 Found 1536 hits with Last Name = 'varnes' and Initial = 'jg'
Found 1536 hits with Last Name = 'varnes' and Initial = 'jg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM12693
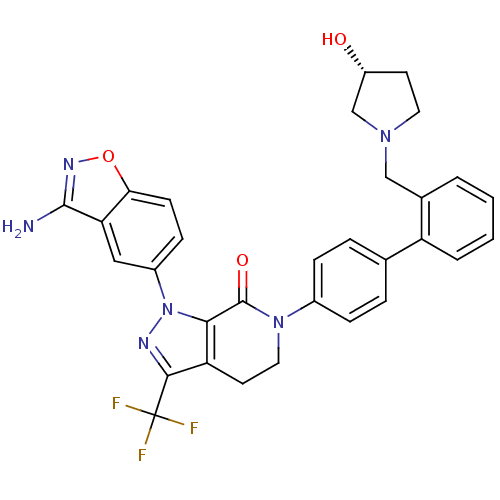
(1-(3-amino-1,2-benzoxazol-5-yl)-6-[4-(2-{[(3R)-3-h...)Show SMILES Nc1noc2ccc(cc12)-n1nc(c2CCN(C(=O)c12)c1ccc(cc1)-c1ccccc1CN1CC[C@@H](O)C1)C(F)(F)F |r| Show InChI InChI=1S/C31H27F3N6O3/c32-31(33,34)28-24-12-14-39(30(42)27(24)40(36-28)21-9-10-26-25(15-21)29(35)37-43-26)20-7-5-18(6-8-20)23-4-2-1-3-19(23)16-38-13-11-22(41)17-38/h1-10,15,22,41H,11-14,16-17H2,(H2,35,37)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228913
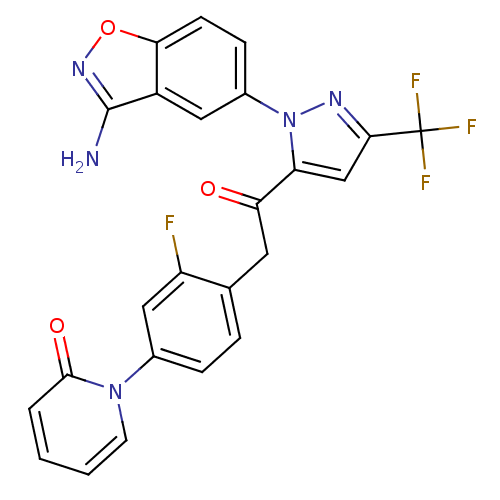
(1-(4-{2-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifl...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(F)(F)F Show InChI InChI=1S/C24H15F4N5O3/c25-17-11-14(32-8-2-1-3-22(32)35)5-4-13(17)9-19(34)18-12-21(24(26,27)28)30-33(18)15-6-7-20-16(10-15)23(29)31-36-20/h1-8,10-12H,9H2,(H2,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228907

(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(CC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C26H18F4N4O4S/c1-39(36,37)23-5-3-2-4-17(23)14-6-7-15(19(27)10-14)11-21(35)20-13-24(26(28,29)30)32-34(20)16-8-9-22-18(12-16)25(31)33-38-22/h2-10,12-13H,11H2,1H3,(H2,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12659
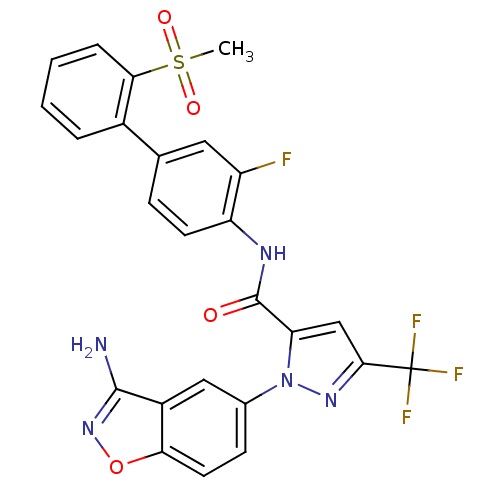
(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-5-[...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H17F4N5O4S/c1-39(36,37)21-5-3-2-4-15(21)13-6-8-18(17(26)10-13)31-24(35)19-12-22(25(27,28)29)32-34(19)14-7-9-20-16(11-14)23(30)33-38-20/h2-12H,1H3,(H2,30,33)(H,31,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228926

(1-(3-fluoro-4-(2-(1-(4-methoxyphenyl)-3-(trifluoro...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(F)(F)F Show InChI InChI=1S/C24H17F4N3O3/c1-34-18-9-7-16(8-10-18)31-20(14-22(29-31)24(26,27)28)21(32)12-15-5-6-17(13-19(15)25)30-11-3-2-4-23(30)33/h2-11,13-14H,12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228914
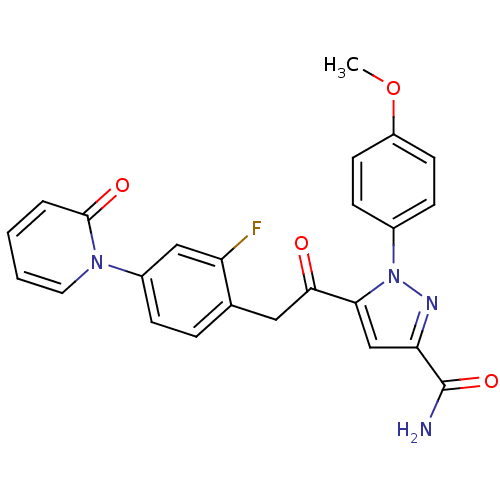
(5-(2-(2-fluoro-4-(2-oxopyridin-1(2H)-yl)phenyl)ace...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-n1ccccc1=O)C(N)=O Show InChI InChI=1S/C24H19FN4O4/c1-33-18-9-7-16(8-10-18)29-21(14-20(27-29)24(26)32)22(30)12-15-5-6-17(13-19(15)25)28-11-3-2-4-23(28)31/h2-11,13-14H,12H2,1H3,(H2,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228909
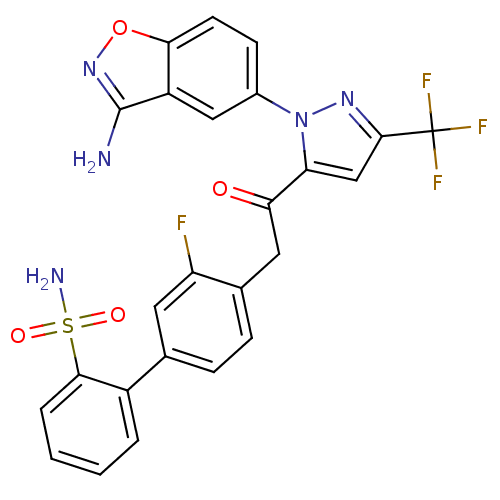
(4'-{2-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluo...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)Cc1ccc(cc1F)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H17F4N5O4S/c26-18-9-13(16-3-1-2-4-22(16)39(31,36)37)5-6-14(18)10-20(35)19-12-23(25(27,28)29)32-34(19)15-7-8-21-17(11-15)24(30)33-38-21/h1-9,11-12H,10H2,(H2,30,33)(H2,31,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12676

