

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20079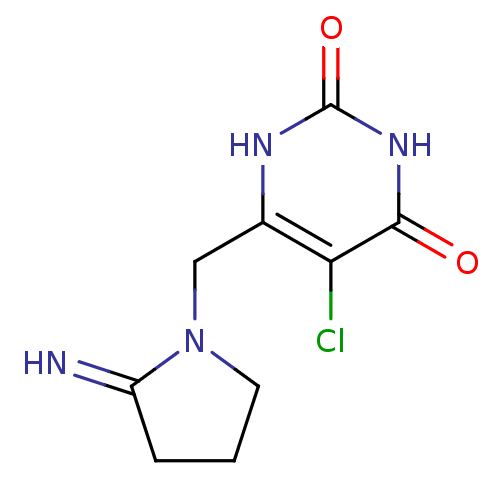 (5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.30 | -52.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20079 (5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20061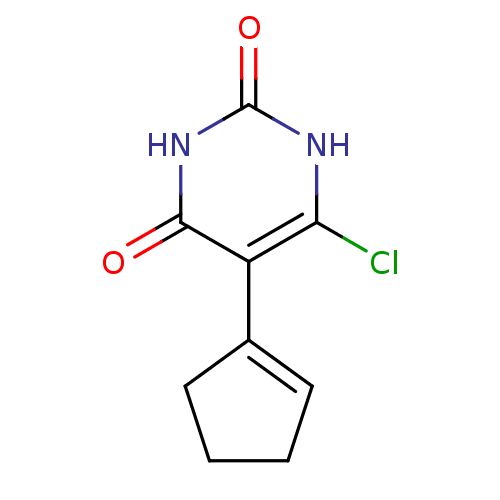 (5-Substituted-6-chlorouracil, 7a | 6-chloro-5-(cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 200 | -39.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50310199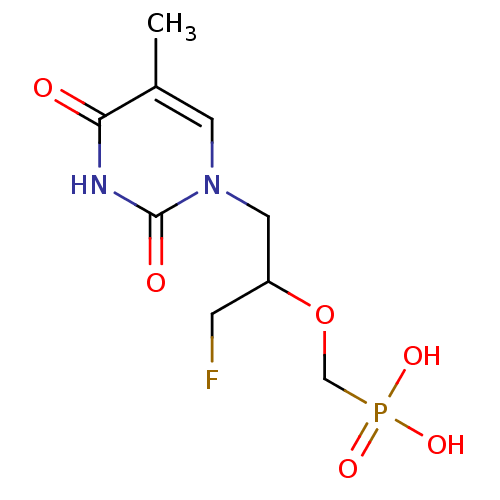 ((1-fluoro-3-(5-methyl-2,4-dioxo-3,4-dihydropyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50310200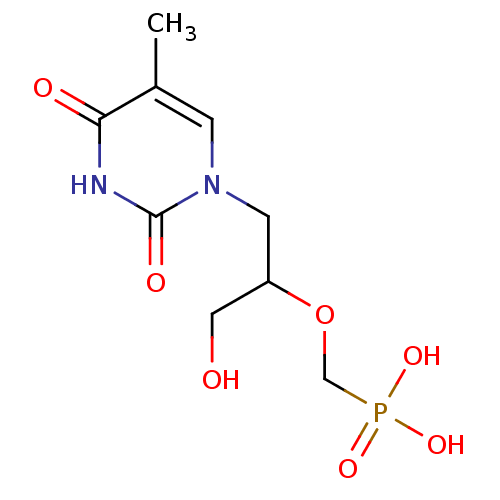 ((1-hydroxy-3-(5-methyl-2,4-dioxo-3,4-dihydropyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50310201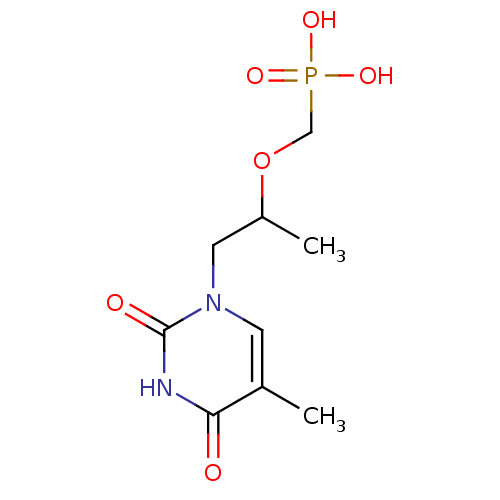 ((1-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50310202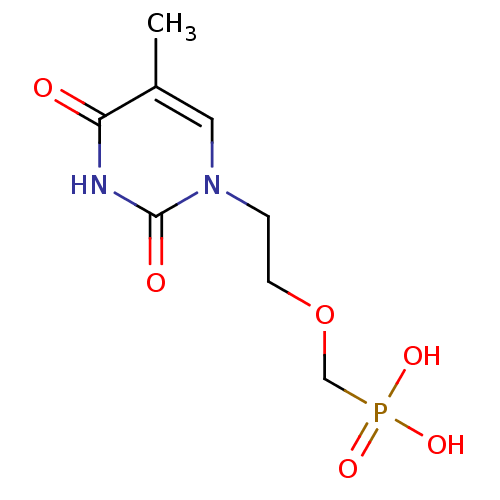 ((2-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201010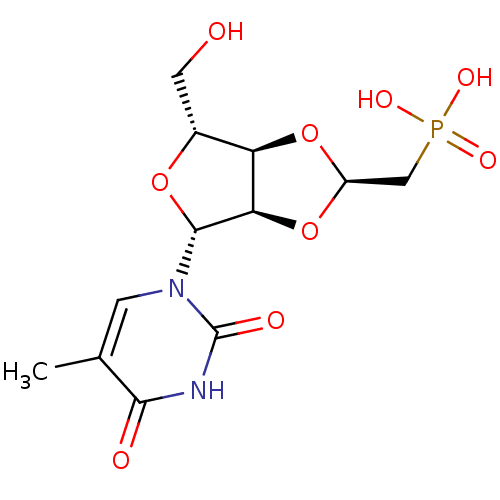 (((2R,3aR,4R,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50310203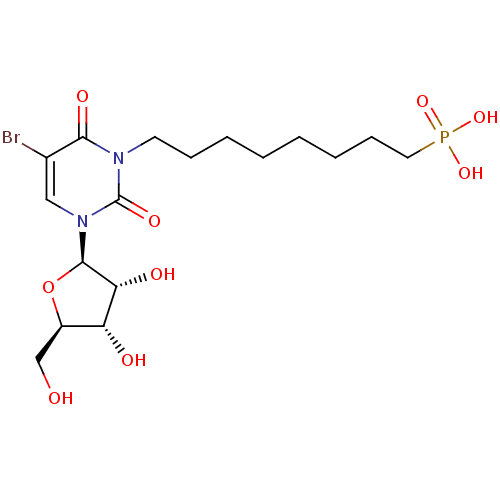 (8-(5-bromo-3-((2R,3R,4S,5R)-3,4-dihydroxy-5-(hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human recombinant thymidine phosphorylase | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20069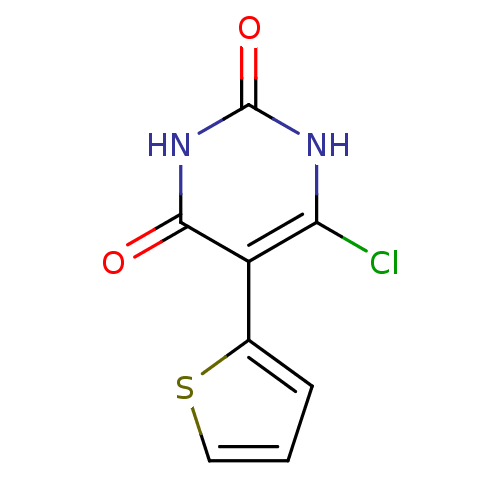 (5-Substituted-6-chlorouracil, 10e | 6-chloro-5-(th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 280 | -38.