 Found 191 hits with Last Name = 'tomaszek' and Initial = 'ta'
Found 191 hits with Last Name = 'tomaszek' and Initial = 'ta' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Procathepsin L
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066647

(CHEMBL113724 | {(S)-3-Methyl-1-[3-oxo-1-(4-phenoxy...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C31H35N3O7S/c1-22(2)19-28(33-31(37)40-21-23-9-5-3-6-10-23)30(36)32-27-17-18-34(20-29(27)35)42(38,39)26-15-13-25(14-16-26)41-24-11-7-4-8-12-24/h3-16,22,27-28H,17-21H2,1-2H3,(H,32,36)(H,33,37)/t27?,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403349
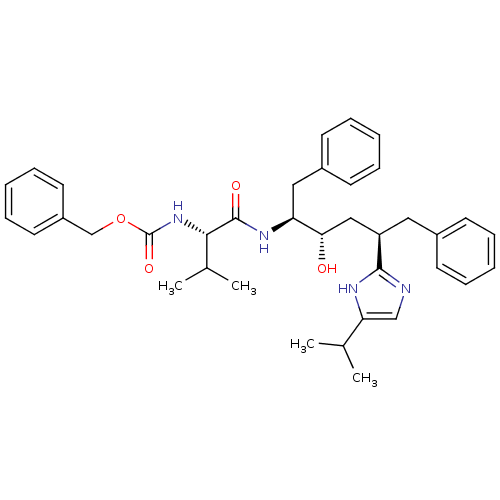
(CHEMBL407551)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C37H46N4O4/c1-25(2)32-23-38-35(39-32)30(20-27-14-8-5-9-15-27)22-33(42)31(21-28-16-10-6-11-17-28)40-36(43)34(26(3)4)41-37(44)45-24-29-18-12-7-13-19-29/h5-19,23,25-26,30-31,33-34,42H,20-22,24H2,1-4H3,(H,38,39)(H,40,43)(H,41,44)/t30-,31+,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037121
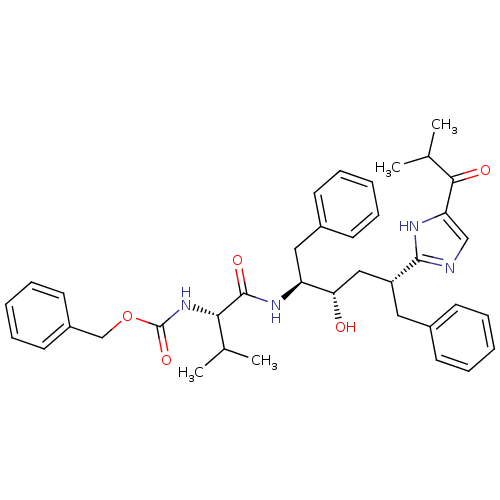
(2-[(1R,3S,4S)-1-BENZYL-4-[N-(BENZYLOXYCARBONYL)-L-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(=O)C(C)C Show InChI InChI=1S/C38H46N4O5/c1-25(2)34(42-38(46)47-24-29-18-12-7-13-19-29)37(45)41-31(21-28-16-10-6-11-17-28)33(43)22-30(20-27-14-8-5-9-15-27)36-39-23-32(40-36)35(44)26(3)4/h5-19,23,25-26,30-31,33-34,43H,20-22,24H2,1-4H3,(H,39,40)(H,41,45)(H,42,46)/t30-,31+,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408519
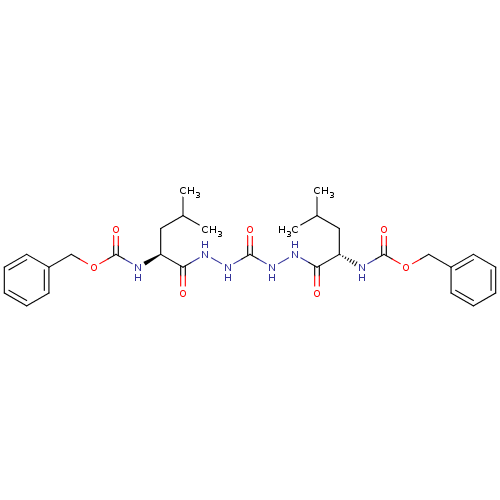
(CHEMBL115357)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C29H40N6O7/c1-19(2)15-23(30-28(39)41-17-21-11-7-5-8-12-21)25(36)32-34-27(38)35-33-26(37)24(16-20(3)4)31-29(40)42-18-22-13-9-6-10-14-22/h5-14,19-20,23-24H,15-18H2,1-4H3,(H,30,39)(H,31,40)(H,32,36)(H,33,37)(H2,34,35,38)/t23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037124
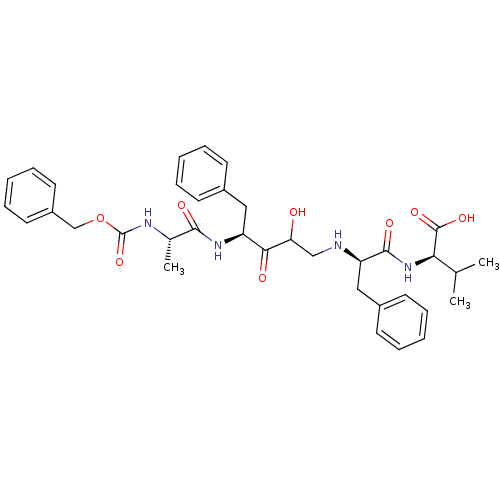
((R)-2-{(R)-2-[(S)-4-((S)-2-Benzyloxycarbonylamino-...)Show SMILES CC(C)[C@@H](NC(=O)[C@@H](Cc1ccccc1)NCC(O)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)OCc1ccccc1)C(O)=O Show InChI InChI=1S/C36H44N4O8/c1-23(2)31(35(45)46)40-34(44)29(20-26-15-9-5-10-16-26)37-21-30(41)32(42)28(19-25-13-7-4-8-14-25)39-33(43)24(3)38-36(47)48-22-27-17-11-6-12-18-27/h4-18,23-24,28-31,37,41H,19-22H2,1-3H3,(H,38,47)(H,39,43)(H,40,44)(H,45,46)/t24-,28-,29+,30?,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408522
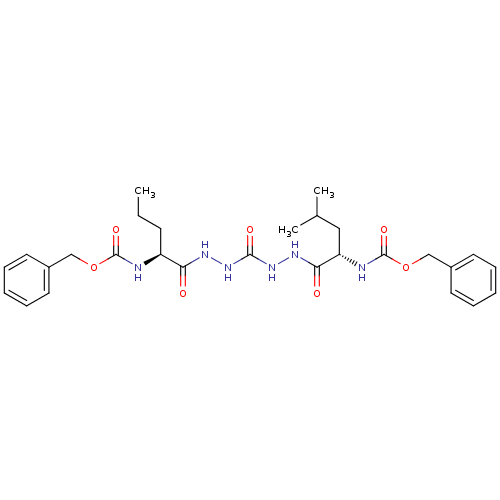
(CHEMBL126820)Show SMILES CCC[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C28H38N6O7/c1-4-11-22(29-27(38)40-17-20-12-7-5-8-13-20)24(35)31-33-26(37)34-32-25(36)23(16-19(2)3)30-28(39)41-18-21-14-9-6-10-15-21/h5-10,12-15,19,22-23H,4,11,16-18H2,1-3H3,(H,29,38)(H,30,39)(H,31,35)(H,32,36)(H2,33,34,37)/t22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403357

