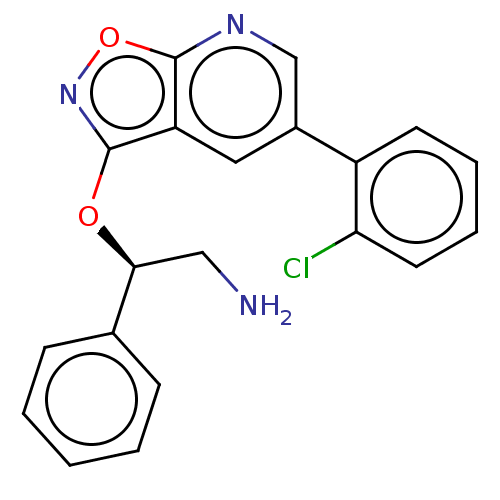 (CHEMBL4471339) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50510286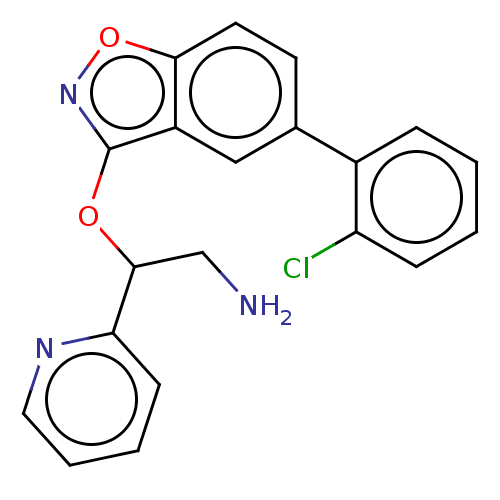 (CHEMBL4467227) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50452872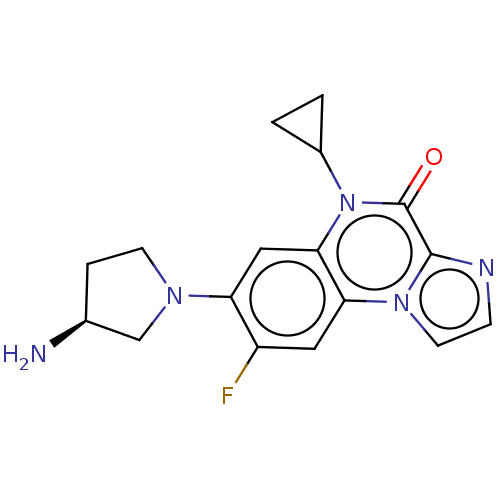 (CHEMBL4210894) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase A2B2 using relaxed pNO1 as substrate | J Med Chem 61: 3565-3581 (2018) Article DOI: 10.1021/acs.jmedchem.7b01892 BindingDB Entry DOI: 10.7270/Q2765HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50510293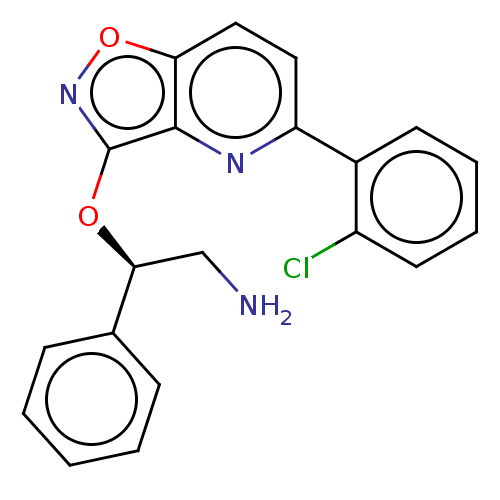 (CHEMBL4528547) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50510289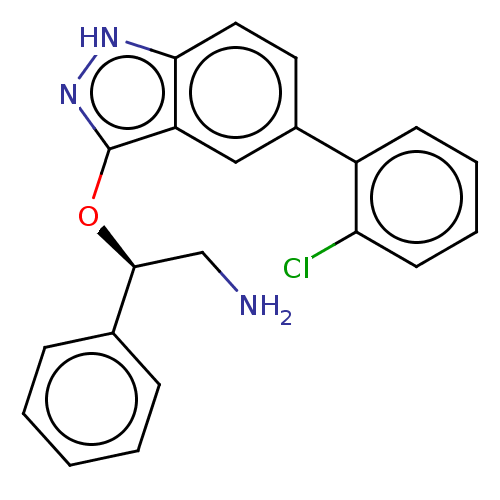 (CHEMBL4588294) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50510287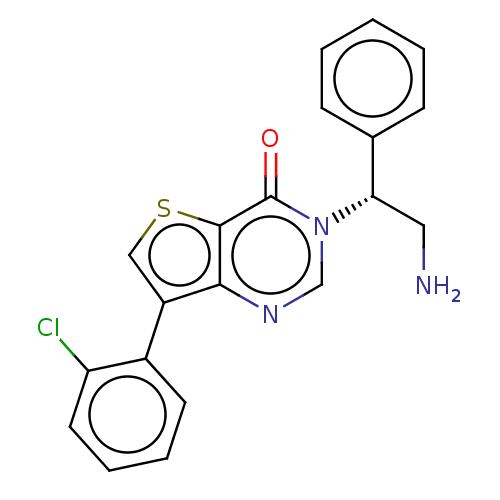 (CHEMBL4468900) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50452870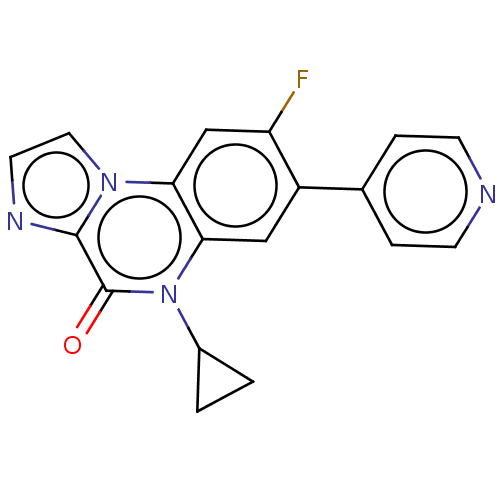 (CHEMBL4203118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase A2B2 using relaxed pNO1 as substrate | J Med Chem 61: 3565-3581 (2018) Article DOI: 10.1021/acs.jmedchem.7b01892 BindingDB Entry DOI: 10.7270/Q2765HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM14054 (N-(4-fluoro-1H-indazol-5-yl)-2-methyl-6-oxo-4-[4-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline | Assay Description The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... | J Med Chem 50: 6-9 (2007) Article DOI: 10.1021/jm0609014 BindingDB Entry DOI: 