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20065 (5-Substituted-6-chlorouracil, 10a | 6-chloro-5-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | -38.0 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20073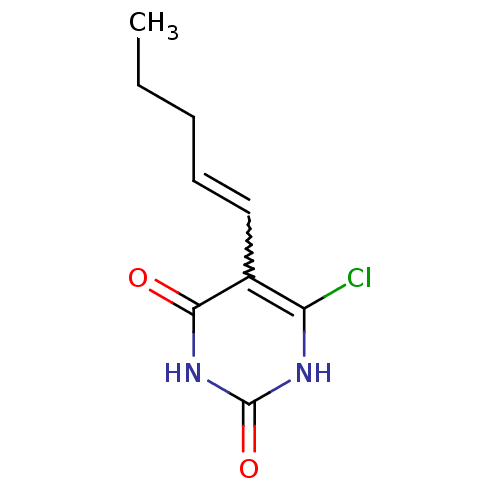 (5-Substituted-6-chlorouracil, 13c | 6-chloro-5-[(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 410 | -37.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20062 (5-Substituted-6-chlorouracil, 7b | 6-chloro-5-(cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 420 | -37.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20064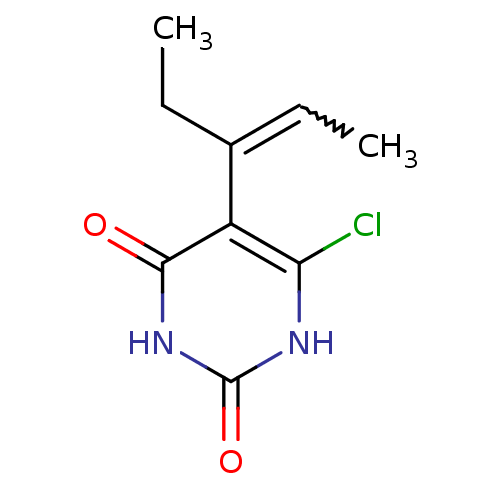 (5-Substituted-6-chlorouracil, 7d | 6-chloro-5-[(2E...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 490 | -37.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20075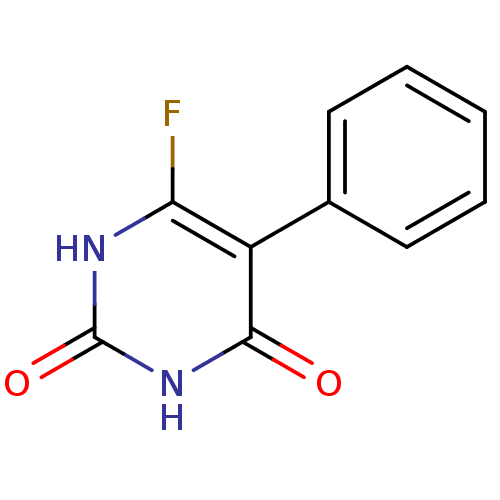 (6-Fluoro-5-phenylpyrimidine-2,4(1H,3H)-dione, 21 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | -37.4 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20072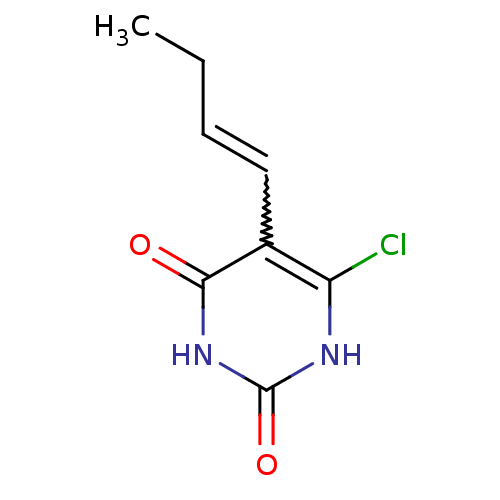 (5-Substituted-6-chlorouracil, 13b | 5-[(1E)-but-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 630 | -36.