(CHEMBL419286)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C31H42N4O3/c1-20(2)27-19-32-30(34-27)25(16-23-12-8-6-9-13-23)18-28(37)26(17-24-14-10-7-11-15-24)35-31(38)29(21(3)4)33-22(5)36/h6-15,19-21,25-26,28-29,37H,16-18H2,1-5H3,(H,32,34)(H,33,36)(H,35,38)/t25-,26+,28+,29+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408520
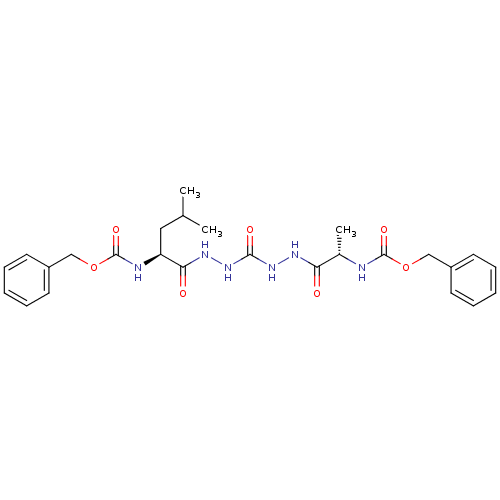
(CHEMBL126352)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C26H34N6O7/c1-17(2)14-21(28-26(37)39-16-20-12-8-5-9-13-20)23(34)30-32-24(35)31-29-22(33)18(3)27-25(36)38-15-19-10-6-4-7-11-19/h4-13,17-18,21H,14-16H2,1-3H3,(H,27,36)(H,28,37)(H,29,33)(H,30,34)(H2,31,32,35)/t18-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM93183
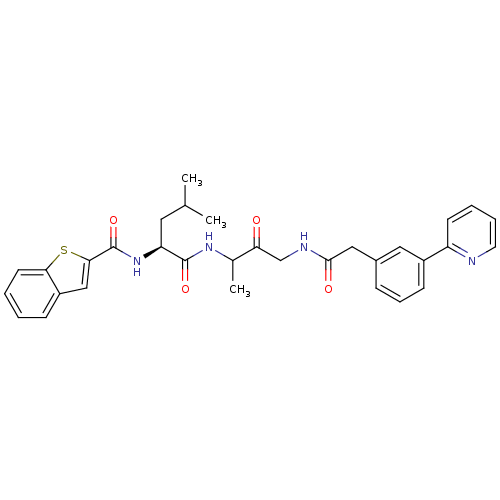
(Cathepsin Inhibitor, Column 6 Row 1)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2s1)C(=O)NC(C)C(=O)CNC(=O)Cc1cccc(c1)-c1ccccn1 |r| Show InChI InChI=1S/C32H34N4O4S/c1-20(2)15-26(36-32(40)29-18-24-10-4-5-13-28(24)41-29)31(39)35-21(3)27(37)19-34-30(38)17-22-9-8-11-23(16-22)25-12-6-7-14-33-25/h4-14,16,18,20-21,26H,15,17,19H2,1-3H3,(H,34,38)(H,35,39)(H,36,40)/t21?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
Inhibitors were assayed against human liver Cathepsin L and B. Inhibitors were also evaluated for inhibition against purified recombinant Cathepsin ... |
J Comb Chem 1: 207-15 (1999)
Article DOI: 10.1021/cc9800374
BindingDB Entry DOI: 10.7270/Q2HQ3XHJ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403360
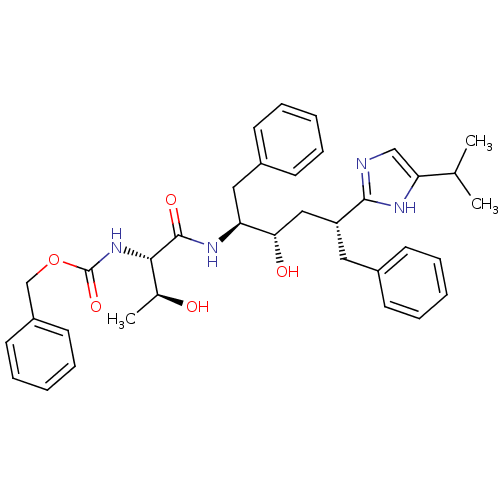
(CHEMBL1790592)Show SMILES CC(C)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)[C@H](C)O)Cc1ccccc1 Show InChI InChI=1S/C36H44N4O5/c1-24(2)31-22-37-34(38-31)29(19-26-13-7-4-8-14-26)21-32(42)30(20-27-15-9-5-10-16-27)39-35(43)33(25(3)41)40-36(44)45-23-28-17-11-6-12-18-28/h4-18,22,24-25,29-30,32-33,41-42H,19-21,23H2,1-3H3,(H,37,38)(H,39,43)(H,40,44)/t25-,29+,30-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098580
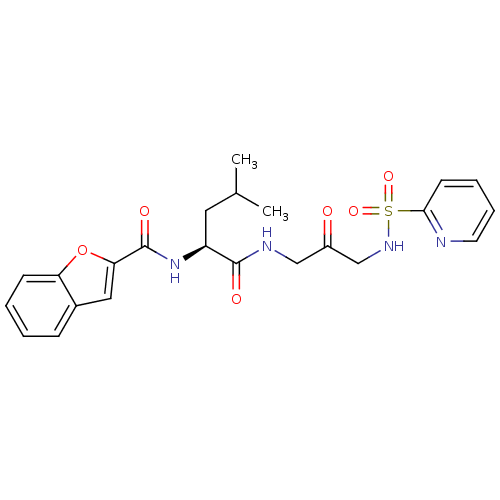
(Benzofuran-2-carboxylic acid {3-methyl-1-[2-oxo-3-...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)NCC(=O)CNS(=O)(=O)c1ccccn1 Show InChI InChI=1S/C23H26N4O6S/c1-15(2)11-18(27-23(30)20-12-16-7-3-4-8-19(16)33-20)22(29)25-13-17(28)14-26-34(31,32)21-9-5-6-10-24-21/h3-10,12,15,18,26H,11,13-14H2,1-2H3,(H,25,29)(H,27,30)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM93180
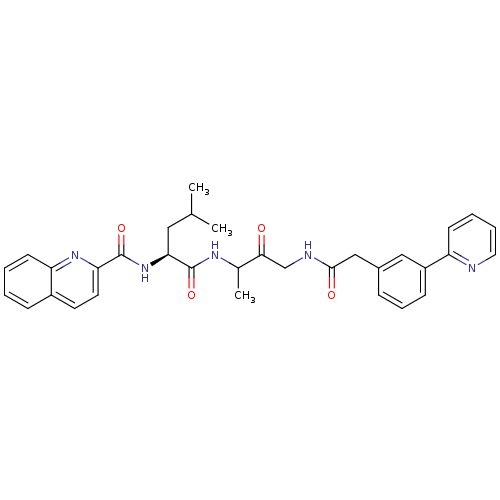
(Cathepsin Inhibitor, Column 5 Row 1)Show SMILES CC(C)C[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)NC(C)C(=O)CNC(=O)Cc1cccc(c1)-c1ccccn1 |r| Show InChI InChI=1S/C33H35N5O4/c1-21(2)17-29(38-32(41)28-15-14-24-10-4-5-13-27(24)37-28)33(42)36-22(3)30(39)20-35-31(40)19-23-9-8-11-25(18-23)26-12-6-7-16-34-26/h4-16,18,21-22,29H,17,19-20H2,1-3H3,(H,35,40)(H,36,42)(H,38,41)/t22?,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