(1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...)Show SMILES CN(C)Cc1nccn1-c1ccc(NC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H20F4N8O2/c1-34(2)12-21-30-7-8-35(21)13-3-5-17(16(25)10-13)31-23(37)18-11-20(24(26,27)28)32-36(18)14-4-6-19-15(9-14)22(29)33-38-19/h3-11H,12H2,1-2H3,(H2,29,33)(H,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224712

(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-oxopyridi...)Show SMILES NC(=O)c1cc(C(=O)N2CCc3cc(ccc23)-n2ccccc2=O)n(n1)-c1ccc2onc(N)c2c1 Show InChI InChI=1S/C25H19N7O4/c26-23-17-12-16(5-7-21(17)36-29-23)32-20(13-18(28-32)24(27)34)25(35)31-10-8-14-11-15(4-6-19(14)31)30-9-2-1-3-22(30)33/h1-7,9,11-13H,8,10H2,(H2,26,29)(H2,27,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228912
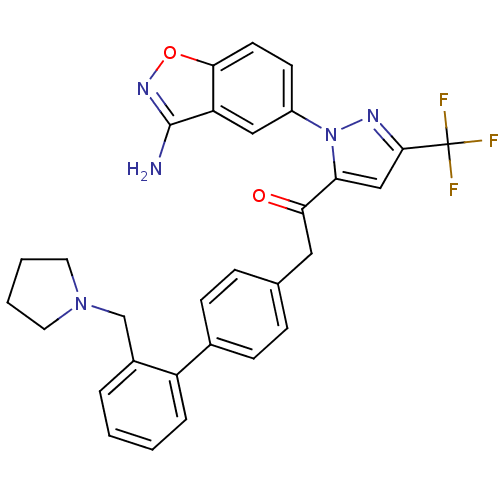
(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)Cc1ccc(cc1)-c1ccccc1CN1CCCC1)C(F)(F)F Show InChI InChI=1S/C30H26F3N5O2/c31-30(32,33)28-17-25(38(35-28)22-11-12-27-24(16-22)29(34)36-40-27)26(39)15-19-7-9-20(10-8-19)23-6-2-1-5-21(23)18-37-13-3-4-14-37/h1-2,5-12,16-17H,3-4,13-15,18H2,(H2,34,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228922

(1-(4-{2-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifl...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)Cc1ccc(cc1)N1CCCCC1=O)C(F)(F)F Show InChI InChI=1S/C24H20F3N5O3/c25-24(26,27)21-13-18(32(29-21)16-8-9-20-17(12-16)23(28)30-35-20)19(33)11-14-4-6-15(7-5-14)31-10-2-1-3-22(31)34/h4-9,12-13H,1-3,10-11H2,(H2,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228919
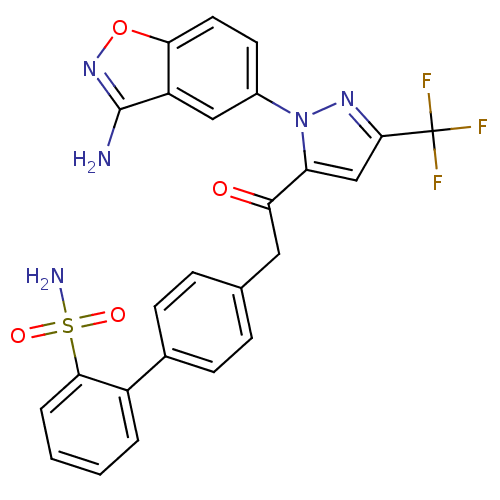
(4'-{2-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluo...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)Cc1ccc(cc1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H18F3N5O4S/c26-25(27,28)23-13-19(33(31-23)16-9-10-21-18(12-16)24(29)32-37-21)20(34)11-14-5-7-15(8-6-14)17-3-1-2-4-22(17)38(30,35)36/h1-10,12-13H,11H2,(H2,29,32)(H2,30,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228917
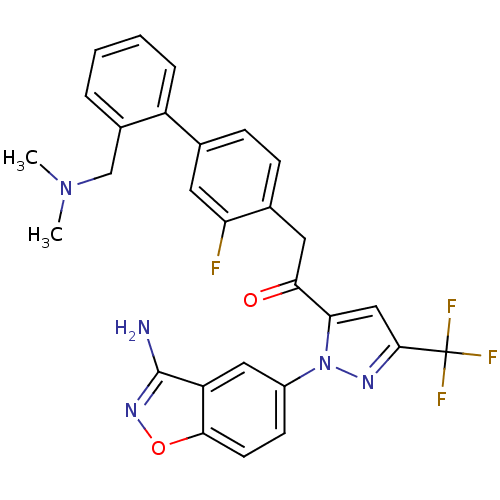
(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES CN(C)Cc1ccccc1-c1ccc(CC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C28H23F4N5O2/c1-36(2)15-18-5-3-4-6-20(18)16-7-8-17(22(29)11-16)12-24(38)23-14-26(28(30,31)32)34-37(23)19-9-10-25-21(13-19)27(33)35-39-25/h3-11,13-14H,12,15H2,1-2H3,(H2,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224704
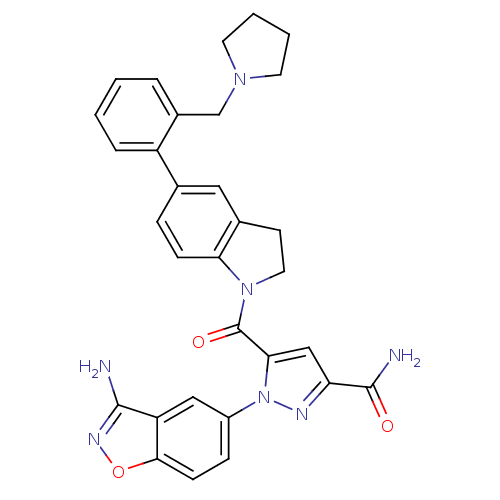
(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-(pyrrolid...)Show SMILES NC(=O)c1cc(C(=O)N2CCc3cc(ccc23)-c2ccccc2CN2CCCC2)n(n1)-c1ccc2onc(N)c2c1 Show InChI InChI=1S/C31H29N7O3/c32-29-24-16-22(8-10-28(24)41-35-29)38-27(17-25(34-38)30(33)39)31(40)37-14-11-20-15-19(7-9-26(20)37)23-6-2-1-5-21(23)18-36-12-3-4-13-36/h1-2,5-10,15-17H,3-4,11-14,18H2,(H2,32,35)(H2,33,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228924