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20068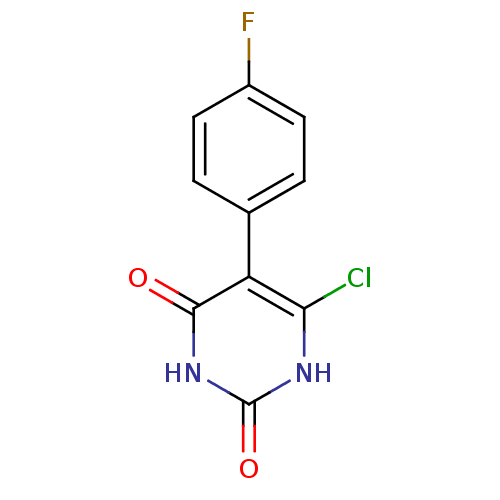 (5-Substituted-6-chlorouracil, 10d | 6-chloro-5-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 710 | -36.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20056 (5-Substituted-6-chlorouracil, 5c | 5-butyl-6-chlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.03E+3 | -35.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20059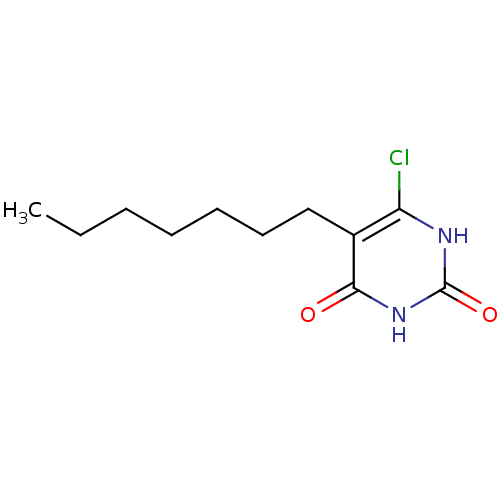 (5-Substituted-6-chlorouracil, 5f | 6-chloro-5-hept...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.06E+3 | -35.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20071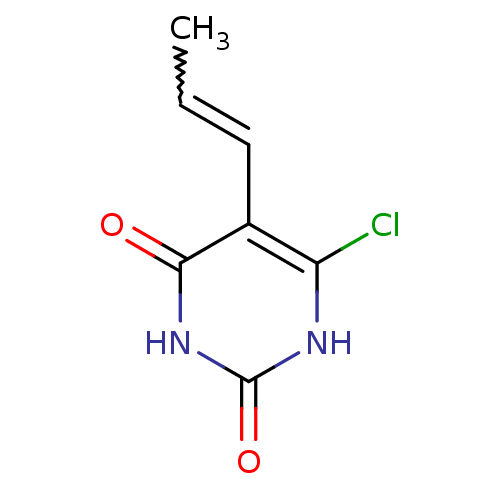 (5-Substituted-6-chlorouracil, 13a | 6-chloro-5-[(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.17E+3 | -35.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20076 (6-Bromo-5-phenylpyrimidine-2,4(1H,3H)-dione, 23 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.21E+3 | -35.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20066 (5-Substituted-6-chlorouracil, 10b | 6-chloro-5-(3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.74E+3 | -34.2 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20058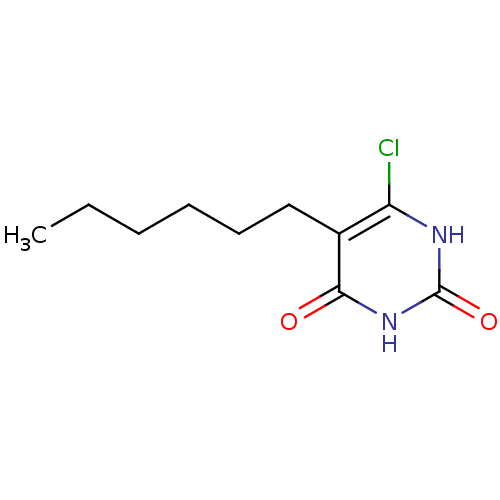 (5-Substituted-6-chlorouracil, 5e | 6-chloro-5-hexy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.83E+3 | -34.