Inhibitors were assayed against human liver Cathepsin L and B. Inhibitors were also evaluated for inhibition against purified recombinant Cathepsin ... |
J Comb Chem 1: 207-15 (1999)
Article DOI: 10.1021/cc9800374
BindingDB Entry DOI: 10.7270/Q2HQ3XHJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098584
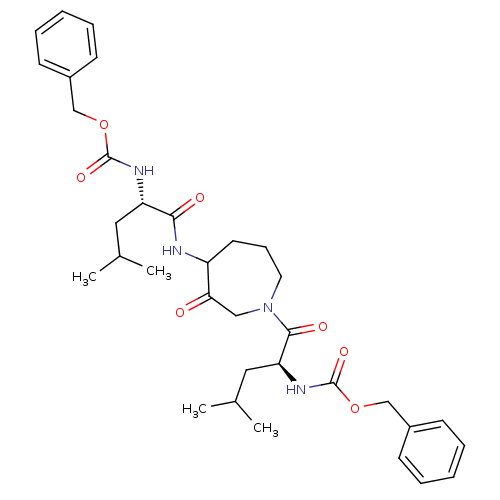
(CHEMBL31947 | {1-[4-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C34H46N4O7/c1-23(2)18-28(36-33(42)44-21-25-12-7-5-8-13-25)31(40)35-27-16-11-17-38(20-30(27)39)32(41)29(19-24(3)4)37-34(43)45-22-26-14-9-6-10-15-26/h5-10,12-15,23-24,27-29H,11,16-22H2,1-4H3,(H,35,40)(H,36,42)(H,37,43)/t27?,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403351
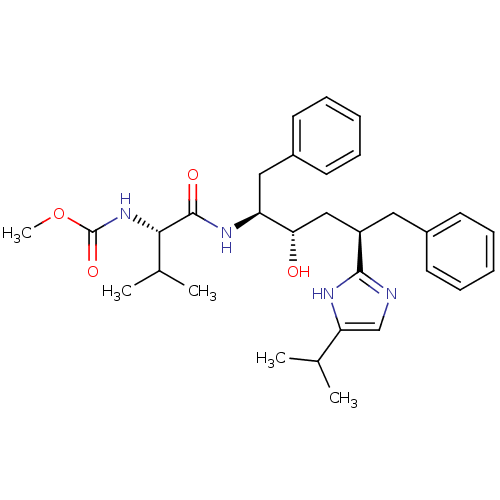
(CHEMBL79698)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C31H42N4O4/c1-20(2)26-19-32-29(33-26)24(16-22-12-8-6-9-13-22)18-27(36)25(17-23-14-10-7-11-15-23)34-30(37)28(21(3)4)35-31(38)39-5/h6-15,19-21,24-25,27-28,36H,16-18H2,1-5H3,(H,32,33)(H,34,37)(H,35,38)/t24-,25+,27+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098579
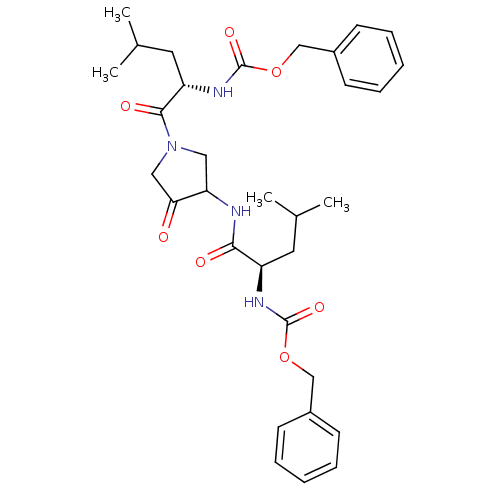
(CHEMBL29483 | {1-[3-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26+,27?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19807
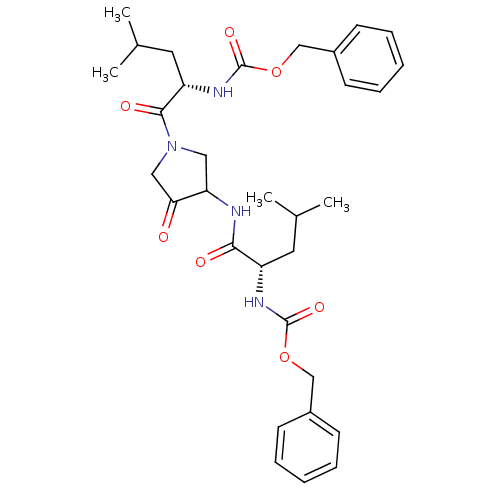
(CHEMBL100563 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C32H42N4O7/c1-21(2)15-25(34-31(40)42-19-23-11-7-5-8-12-23)29(38)33-27-17-36(18-28(27)37)30(39)26(16-22(3)4)35-32(41)43-20-24-13-9-6-10-14-24/h5-14,21-22,25-27H,15-20H2,1-4H3,(H,33,38)(H,34,40)(H,35,41)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098575
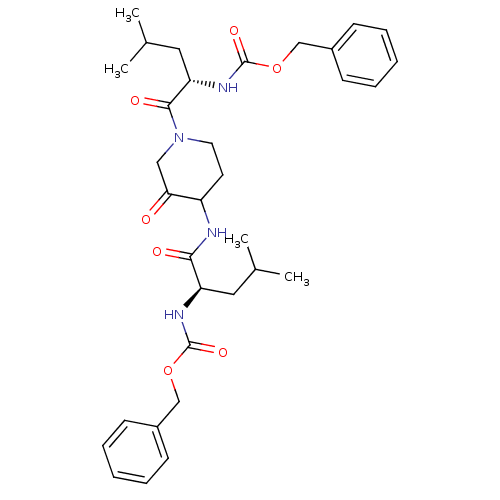
(CHEMBL281086 | {1-[4-(2-Benzyloxycarbonylamino-4-m...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM19810
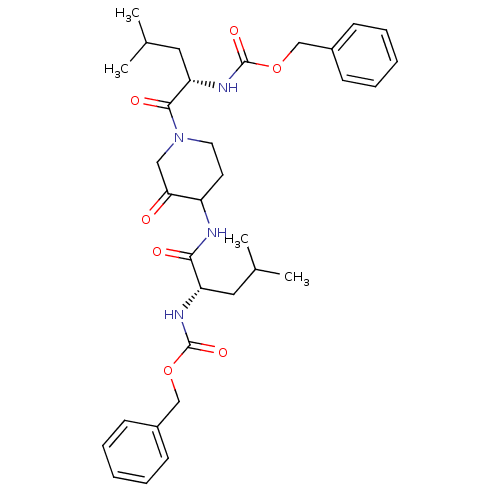
(CHEMBL118449 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403358
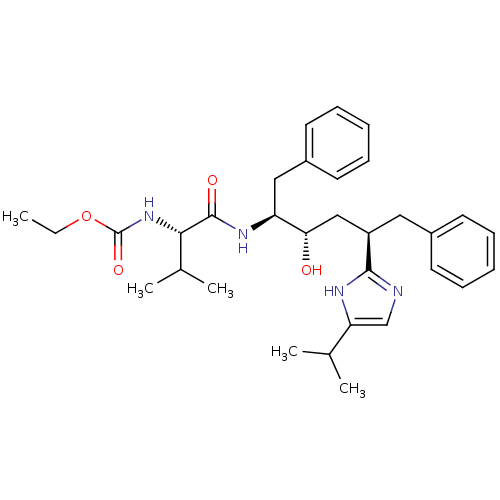
(CHEMBL78531)Show SMILES CCOC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C32H44N4O4/c1-6-40-32(39)36-29(22(4)5)31(38)35-26(18-24-15-11-8-12-16-24)28(37)19-25(17-23-13-9-7-10-14-23)30-33-20-27(34-30)21(2)3/h7-16,20-22,25-26,28-29,37H,6,17-19H2,1-5H3,(H,33,34)(H,35,38)(H,36,39)/t25-,26+,28+,29+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408521
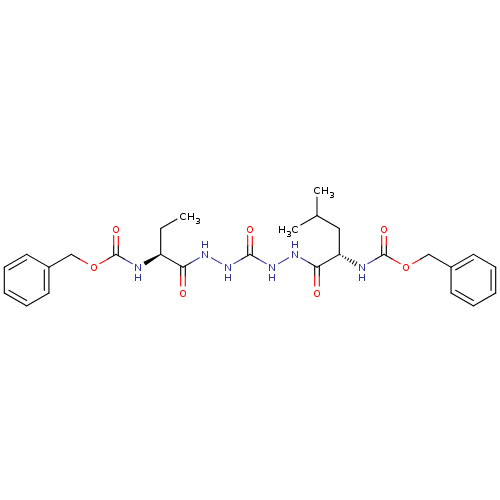
(CHEMBL129773)Show SMILES CC[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C27H36N6O7/c1-4-21(28-26(37)39-16-19-11-7-5-8-12-19)23(34)30-32-25(36)33-31-24(35)22(15-18(2)3)29-27(38)40-17-20-13-9-6-10-14-20/h5-14,18,21-22H,4,15-17H2,1-3H3,(H,28,37)(H,29,38)(H,30,34)(H,31,35)(H2,32,33,36)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066648