(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(CC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)cc1 Show InChI InChI=1S/C26H19F3N4O4S/c1-38(35,36)23-5-3-2-4-18(23)16-8-6-15(7-9-16)12-21(34)20-14-24(26(27,28)29)31-33(20)17-10-11-22-19(13-17)25(30)32-37-22/h2-11,13-14H,12H2,1H3,(H2,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224711

(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-(methylsu...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc2N(CCc2c1)C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(N)=O Show InChI InChI=1S/C27H22N6O5S/c1-39(36,37)24-5-3-2-4-18(24)15-6-8-21-16(12-15)10-11-32(21)27(35)22-14-20(26(29)34)30-33(22)17-7-9-23-19(13-17)25(28)31-38-23/h2-9,12-14H,10-11H2,1H3,(H2,28,31)(H2,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228915
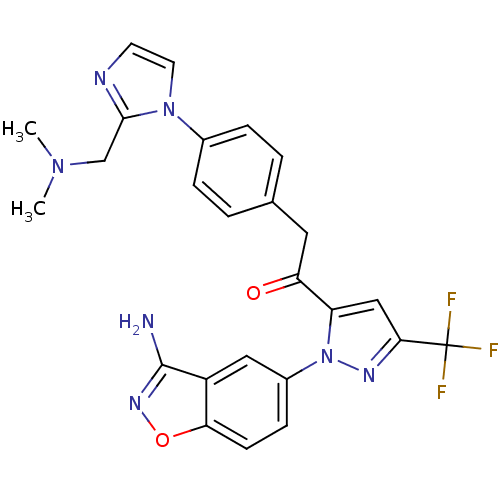
(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES CN(C)Cc1nccn1-c1ccc(CC(=O)c2cc(nn2-c2ccc3onc(N)c3c2)C(F)(F)F)cc1 Show InChI InChI=1S/C25H22F3N7O2/c1-33(2)14-23-30-9-10-34(23)16-5-3-15(4-6-16)11-20(36)19-13-22(25(26,27)28)31-35(19)17-7-8-21-18(12-17)24(29)32-37-21/h3-10,12-13H,11,14H2,1-2H3,(H2,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228906

(1-(4-(2-(1-(4-methoxyphenyl)-3-(trifluoromethyl)-1...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1)N1CCCCC1=O)C(F)(F)F Show InChI InChI=1S/C24H22F3N3O3/c1-33-19-11-9-18(10-12-19)30-20(15-22(28-30)24(25,26)27)21(31)14-16-5-7-17(8-6-16)29-13-3-2-4-23(29)32/h5-12,15H,2-4,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224697
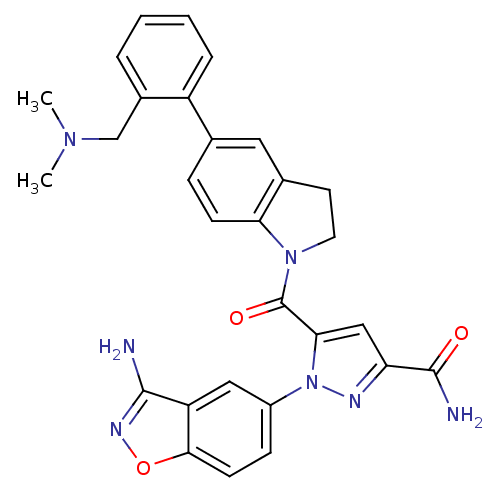
(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-((dimethy...)Show SMILES CN(C)Cc1ccccc1-c1ccc2N(CCc2c1)C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(N)=O Show InChI InChI=1S/C29H27N7O3/c1-34(2)16-19-5-3-4-6-21(19)17-7-9-24-18(13-17)11-12-35(24)29(38)25-15-23(28(31)37)32-36(25)20-8-10-26-22(14-20)27(30)33-39-26/h3-10,13-15H,11-12,16H2,1-2H3,(H2,30,33)(H2,31,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224693
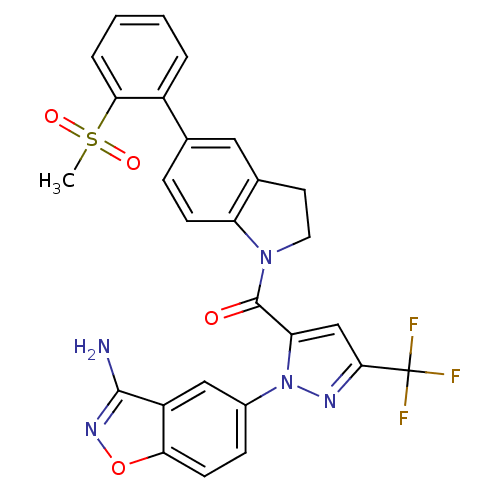
((1-(3-aminobenzo[d]isoxazol-5-yl)-3-(trifluorometh...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc2N(CCc2c1)C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(F)(F)F Show InChI InChI=1S/C27H20F3N5O4S/c1-40(37,38)23-5-3-2-4-18(23)15-6-8-20-16(12-15)10-11-34(20)26(36)21-14-24(27(28,29)30)32-35(21)17-7-9-22-19(13-17)25(31)33-39-22/h2-9,12-14H,10-11H2,1H3,(H2,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228921
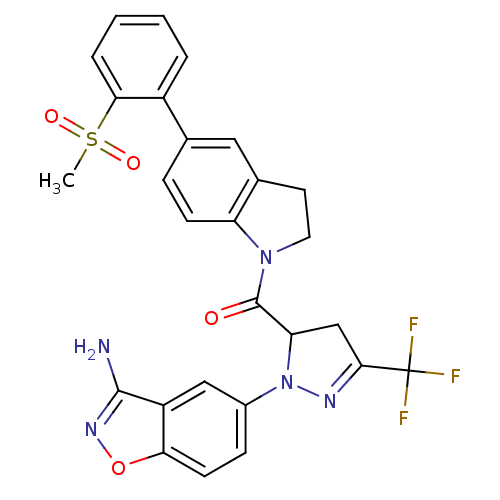
((1-(3-aminobenzo[d]isoxazol-5-yl)-3-(trifluorometh...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc2N(CCc2c1)C(=O)C1CC(=NN1c1ccc2onc(N)c2c1)C(F)(F)F |c:26| Show InChI InChI=1S/C27H22F3N5O4S/c1-40(37,38)23-5-3-2-4-18(23)15-6-8-20-16(12-15)10-11-34(20)26(36)21-14-24(27(28,29)30)32-35(21)17-7-9-22-19(13-17)25(31)33-39-22/h2-9,12-13,21H,10-11,14H2,1H3,(H2,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224705