10.7270/Q298858V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50510290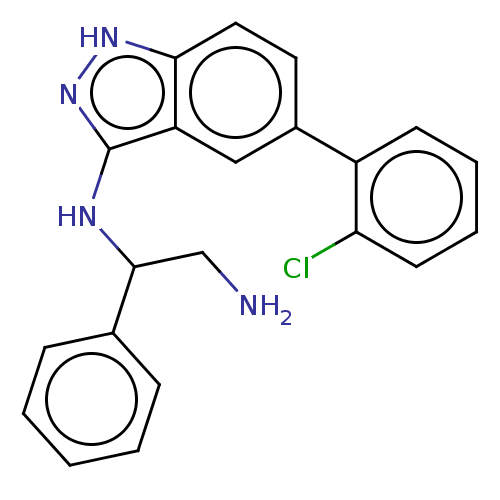 (CHEMBL4583055) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50510293 (CHEMBL4528547) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human ERG by QPatch assay | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50510296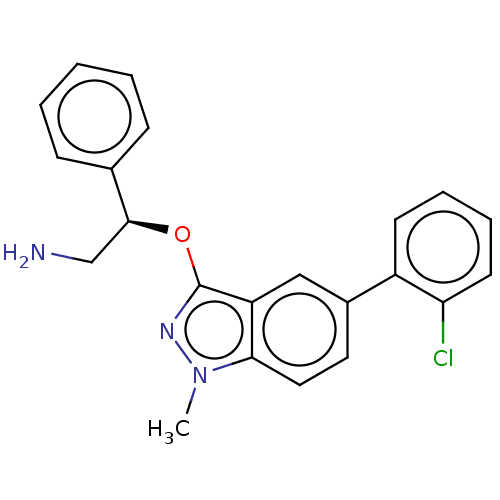 (CHEMBL4592804) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50452869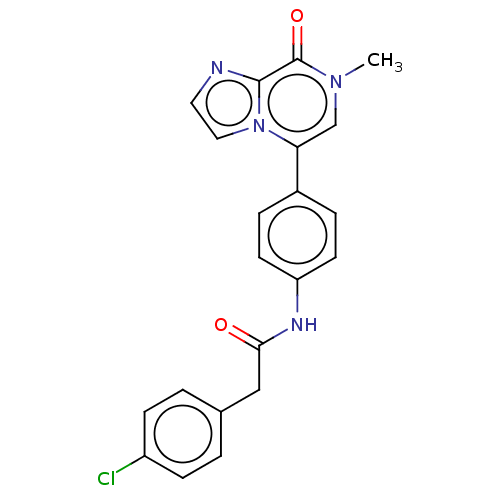 (CHEMBL4206009) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of Escherichia coli DNA gyrase A2B2 using relaxed pNO1 as substrate | J Med Chem 61: 3565-3581 (2018) Article DOI: 10.1021/acs.jmedchem.7b01892 BindingDB Entry DOI: 10.7270/Q2765HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50510295 (CHEMBL4443422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human ERG by QPatch assay | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50510291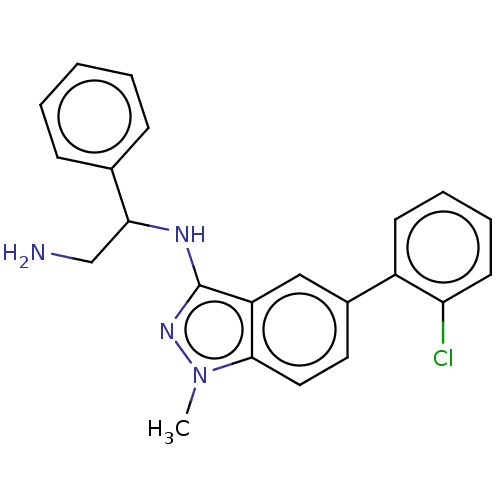 (CHEMBL4553177) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50510294 (CHEMBL4592805) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human ERG by QPatch assay | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A/B (Escherichia coli (strain K12)) | BDBM50510297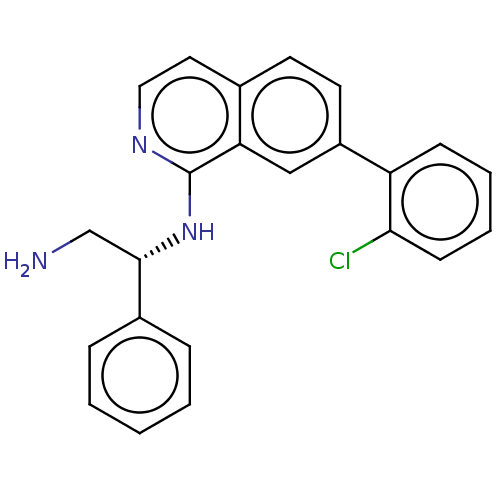 (CHEMBL4577405) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM50510295 (CHEMBL4443422) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human NaV1.5 expressed in HEK293 cells assessed as decrease in sodium current amplitude at -120 mV holding potential by Qpatch assay | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50452870 (CHEMBL4203118) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of CYP3A4-mediated testosterone metabolism in human liver microsomes | J Med Chem 61: 3565-3581 (2018) Article DOI: 10.1021/acs.jmedchem.7b01892 BindingDB Entry DOI: 10.7270/Q2765HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50452870 (CHEMBL4203118) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of CYP3A4-mediated midazolam metabolism in human liver microsomes | J Med Chem 61: 3565-3581 (2018) Article DOI: 10.1021/acs.jmedchem.7b01892 BindingDB Entry DOI: 10.7270/Q2765HX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM50510293 (CHEMBL4528547) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human NaV1.5 expressed in HEK293 cells assessed as decrease in sodium current amplitude at -120 mV holding potential by Qpatch assay | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM50510288 (CHEMBL4445556) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human NaV1.5 expressed in HEK293 cells assessed as decrease in sodium current amplitude at -120 mV holding potential by Qpatch assay | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50510288 (CHEMBL4445556) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human ERG by QPatch assay | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 BindingDB Entry DOI: 10.7270/Q2NC64HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 65 total ) | Next | Last >> |