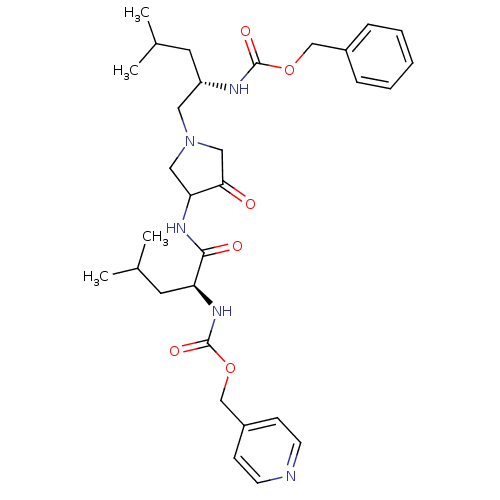
(((S)-3-Methyl-1-{3-[(S)-4-methyl-2-(pyridin-4-ylme...)Show SMILES CC(C)C[C@@H](CN1CC(NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccncc2)C(=O)C1)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H43N5O6/c1-21(2)14-25(33-30(39)41-19-23-8-6-5-7-9-23)16-36-17-27(28(37)18-36)34-29(38)26(15-22(3)4)35-31(40)42-20-24-10-12-32-13-11-24/h5-13,21-22,25-27H,14-20H2,1-4H3,(H,33,39)(H,34,38)(H,35,40)/t25-,26-,27?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin S
(Homo sapiens (Human)) | BDBM19769

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C26H30N4O6S/c1-17(2)14-20(29-26(33)23-15-18-8-3-4-10-22(18)36-23)25(32)28-19-9-7-13-30(16-21(19)31)37(34,35)24-11-5-6-12-27-24/h3-6,8,10-12,15,17,19-20H,7,9,13-14,16H2,1-2H3,(H,28,32)(H,29,33)/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin S |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403352

(CHEMBL81517)Show SMILES CC(C)[C@H](NC=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C30H40N4O3/c1-20(2)26-18-31-29(33-26)24(15-22-11-7-5-8-12-22)17-27(36)25(16-23-13-9-6-10-14-23)34-30(37)28(21(3)4)32-19-35/h5-14,18-21,24-25,27-28,36H,15-17H2,1-4H3,(H,31,33)(H,32,35)(H,34,37)/t24-,25+,27+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098581

(5-(2-Morpholin-4-yl-ethoxy)-benzofuran-2-carboxyli...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Rattus norvegicus) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Rat cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin K
(Homo sapiens (Human)) | BDBM93174
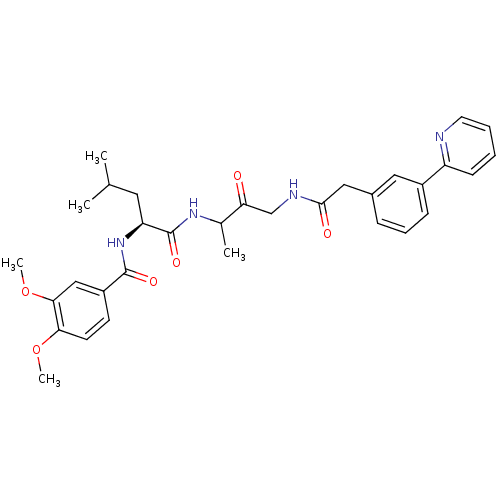
(Cathepsin Inhibitor, Column 3 Row 1)Show SMILES COc1ccc(cc1OC)C(=O)N[C@@H](CC(C)C)C(=O)NC(C)C(=O)CNC(=O)Cc1cccc(c1)-c1ccccn1 |r| Show InChI InChI=1S/C32H38N4O6/c1-20(2)15-26(36-31(39)24-12-13-28(41-4)29(18-24)42-5)32(40)35-21(3)27(37)19-34-30(38)17-22-9-8-10-23(16-22)25-11-6-7-14-33-25/h6-14,16,18,20-21,26H,15,17,19H2,1-5H3,(H,34,38)(H,35,40)(H,36,39)/t21?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
Inhibitors were assayed against human liver Cathepsin L and B. Inhibitors were also evaluated for inhibition against purified recombinant Cathepsin ... |
J Comb Chem 1: 207-15 (1999)
Article DOI: 10.1021/cc9800374
BindingDB Entry DOI: 10.7270/Q2HQ3XHJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM19782
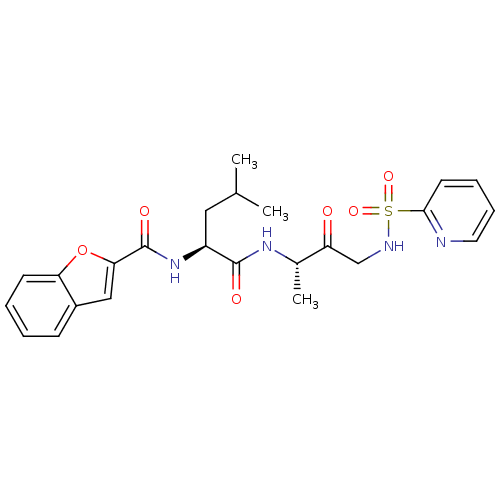
((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(2...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@@H](C)C(=O)CNS(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C24H28N4O6S/c1-15(2)12-18(28-24(31)21-13-17-8-4-5-9-20(17)34-21)23(30)27-16(3)19(29)14-26-35(32,33)22-10-6-7-11-25-22/h4-11,13,15-16,18,26H,12,14H2,1-3H3,(H,27,30)(H,28,31)/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403348