(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-((dimethy...)Show SMILES CN(C)Cc1nccn1-c1ccc2N(CCc2c1)C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(N)=O Show InChI InChI=1S/C26H25N9O3/c1-32(2)14-23-29-8-10-33(23)16-3-5-20-15(11-16)7-9-34(20)26(37)21-13-19(25(28)36)30-35(21)17-4-6-22-18(12-17)24(27)31-38-22/h3-6,8,10-13H,7,9,14H2,1-2H3,(H2,27,31)(H2,28,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228925
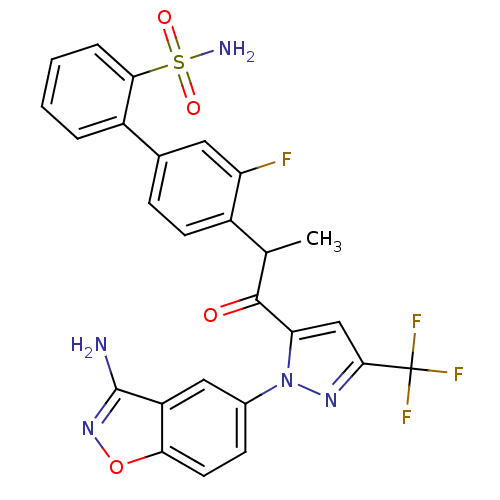
(4'-{2-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluo...)Show SMILES CC(C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(F)(F)F)c1ccc(cc1F)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C26H19F4N5O4S/c1-13(16-8-6-14(10-19(16)27)17-4-2-3-5-22(17)40(32,37)38)24(36)20-12-23(26(28,29)30)33-35(20)15-7-9-21-18(11-15)25(31)34-39-21/h2-13H,1H3,(H2,31,34)(H2,32,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224698
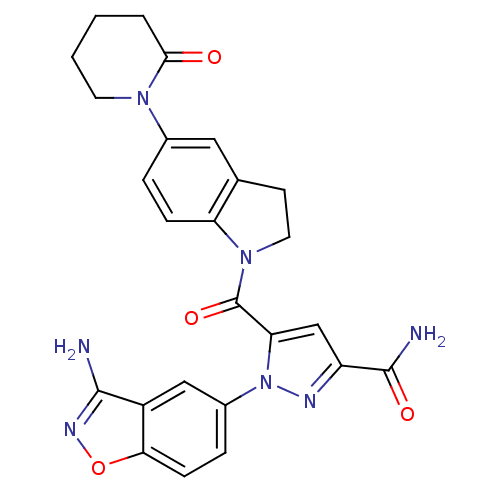
(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-oxopiperi...)Show SMILES NC(=O)c1cc(C(=O)N2CCc3cc(ccc23)N2CCCCC2=O)n(n1)-c1ccc2onc(N)c2c1 Show InChI InChI=1S/C25H23N7O4/c26-23-17-12-16(5-7-21(17)36-29-23)32-20(13-18(28-32)24(27)34)25(35)31-10-8-14-11-15(4-6-19(14)31)30-9-2-1-3-22(30)33/h4-7,11-13H,1-3,8-10H2,(H2,26,29)(H2,27,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224696
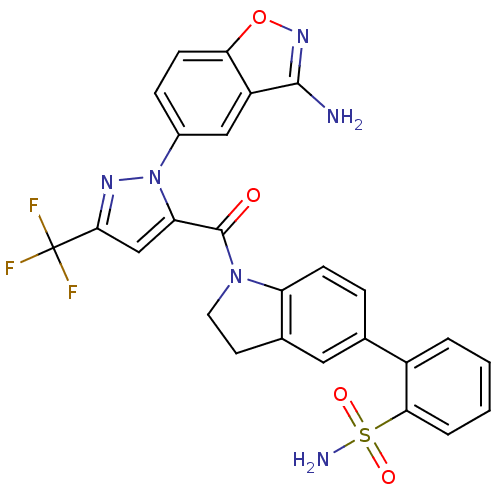
(2-(1-(1-(3-aminobenzo[d]isoxazol-5-yl)-3-(trifluor...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)N1CCc2cc(ccc12)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C26H19F3N6O4S/c27-26(28,29)23-13-20(35(32-23)16-6-8-21-18(12-16)24(30)33-39-21)25(36)34-10-9-15-11-14(5-7-19(15)34)17-3-1-2-4-22(17)40(31,37)38/h1-8,11-13H,9-10H2,(H2,30,33)(H2,31,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228911
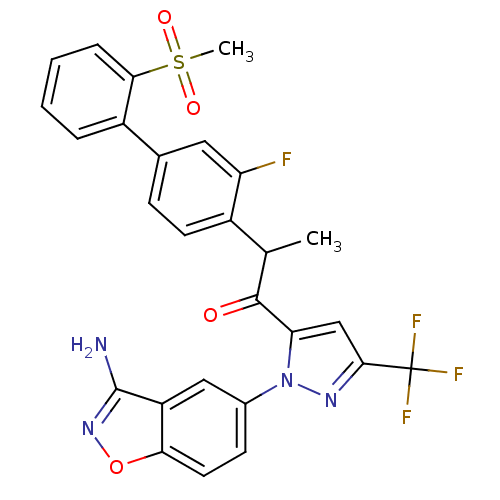
(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES CC(C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(F)(F)F)c1ccc(cc1F)-c1ccccc1S(C)(=O)=O Show InChI InChI=1S/C27H20F4N4O4S/c1-14(17-9-7-15(11-20(17)28)18-5-3-4-6-23(18)40(2,37)38)25(36)21-13-24(27(29,30)31)33-35(21)16-8-10-22-19(12-16)26(32)34-39-22/h3-14H,1-2H3,(H2,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228910