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20070 (5-Substituted-6-chlorouracil, 10f | 6-chloro-5-(py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.01E+3 | -32.8 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20063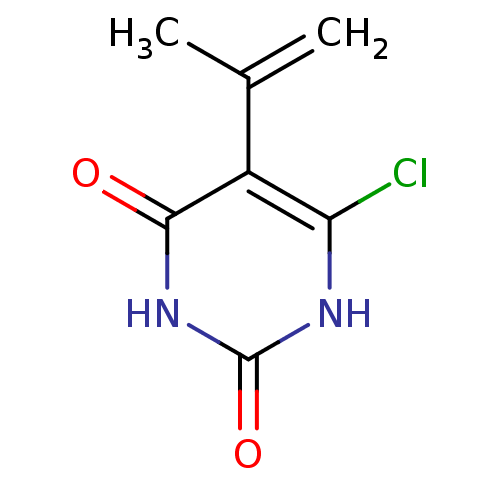 (5-Substituted-6-chlorouracil, 7c | 6-chloro-5-(pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.34E+3 | -32.5 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20057 (5-Substituted-6-chlorouracil, 5d | 6-chloro-5-pent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.67E+3 | -32.3 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20067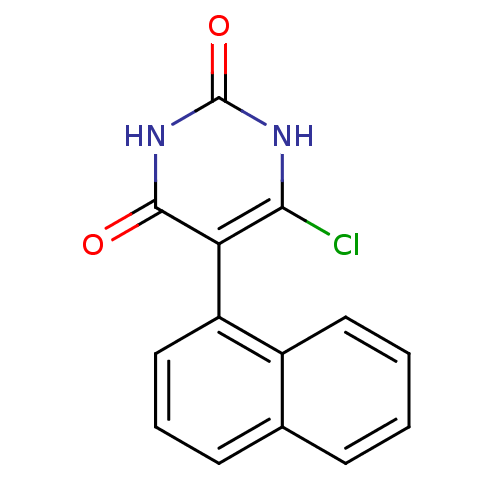 (5-Substituted-6-chlorouracil, 10c | 6-chloro-5-(na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.59E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20060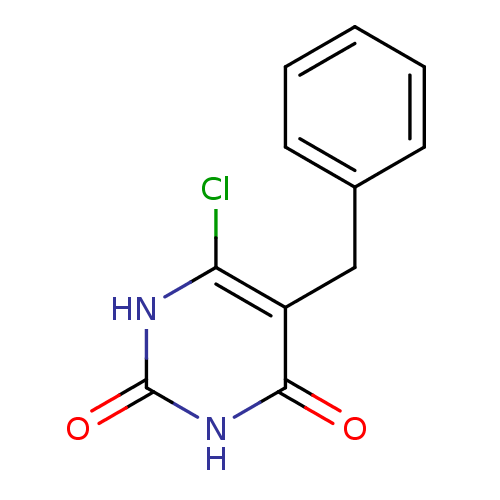 (5-Substituted-6-chlorouracil, 5g | 5-benzyl-6-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.65E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20055 (5-Substituted-6-chlorouracil, 5b | 6-chloro-5-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.81E+3 | -31.1 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20077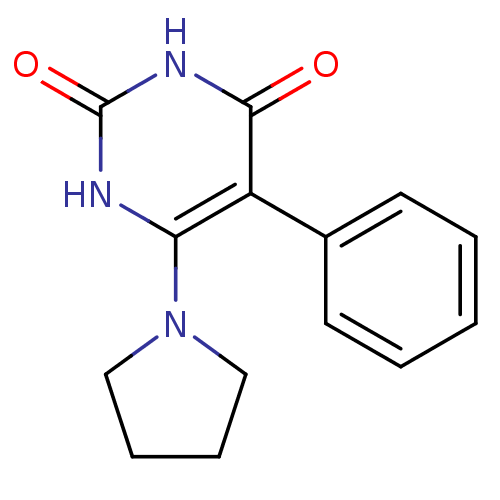 (5-Phenyl-6-pyrrolidin-1-ylpyrimidine-2,4(1H,3H)-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.00E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20078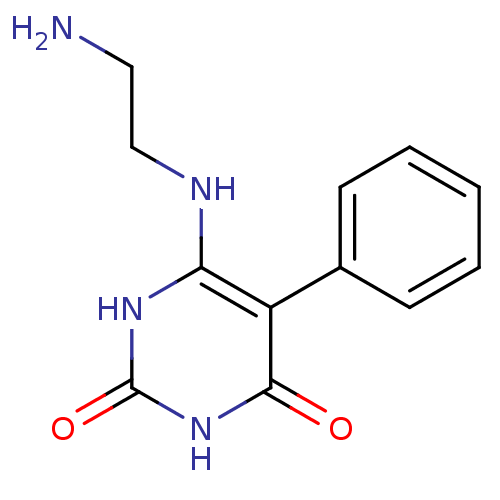 (6-[(2-Aminoethyl)amino]-5-phenylpyrimidine-2,4(1H,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.00E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20054 (5-Substituted-6-chlorouracil, 5a | 6-chloro-5-ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | >2.00E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM20074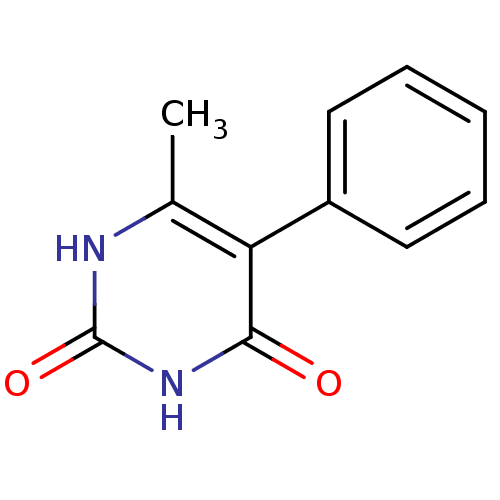 (6-Methyl-5-phenylpyrimidine-2,4(1H,3H)-dione, 16 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >2.00E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 6.4 | 37 |
Gilead Sciences Inc. | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | J Med Chem 50: 6016-23 (2007) Article DOI: 10.1021/jm070644i BindingDB Entry DOI: 10.7270/Q2M043PX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Rattus norvegicus) | BDBM50378690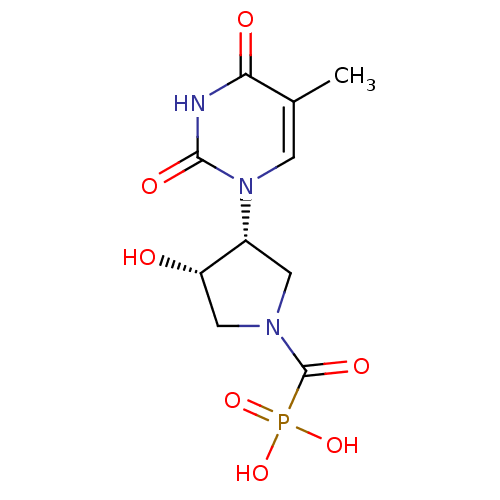 (CHEMBL597306) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphate | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Rattus norvegicus) | BDBM50378691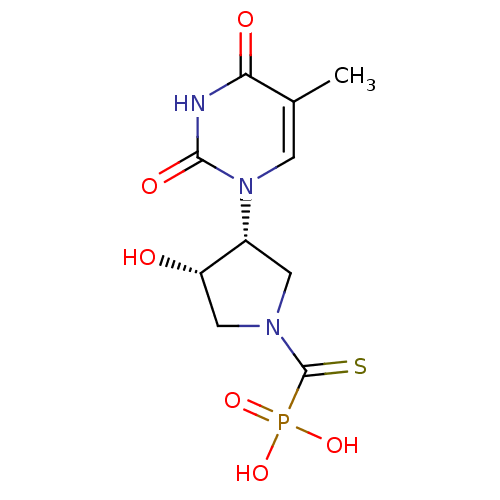 (CHEMBL609919) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphate | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Rattus norvegicus) | BDBM50378689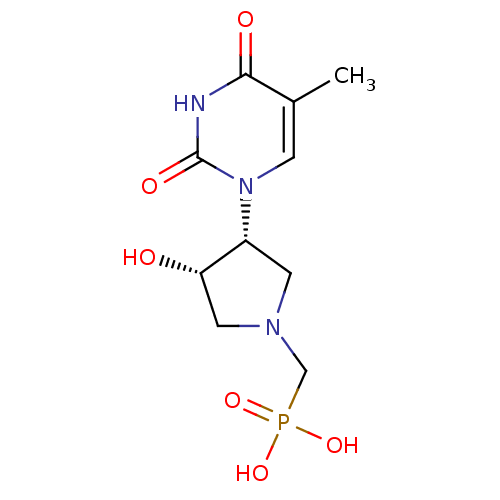 (CHEMBL599563) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphate | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Rattus norvegicus) | BDBM50378684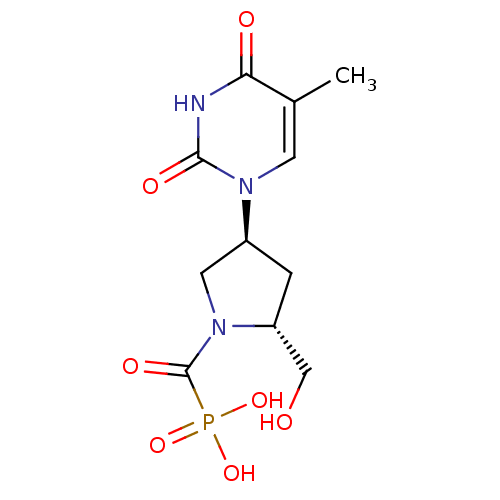 (CHEMBL599543) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphate | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50350205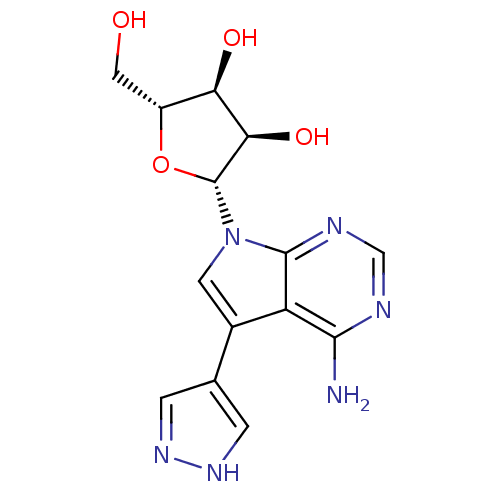 (CHEMBL1814774) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human ADK using [3H]-adenosine by scintillation counting | J Med Chem 54: 5498-507 (2011) Article DOI: 10.1021/jm2005173 BindingDB Entry DOI: 10.7270/Q2BK1CQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Rattus norvegicus) | BDBM50378687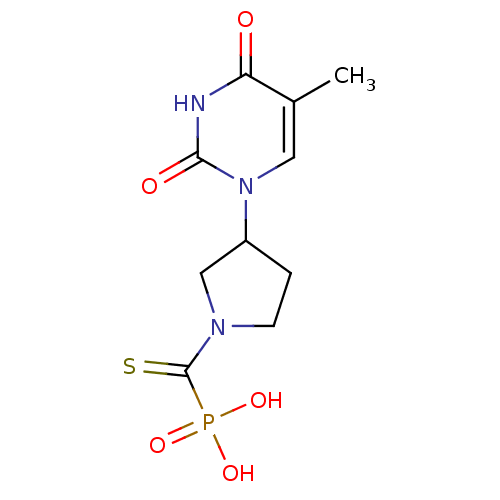 (CHEMBL598328) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphate | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50350207 (CHEMBL1814776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human ADK expressed in Escherichia coli BL21(DE3) cells using [3H]adenosine by liquid scintillation counting method | J Med Chem 57: 8268-79 (2014) Article DOI: 10.1021/jm500497v BindingDB Entry DOI: 10.7270/Q29888MK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50350207 (CHEMBL1814776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human ADK using [3H]-adenosine by scintillation counting | J Med Chem 54: 5498-507 (2011) Article DOI: 10.1021/jm2005173 BindingDB Entry DOI: 10.7270/Q2BK1CQX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thymidine phosphorylase (Rattus norvegicus) | BDBM50378688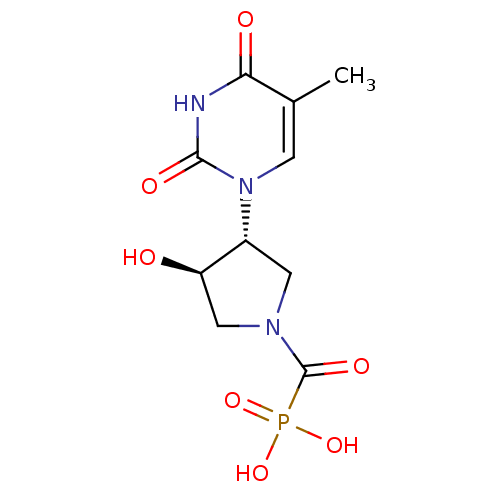 (CHEMBL599562) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphate | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Rattus norvegicus) | BDBM50378685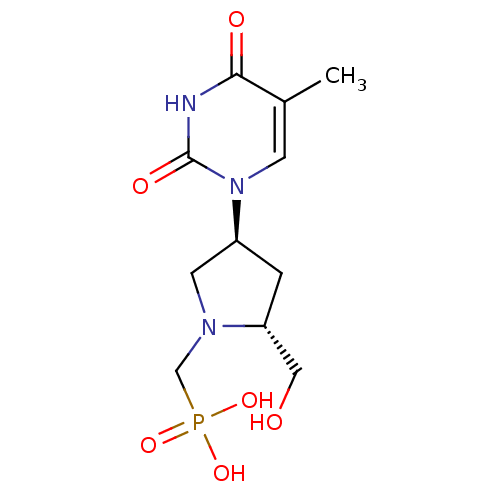 (CHEMBL599544) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphate | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Rattus norvegicus) | BDBM50378692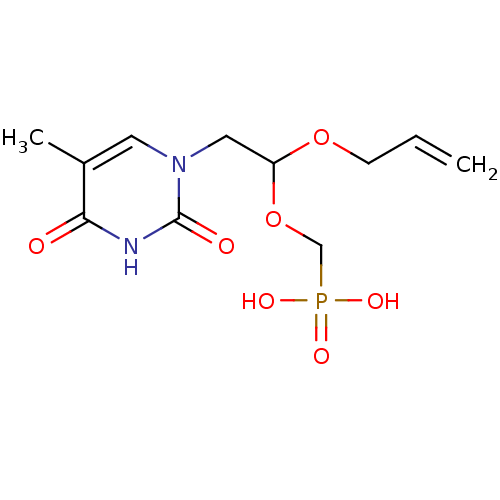 (CHEMBL611383) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphate | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Rattus norvegicus) | BDBM50378686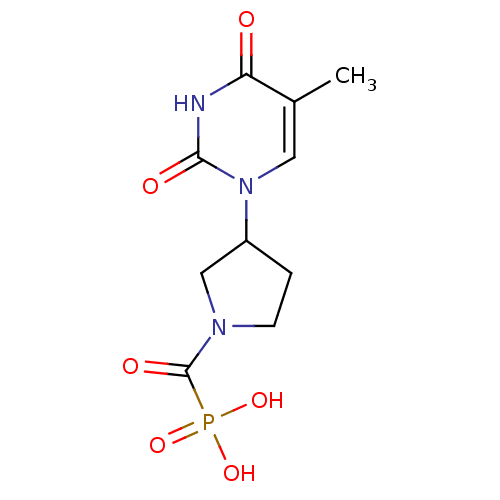 (CHEMBL598327) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphate | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Rattus norvegicus) | BDBM50378683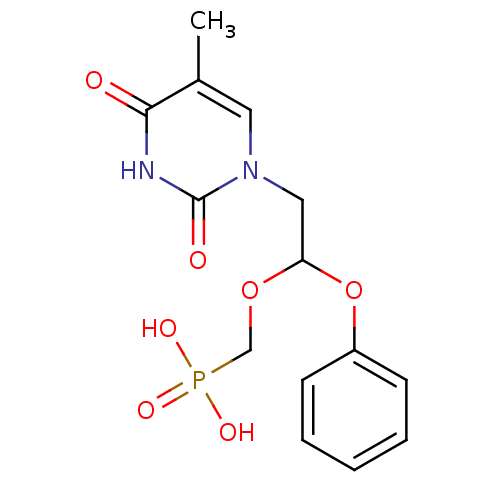 (CHEMBL599132) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of thymidine phosphorylase in T cell lymphomas of Sprague-Dawley rat in presence of 100 uM thymidine and 200 uM phosphate | Bioorg Med Chem Lett 20: 862-5 (2010) Article DOI: 10.1016/j.bmcl.2009.12.081 BindingDB Entry DOI: 10.7270/Q2930V4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50350195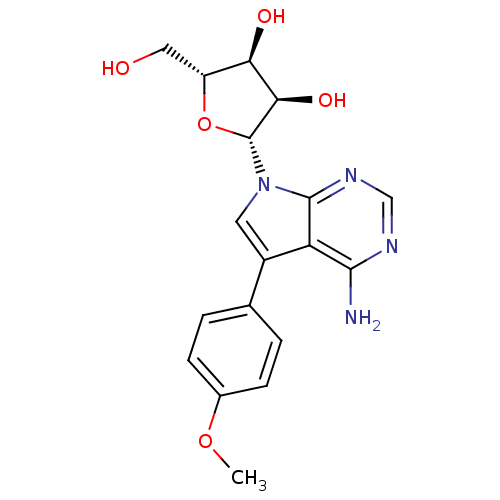 (CHEMBL1814763) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human ADK using [3H]-adenosine by scintillation counting | J Med Chem 54: 5498-507 (2011) Article DOI: 10.1021/jm2005173 BindingDB Entry DOI: 