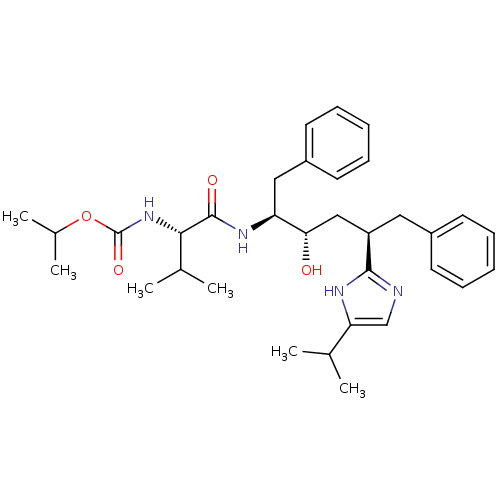
(CHEMBL421709)Show SMILES CC(C)OC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C33H46N4O4/c1-21(2)28-20-34-31(35-28)26(17-24-13-9-7-10-14-24)19-29(38)27(18-25-15-11-8-12-16-25)36-32(39)30(22(3)4)37-33(40)41-23(5)6/h7-16,20-23,26-27,29-30,38H,17-19H2,1-6H3,(H,34,35)(H,36,39)(H,37,40)/t26-,27+,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM93171

(Cathepsin Inhibitor, Column 2 Row 1)Show SMILES CC(C)C[C@H](NC(=O)c1ccc(cc1)C(F)(F)F)C(=O)NC(C)C(=O)CNC(=O)Cc1cccc(c1)-c1ccccn1 |r| Show InChI InChI=1S/C31H33F3N4O4/c1-19(2)15-26(38-29(41)22-10-12-24(13-11-22)31(32,33)34)30(42)37-20(3)27(39)18-36-28(40)17-21-7-6-8-23(16-21)25-9-4-5-14-35-25/h4-14,16,19-20,26H,15,17-18H2,1-3H3,(H,36,40)(H,37,42)(H,38,41)/t20?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
Inhibitors were assayed against human liver Cathepsin L and B. Inhibitors were also evaluated for inhibition against purified recombinant Cathepsin ... |
J Comb Chem 1: 207-15 (1999)
Article DOI: 10.1021/cc9800374
BindingDB Entry DOI: 10.7270/Q2HQ3XHJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50079596
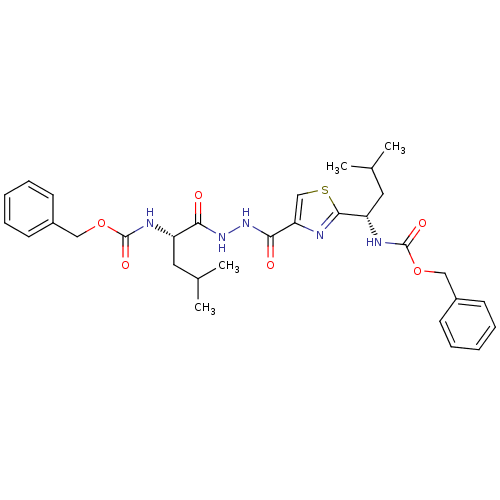
(((S)-1-{N'-[2-((S)-1-Benzyloxycarbonylamino-3-meth...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)c1csc(n1)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H39N5O6S/c1-20(2)15-24(33-30(39)41-17-22-11-7-5-8-12-22)27(37)35-36-28(38)26-19-43-29(32-26)25(16-21(3)4)34-31(40)42-18-23-13-9-6-10-14-23/h5-14,19-21,24-25H,15-18H2,1-4H3,(H,33,39)(H,34,40)(H,35,37)(H,36,38)/t24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human osteoclast cathepsin K |
Bioorg Med Chem Lett 9: 1907-10 (1999)
BindingDB Entry DOI: 10.7270/Q2M61JGJ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408515
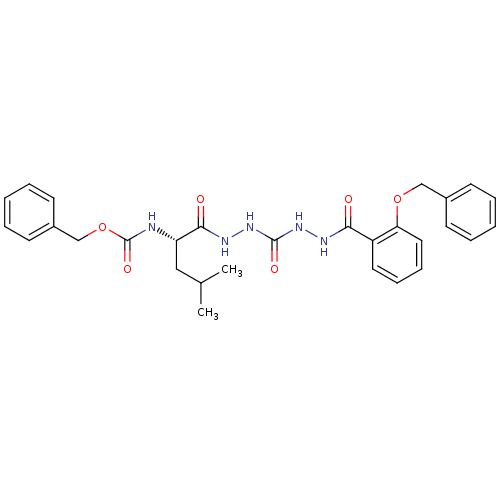
(CHEMBL338770)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)c1ccccc1OCc1ccccc1 Show InChI InChI=1S/C29H33N5O6/c1-20(2)17-24(30-29(38)40-19-22-13-7-4-8-14-22)27(36)32-34-28(37)33-31-26(35)23-15-9-10-16-25(23)39-18-21-11-5-3-6-12-21/h3-16,20,24H,17-19H2,1-2H3,(H,30,38)(H,31,35)(H,32,36)(H2,33,34,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50098576

(5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2cc(OCCN3CCOCC3)ccc2o1)C(=O)N[C@H]1CCCN(CC1=O)C(=O)Cc1cccc(c1)-c1ccccn1 Show InChI InChI=1S/C40H47N5O7/c1-27(2)21-34(43-40(49)37-25-30-24-31(11-12-36(30)52-37)51-20-17-44-15-18-50-19-16-44)39(48)42-33-10-6-14-45(26-35(33)46)38(47)23-28-7-5-8-29(22-28)32-9-3-4-13-41-32/h3-5,7-9,11-13,22,24-25,27,33-34H,6,10,14-21,23,26H2,1-2H3,(H,42,48)(H,43,49)/t33-,34-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin S |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50098578