(2-[2'-((S)-3-hydroxy-pyrrolidin-1-ylmethyl)-biphen...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1)-c1ccccc1CN1CC[C@H](O)C1)C(F)(F)F Show InChI InChI=1S/C30H28F3N3O3/c1-39-25-12-10-23(11-13-25)36-27(17-29(34-36)30(31,32)33)28(38)16-20-6-8-21(9-7-20)26-5-3-2-4-22(26)18-35-15-14-24(37)19-35/h2-13,17,24,37H,14-16,18-19H2,1H3/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228920

(1-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluorome...)Show SMILES CC(C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(F)(F)F)c1ccc(cc1)-c1ccccc1S(C)(=O)=O Show InChI InChI=1S/C27H21F3N4O4S/c1-15(16-7-9-17(10-8-16)19-5-3-4-6-23(19)39(2,36)37)25(35)21-14-24(27(28,29)30)32-34(21)18-11-12-22-20(13-18)26(31)33-38-22/h3-15H,1-2H3,(H2,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224699
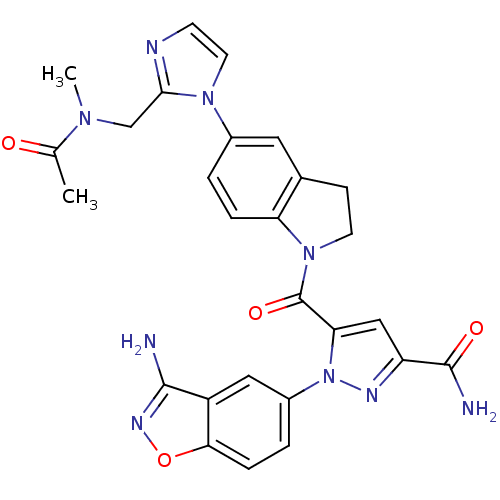
(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-((N-methy...)Show SMILES CN(Cc1nccn1-c1ccc2N(CCc2c1)C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(N)=O)C(C)=O Show InChI InChI=1S/C27H25N9O4/c1-15(37)33(2)14-24-30-8-10-34(24)17-3-5-21-16(11-17)7-9-35(21)27(39)22-13-20(26(29)38)31-36(22)18-4-6-23-19(12-18)25(28)32-40-23/h3-6,8,10-13H,7,9,14H2,1-2H3,(H2,28,32)(H2,29,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224708

((1-(3-aminobenzo[d]isoxazol-5-yl)-3-(trifluorometh...)Show SMILES Nc1noc2ccc(cc12)-n1nc(cc1C(=O)N1CCc2cc(ccc12)-c1ccccc1CN1CCCC1)C(F)(F)F Show InChI InChI=1S/C31H27F3N6O2/c32-31(33,34)28-17-26(40(36-28)22-8-10-27-24(16-22)29(35)37-42-27)30(41)39-14-11-20-15-19(7-9-25(20)39)23-6-2-1-5-21(23)18-38-12-3-4-13-38/h1-2,5-10,15-17H,3-4,11-14,18H2,(H2,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224703

(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-(dimethyl...)Show SMILES CN(C)CC(=O)N(C)c1ccc2N(CCc2c1)C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(N)=O Show InChI InChI=1S/C25H26N8O4/c1-30(2)13-22(34)31(3)15-4-6-19-14(10-15)8-9-32(19)25(36)20-12-18(24(27)35)28-33(20)16-5-7-21-17(11-16)23(26)29-37-21/h4-7,10-12H,8-9,13H2,1-3H3,(H2,26,29)(H2,27,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224700