10.7270/Q2BK1CQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50350199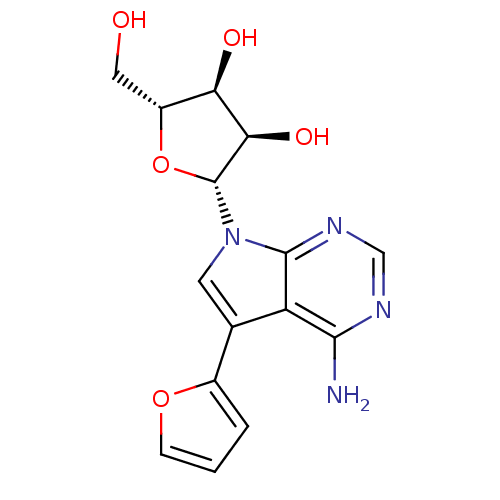 (CHEMBL1814767) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human ADK using [3H]-adenosine by scintillation counting | J Med Chem 54: 5498-507 (2011) Article DOI: 10.1021/jm2005173 BindingDB Entry DOI: 10.7270/Q2BK1CQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Escherichia coli) | BDBM20033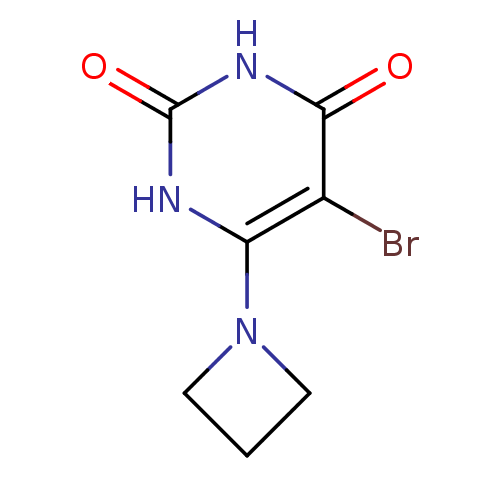 (6-(azetidin-1-yl)-5-bromo-1,3-diazinane-2,4-dione ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic | Assay Description The enzyme reaction was started by the addition of enzyme to the reaction mixture containing substrate and test compounds. The reaction was stopped b... | Bioorg Med Chem Lett 16: 1335-7 (2006) Article DOI: 10.1016/j.bmcl.2005.11.050 BindingDB Entry DOI: 10.7270/Q2QR4VCH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50028657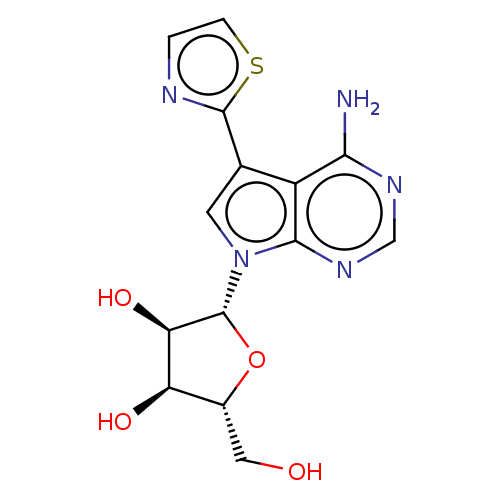 (CHEMBL1814778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Academy of Sciences of the Czech Republic Curated by ChEMBL | Assay Description Inhibition of human ADK expressed in Escherichia coli BL21(DE3) cells using [3H]adenosine by liquid scintillation counting method | J Med Chem 57: 8268-79 (2014) Article DOI: 10.1021/jm500497v BindingDB Entry DOI: 10.7270/Q29888MK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 148 total ) | Next | Last >> |