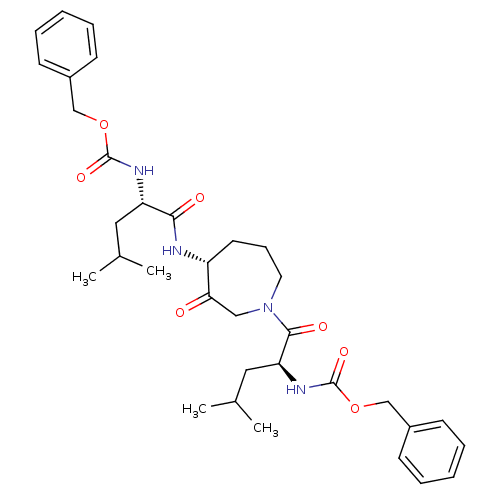
(CHEMBL286034 | {1-[4-(2-Benzyloxycarbonylamino-4-m...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H]1CCCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C34H46N4O7/c1-23(2)18-28(36-33(42)44-21-25-12-7-5-8-13-25)31(40)35-27-16-11-17-38(20-30(27)39)32(41)29(19-24(3)4)37-34(43)45-22-26-14-9-6-10-15-26/h5-10,12-15,23-24,27-29H,11,16-22H2,1-4H3,(H,35,40)(H,36,42)(H,37,43)/t27-,28+,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM93184
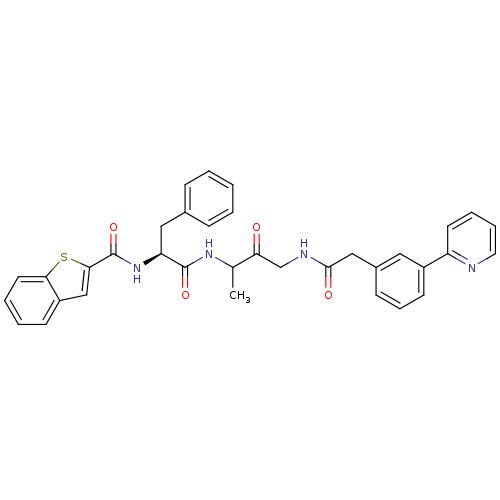
(Cathepsin Inhibitor, Column 6 Row 2)Show SMILES CC(NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cc2ccccc2s1)C(=O)CNC(=O)Cc1cccc(c1)-c1ccccn1 |r| Show InChI InChI=1S/C35H32N4O4S/c1-23(30(40)22-37-33(41)20-25-12-9-14-26(18-25)28-15-7-8-17-36-28)38-34(42)29(19-24-10-3-2-4-11-24)39-35(43)32-21-27-13-5-6-16-31(27)44-32/h2-18,21,23,29H,19-20,22H2,1H3,(H,37,41)(H,38,42)(H,39,43)/t23?,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
Inhibitors were assayed against human liver Cathepsin L and B. Inhibitors were also evaluated for inhibition against purified recombinant Cathepsin ... |
J Comb Chem 1: 207-15 (1999)
Article DOI: 10.1021/cc9800374
BindingDB Entry DOI: 10.7270/Q2HQ3XHJ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM19810

(CHEMBL118449 | benzyl N-[(1S)-1-({1-[(2S)-2-{[(ben...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50098575

(CHEMBL281086 | {1-[4-(2-Benzyloxycarbonylamino-4-m...)Show SMILES CC(C)C[C@@H](NC(=O)OCc1ccccc1)C(=O)NC1CCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C33H44N4O7/c1-22(2)17-27(35-32(41)43-20-24-11-7-5-8-12-24)30(39)34-26-15-16-37(19-29(26)38)31(40)28(18-23(3)4)36-33(42)44-21-25-13-9-6-10-14-25/h5-14,22-23,26-28H,15-21H2,1-4H3,(H,34,39)(H,35,41)(H,36,42)/t26?,27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin L |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin L
(Homo sapiens (Human)) | BDBM93184

(Cathepsin Inhibitor, Column 6 Row 2)Show SMILES CC(NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cc2ccccc2s1)C(=O)CNC(=O)Cc1cccc(c1)-c1ccccn1 |r| Show InChI InChI=1S/C35H32N4O4S/c1-23(30(40)22-37-33(41)20-25-12-9-14-26(18-25)28-15-7-8-17-36-28)38-34(42)29(19-24-10-3-2-4-11-24)39-35(43)32-21-27-13-5-6-16-31(27)44-32/h2-18,21,23,29H,19-20,22H2,1H3,(H,37,41)(H,38,42)(H,39,43)/t23?,29-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
Inhibitors were assayed against human liver Cathepsin L and B. Inhibitors were also evaluated for inhibition against purified recombinant Cathepsin ... |
J Comb Chem 1: 207-15 (1999)
Article DOI: 10.1021/cc9800374
BindingDB Entry DOI: 10.7270/Q2HQ3XHJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM93177