(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-((N-methy...)Show SMILES CN(Cc1nccn1-c1ccc2N(CCc2c1)C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(N)=O)S(C)(=O)=O Show InChI InChI=1S/C26H25N9O5S/c1-32(41(2,38)39)14-23-29-8-10-33(23)16-3-5-20-15(11-16)7-9-34(20)26(37)21-13-19(25(28)36)30-35(21)17-4-6-22-18(12-17)24(27)31-40-22/h3-6,8,10-13H,7,9,14H2,1-2H3,(H2,27,31)(H2,28,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228908
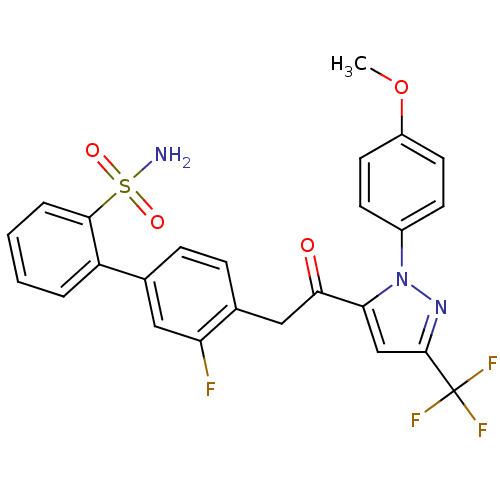
(3'-fluoro-4'-{2-[2-(4-methoxy-phenyl)-5-trifluorom...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1F)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H19F4N3O4S/c1-36-18-10-8-17(9-11-18)32-21(14-24(31-32)25(27,28)29)22(33)13-16-7-6-15(12-20(16)26)19-4-2-3-5-23(19)37(30,34)35/h2-12,14H,13H2,1H3,(H2,30,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50205861
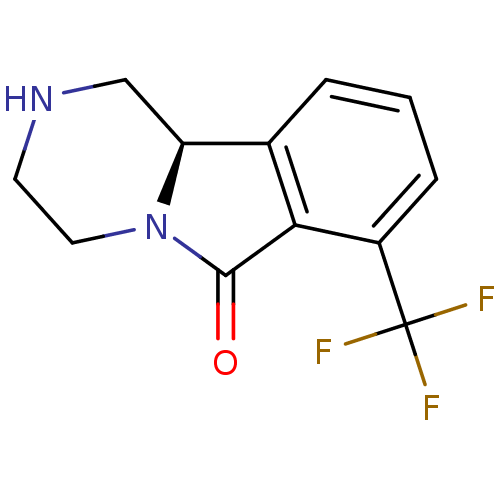
((R)-1,3,4,10b-tetrahydro-7-trifluoromethylpyrazino...)Show InChI InChI=1S/C12H11F3N2O/c13-12(14,15)8-3-1-2-7-9-6-16-4-5-17(9)11(18)10(7)8/h1-3,9,16H,4-6H2/t9-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2C expressed in HEK293E cells |
J Med Chem 50: 1365-79 (2007)
Article DOI: 10.1021/jm0612968
BindingDB Entry DOI: 10.7270/Q2XP74M8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50205871
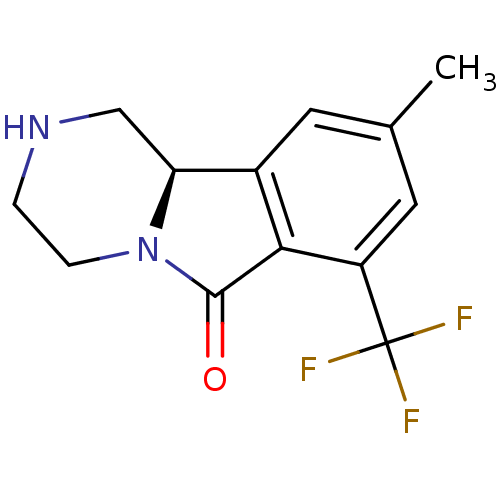
((R)-9-methyl-1,3,4,10b-tetrahydro-7-trifluoromethy...)Show InChI InChI=1S/C13H13F3N2O/c1-7-4-8-10-6-17-2-3-18(10)12(19)11(8)9(5-7)13(14,15)16/h4-5,10,17H,2-3,6H2,1H3/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2C expressed in HEK293E cells |
J Med Chem 50: 1365-79 (2007)
Article DOI: 10.1021/jm0612968
BindingDB Entry DOI: 10.7270/Q2XP74M8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228923
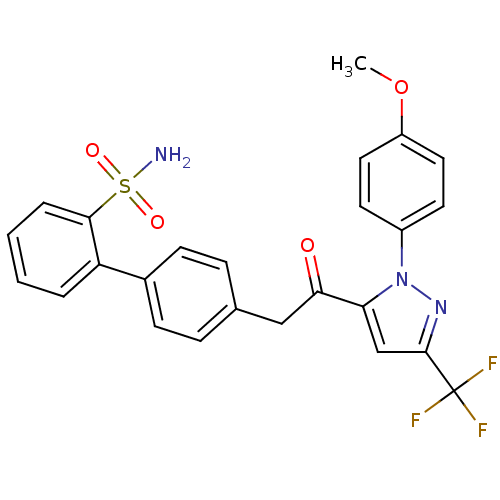
(4'-{2-[2-(4-methoxy-phenyl)-5-trifluoromethyl-2H-p...)Show SMILES COc1ccc(cc1)-n1nc(cc1C(=O)Cc1ccc(cc1)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C25H20F3N3O4S/c1-35-19-12-10-18(11-13-19)31-21(15-24(30-31)25(26,27)28)22(32)14-16-6-8-17(9-7-16)20-4-2-3-5-23(20)36(29,33)34/h2-13,15H,14H2,1H3,(H2,29,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224714
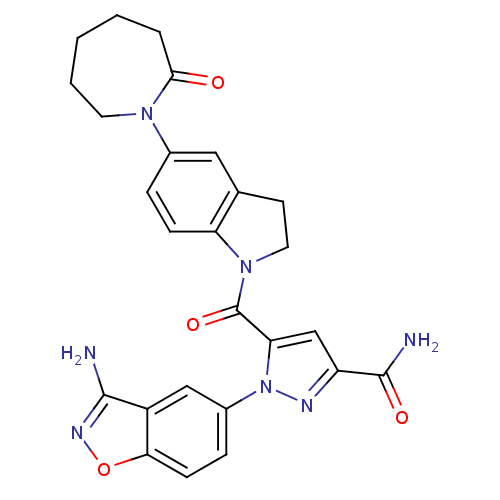
(1-(3-aminobenzo[d]isoxazol-5-yl)-5-(5-(2-oxoazepan...)Show SMILES NC(=O)c1cc(C(=O)N2CCc3cc(ccc23)N2CCCCCC2=O)n(n1)-c1ccc2onc(N)c2c1 Show InChI InChI=1S/C26H25N7O4/c27-24-18-13-17(6-8-22(18)37-30-24)33-21(14-19(29-33)25(28)35)26(36)32-11-9-15-12-16(5-7-20(15)32)31-10-3-1-2-4-23(31)34/h5-8,12-14H,1-4,9-11H2,(H2,27,30)(H2,28,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001915
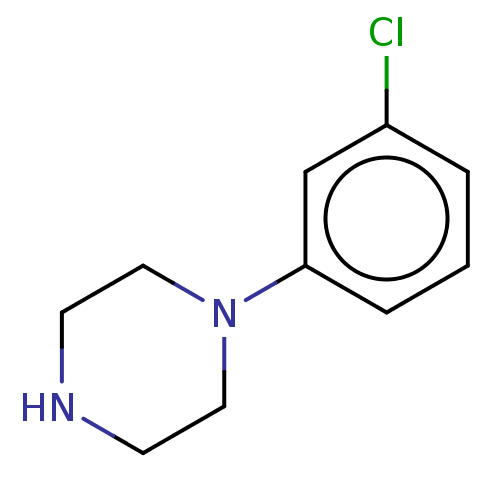
(1-(3-chlorophenyl)piperazine | CHEMBL478 | m-Chlor...)Show InChI InChI=1S/C10H13ClN2/c11-9-2-1-3-10(8-9)13-6-4-12-5-7-13/h1-3,8,12H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2C expressed in HEK293E cells |
J Med Chem 50: 1365-79 (2007)
Article DOI: 10.1021/jm0612968
BindingDB Entry DOI: 10.7270/Q2XP74M8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50205875
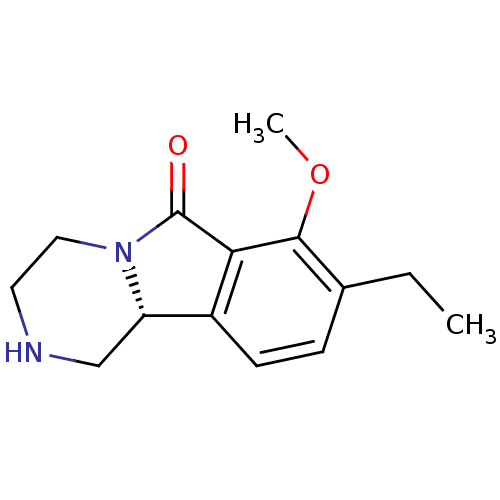
((R)-8-ethyl-7-methoxy-1,3,4,10b-tetrahydropyrazino...)Show InChI InChI=1S/C14H18N2O2/c1-3-9-4-5-10-11-8-15-6-7-16(11)14(17)12(10)13(9)18-2/h4-5,11,15H,3,6-8H2,1-2H3/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT2B expressed in HEK293E cells |
J Med Chem 50: 1365-79 (2007)
Article DOI: 10.1021/jm0612968
BindingDB Entry DOI: 10.7270/Q2XP74M8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50205864
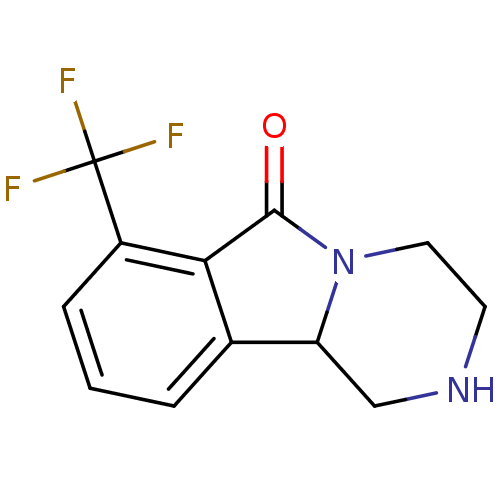
((+/-)-1,3,4,10b-tetrahydro-7-trifluoromethylpyrazi...)Show InChI InChI=1S/C12H11F3N2O/c13-12(14,15)8-3-1-2-7-9-6-16-4-5-17(9)11(18)10(7)8/h1-3,9,16H,4-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2C expressed in HEK293E cells |
J Med Chem 50: 1365-79 (2007)
Article DOI: 10.1021/jm0612968
BindingDB Entry DOI: 10.7270/Q2XP74M8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50224694