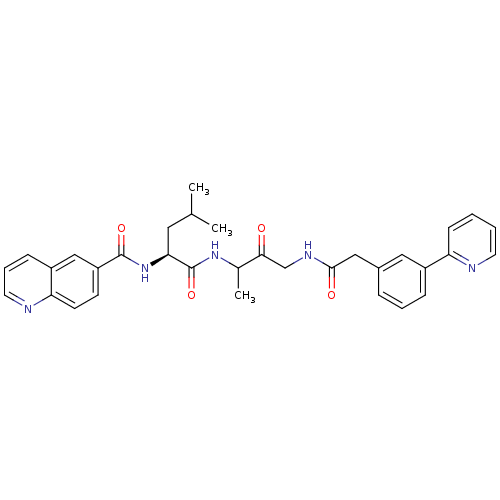
(Cathepsin Inhibitor, Column 4 Row 1)Show SMILES CC(C)C[C@H](NC(=O)c1ccc2ncccc2c1)C(=O)NC(C)C(=O)CNC(=O)Cc1cccc(c1)-c1ccccn1 |r| Show InChI InChI=1S/C33H35N5O4/c1-21(2)16-29(38-32(41)26-12-13-28-25(19-26)10-7-15-35-28)33(42)37-22(3)30(39)20-36-31(40)18-23-8-6-9-24(17-23)27-11-4-5-14-34-27/h4-15,17,19,21-22,29H,16,18,20H2,1-3H3,(H,36,40)(H,37,42)(H,38,41)/t22?,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
Inhibitors were assayed against human liver Cathepsin L and B. Inhibitors were also evaluated for inhibition against purified recombinant Cathepsin ... |
J Comb Chem 1: 207-15 (1999)
Article DOI: 10.1021/cc9800374
BindingDB Entry DOI: 10.7270/Q2HQ3XHJ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM19782

((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(2...)Show SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@@H](C)C(=O)CNS(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C24H28N4O6S/c1-15(2)12-18(28-24(31)21-13-17-8-4-5-9-20(17)34-21)23(30)27-16(3)19(29)14-26-35(32,33)22-10-6-7-11-25-22/h4-11,13,15-16,18,26H,12,14H2,1-3H3,(H,27,30)(H,28,31)/t16-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin S |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066650
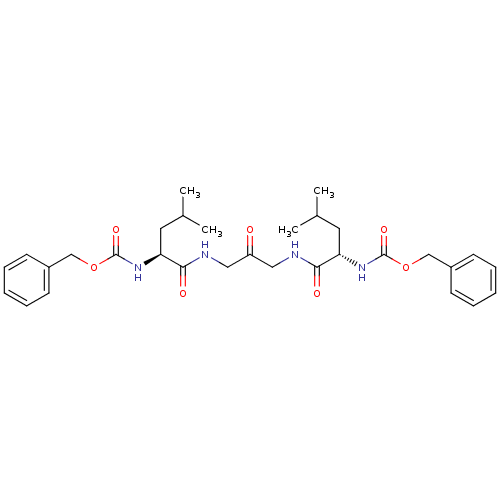
(1,3-BIS[[N-[(PHENYLMETHOXY)CARBONYL]-L-LEUCYL]AMIN...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NCC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H42N4O7/c1-21(2)15-26(34-30(39)41-19-23-11-7-5-8-12-23)28(37)32-17-25(36)18-33-29(38)27(16-22(3)4)35-31(40)42-20-24-13-9-6-10-14-24/h5-14,21-22,26-27H,15-20H2,1-4H3,(H,32,37)(H,33,38)(H,34,39)(H,35,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of Human cathepsin K |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066650

(1,3-BIS[[N-[(PHENYLMETHOXY)CARBONYL]-L-LEUCYL]AMIN...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NCC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H42N4O7/c1-21(2)15-26(34-30(39)41-19-23-11-7-5-8-12-23)28(37)32-17-25(36)18-33-29(38)27(16-22(3)4)35-31(40)42-20-24-13-9-6-10-14-24/h5-14,21-22,26-27H,15-20H2,1-4H3,(H,32,37)(H,33,38)(H,34,39)(H,35,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066650

(1,3-BIS[[N-[(PHENYLMETHOXY)CARBONYL]-L-LEUCYL]AMIN...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NCC(=O)CNC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H42N4O7/c1-21(2)15-26(34-30(39)41-19-23-11-7-5-8-12-23)28(37)32-17-25(36)18-33-29(38)27(16-22(3)4)35-31(40)42-20-24-13-9-6-10-14-24/h5-14,21-22,26-27H,15-20H2,1-4H3,(H,32,37)(H,33,38)(H,34,39)(H,35,40)/t26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory activity measured against cathepsin K. |
J Med Chem 41: 4567-76 (1998)
Article DOI: 10.1021/jm980249f
BindingDB Entry DOI: 10.7270/Q26974TN |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM93175
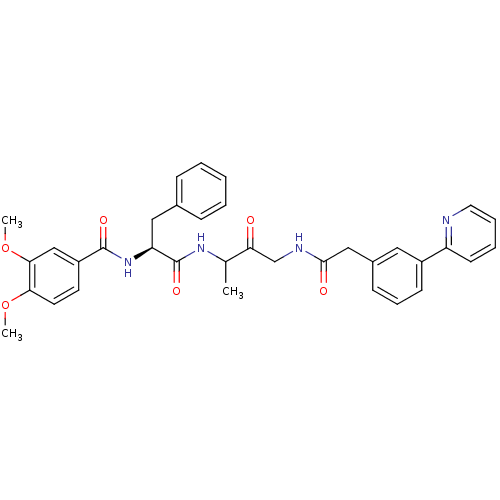
(Cathepsin Inhibitor, Column 3 Row 2)Show SMILES COc1ccc(cc1OC)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC(C)C(=O)CNC(=O)Cc1cccc(c1)-c1ccccn1 |r| Show InChI InChI=1S/C35H36N4O6/c1-23(30(40)22-37-33(41)20-25-12-9-13-26(18-25)28-14-7-8-17-36-28)38-35(43)29(19-24-10-5-4-6-11-24)39-34(42)27-15-16-31(44-2)32(21-27)45-3/h4-18,21,23,29H,19-20,22H2,1-3H3,(H,37,41)(H,38,43)(H,39,42)/t23?,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
SmithKline Beecham Pharmaceuticals
| Assay Description
Inhibitors were assayed against human liver Cathepsin L and B. Inhibitors were also evaluated for inhibition against purified recombinant Cathepsin ... |
J Comb Chem 1: 207-15 (1999)
Article DOI: 10.1021/cc9800374
BindingDB Entry DOI: 10.7270/Q2HQ3XHJ |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50066645
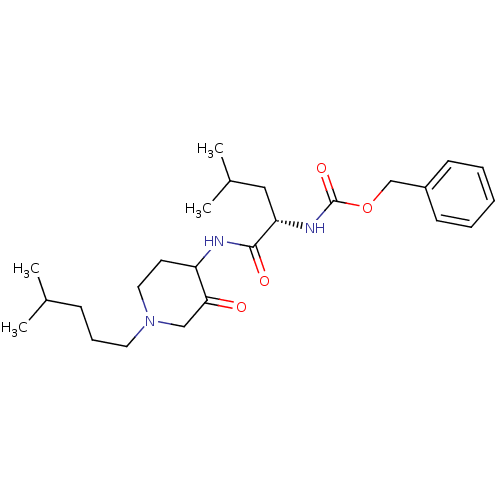
(CHEMBL432634 | {(S)-3-Methyl-1-[1-(4-methyl-pentyl...)Show SMILES CC(C)CCCN1CCC(NC(=O)[C@H](CC(C)C)NC(=O)OCc2ccccc2)C(=O)C1 Show InChI InChI=1S/C25H39N3O4/c1-18(2)9-8-13-28-14-12-21(23(29)16-28)26-24(30)22(15-19(3)4)27-25(31)32-17-20-10-6-5-7-11-20/h5-7,10-11,18-19,21-22H,8-9,12-17H2,1-4H3,(H,26,30)(H,27,31)/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K |
J Med Chem 41: 3563-7 (1998)
Article DOI: 10.1021/jm980295f
BindingDB Entry DOI: 10.7270/Q2G15ZZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin S
(Homo sapiens (Human)) | BDBM50098584

(CHEMBL31947 | {1-[4-(2-Benzyloxycarbonylamino-4-me...)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NC1CCCN(CC1=O)C(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C34H46N4O7/c1-23(2)18-28(36-33(42)44-21-25-12-7-5-8-13-25)31(40)35-27-16-11-17-38(20-30(27)39)32(41)29(19-24(3)4)37-34(43)45-22-26-14-9-6-10-15-26/h5-10,12-15,23-24,27-29H,11,16-22H2,1-4H3,(H,35,40)(H,36,42)(H,37,43)/t27?,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Human cathepsin S |
J Med Chem 44: 1380-95 (2001)
BindingDB Entry DOI: 10.7270/Q2QR4WDC |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50408517
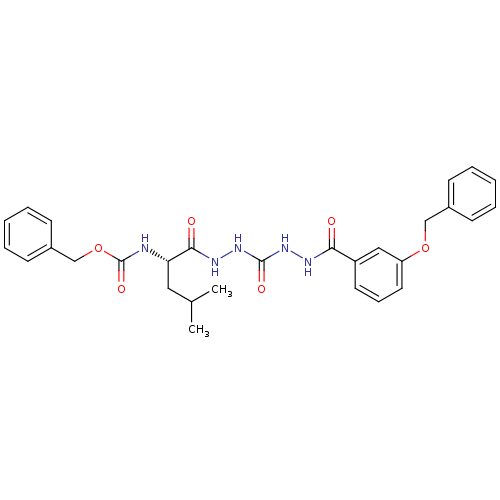
(CHEMBL340191)Show SMILES CC(C)C[C@H](NC(=O)OCc1ccccc1)C(=O)NNC(=O)NNC(=O)c1cccc(OCc2ccccc2)c1 |r| Show InChI InChI=1S/C29H33N5O6/c1-20(2)16-25(30-29(38)40-19-22-12-7-4-8-13-22)27(36)32-34-28(37)33-31-26(35)23-14-9-15-24(17-23)39-18-21-10-5-3-6-11-21/h3-15,17,20,25H,16,18-19H2,1-2H3,(H,30,38)(H,31,35)(H,32,36)(H2,33,34,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Relative binding affinity was measured for Cathepsin K |
J Med Chem 41: 3923-7 (1998)
Article DOI: 10.1021/jm980474x
BindingDB Entry DOI: 10.7270/Q2Q81F87 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data