((1-(3-aminobenzo[d]isoxazol-5-yl)-3-(trifluorometh...)Show SMILES CN(C)Cc1ccccc1-c1ccc2N(CCc2c1)C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(F)(F)F Show InChI InChI=1S/C29H25F3N6O2/c1-36(2)16-19-5-3-4-6-21(19)17-7-9-23-18(13-17)11-12-37(23)28(39)24-15-26(29(30,31)32)34-38(24)20-8-10-25-22(14-20)27(33)35-40-25/h3-10,13-15H,11-12,16H2,1-2H3,(H2,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 17: 6481-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.091
BindingDB Entry DOI: 10.7270/Q2GX4B9Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50205895

((+/-)-1,3,4,10b-tetrahydro-7-trifluoromethoxypyraz...)Show InChI InChI=1S/C12H11F3N2O2/c13-12(14,15)19-9-3-1-2-7-8-6-16-4-5-17(8)11(18)10(7)9/h1-3,8,16H,4-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2C expressed in HEK293E cells |
J Med Chem 50: 1365-79 (2007)
Article DOI: 10.1021/jm0612968
BindingDB Entry DOI: 10.7270/Q2XP74M8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50205882
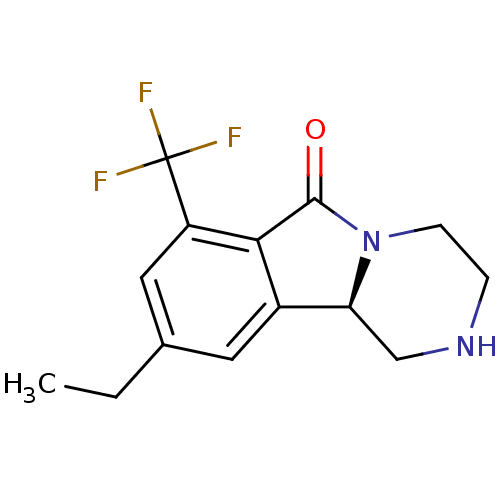
((R)-9-ethyl-1,3,4,10b-tetrahydro-7-trifluoromethyl...)Show InChI InChI=1S/C14H15F3N2O/c1-2-8-5-9-11-7-18-3-4-19(11)13(20)12(9)10(6-8)14(15,16)17/h5-6,11,18H,2-4,7H2,1H3/t11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2C expressed in HEK293E cells |
J Med Chem 50: 1365-79 (2007)
Article DOI: 10.1021/jm0612968
BindingDB Entry DOI: 10.7270/Q2XP74M8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50205877
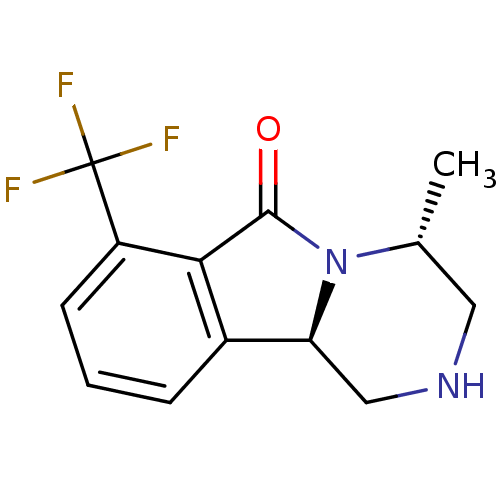
((4S,10bR)-4-methyl-1,3,4,10b-tetrahydro-7-trifluor...)Show InChI InChI=1S/C13H13F3N2O/c1-7-5-17-6-10-8-3-2-4-9(13(14,15)16)11(8)12(19)18(7)10/h2-4,7,10,17H,5-6H2,1H3/t7-,10+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2C expressed in HEK293E cells |
J Med Chem 50: 1365-79 (2007)
Article DOI: 10.1021/jm0612968
BindingDB Entry DOI: 10.7270/Q2XP74M8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50228918
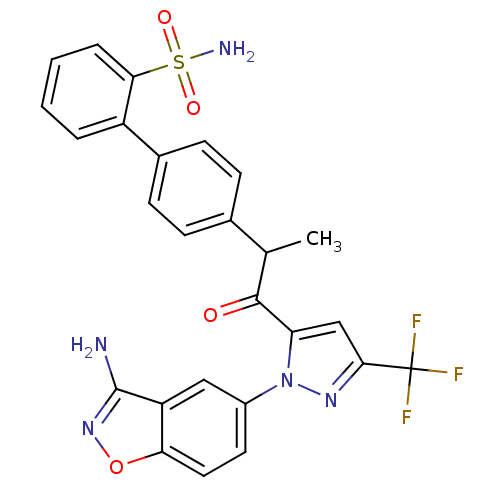
(4'-{2-[2-(3-amino-benzo[d]isoxazol-5-yl)-5-trifluo...)Show SMILES CC(C(=O)c1cc(nn1-c1ccc2onc(N)c2c1)C(F)(F)F)c1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C26H20F3N5O4S/c1-14(15-6-8-16(9-7-15)18-4-2-3-5-22(18)39(31,36)37)24(35)20-13-23(26(27,28)29)32-34(20)17-10-11-21-19(12-17)25(30)33-38-21/h2-14H,1H3,(H2,30,33)(H2,31,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 749-54 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.040
BindingDB Entry DOI: 10.7270/Q2DV1KQS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50205899
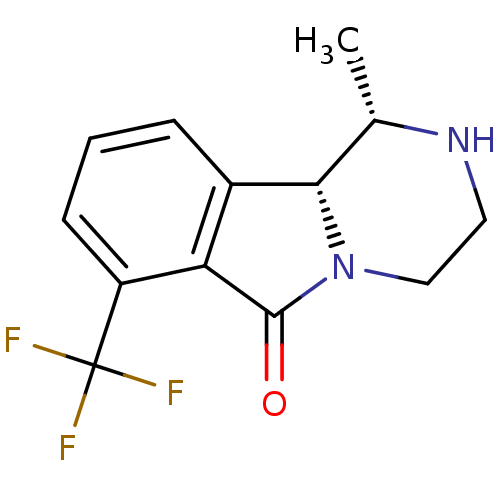
((1S,10bR)-1-methyl-1,3,4,10b-tetrahydro-7-trifluor...)Show InChI InChI=1S/C13H13F3N2O/c1-7-11-8-3-2-4-9(13(14,15)16)10(8)12(19)18(11)6-5-17-7/h2-4,7,11,17H,5-6H2,1H3/t7-,11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT2B expressed in HEK293E cells |
J Med Chem 50: 1365-79 (2007)
Article DOI: 10.1021/jm0612968
BindingDB Entry DOI: 10.7270/Q2XP74M8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50001915

(1-(3-chlorophenyl)piperazine | CHEMBL478 | m-Chlor...)Show InChI InChI=1S/C10H13ClN2/c11-9-2-1-3-10(8-9)13-6-4-12-5-7-13/h1-3,8,12H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT2B expressed in HEK293E cells |
J Med Chem 50: 1365-79 (2007)
Article DOI: 10.1021/jm0612968
BindingDB Entry DOI: 10.7270/Q2XP74M8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50205899

((1S,10bR)-1-methyl-1,3,4,10b-tetrahydro-7-trifluor...)Show InChI InChI=1S/C13H13F3N2O/c1-7-11-8-3-2-4-9(13(14,15)16)10(8)12(19)18(11)6-5-17-7/h2-4,7,11,17H,5-6H2,1H3/t7-,11-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2C expressed in HEK293E cells |
J Med Chem 50: 1365-79 (2007)
Article DOI: 10.1021/jm0612968
BindingDB Entry DOI: 10.7270/Q2XP74M8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50205877

((4S,10bR)-4-methyl-1,3,4,10b-tetrahydro-7-trifluor...)Show InChI InChI=1S/C13H13F3N2O/c1-7-5-17-6-10-8-3-2-4-9(13(14,15)16)11(8)12(19)18(7)10/h2-4,7,10,17H,5-6H2,1H3/t7-,10+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT2B expressed in HEK293E cells |
J Med Chem 50: 1365-79 (2007)
Article DOI: 10.1021/jm0612968
BindingDB Entry DOI: 10.7270/Q2XP74M8 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50205888

((R)-9-(1-methylcyclopropyl)-1,3,4,10b-tetrahydro-7...)Show SMILES CC1(CC1)c1cc2[C@@H]3CNCCN3C(=O)c2c(c1)C(F)(F)F Show InChI InChI=1S/C16H17F3N2O/c1-15(2-3-15)9-6-10-12-8-20-4-5-21(12)14(22)13(10)11(7-9)16(17,18)19/h6-7,12,20H,2-5,8H2,1H3/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human recombinant 5HT2B expressed in HEK293E cells |
J Med Chem 50: 1365-79 (2007)
Article DOI: 10.1021/jm0612968
BindingDB Entry DOI: 10.7270/Q2XP74M8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data