

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein [1027-1206,R1052K] (Hepatitis C virus) | BDBM92407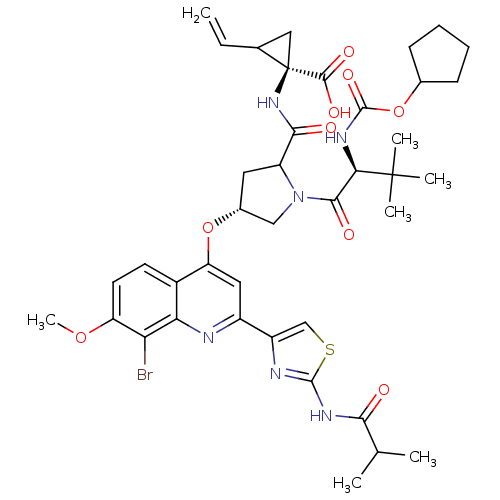 (BI201335) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | 0.000440 | 8.50E+5 | 8.0 | n/a |
Boehringer Ingelheim (Canada) Ltd. | Assay Description Kinetic analysis of NS3 inhibitor binding experiment were performed using the KinTek stopped flow instrument (SF-2005; excitation, 325 nm; and emissi... | J Biol Chem 286: 11434-43 (2011) Article DOI: 10.1074/jbc.M110.211417 BindingDB Entry DOI: 10.7270/Q2WM1C0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C Virus) | BDBM92407 (BI201335) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | 0.000530 | 5.40E+5 | 8.0 | n/a |
Boehringer Ingelheim (Canada) Ltd. | Assay Description Kinetic analysis of NS3 inhibitor binding experiment were performed using the KinTek stopped flow instrument (SF-2005; excitation, 325 nm; and emissi... | J Biol Chem 286: 11434-43 (2011) Article DOI: 10.1074/jbc.M110.211417 BindingDB Entry DOI: 10.7270/Q2WM1C0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1027-1206,R1052K] (Hepatitis C virus) | BDBM92408 (NS3 protease inhibitor, analog 1) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.89 | n/a | n/a | n/a | n/a | 0.00220 | 1.10E+6 | 8.0 | n/a |
Boehringer Ingelheim (Canada) Ltd. | Assay Description Kinetic analysis of NS3 inhibitor binding experiment were performed using the KinTek stopped flow instrument (SF-2005; excitation, 325 nm; and emissi... | J Biol Chem 286: 11434-43 (2011) Article DOI: 10.1074/jbc.M110.211417 BindingDB Entry DOI: 10.7270/Q2WM1C0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137912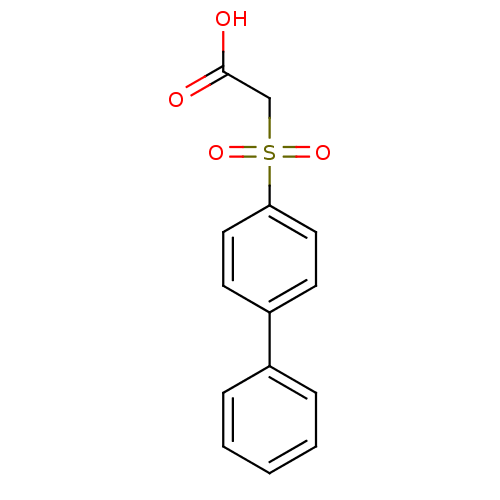 ((Biphenyl-4-sulfinyl)-acetic acid | (Biphenyl-4-su...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137910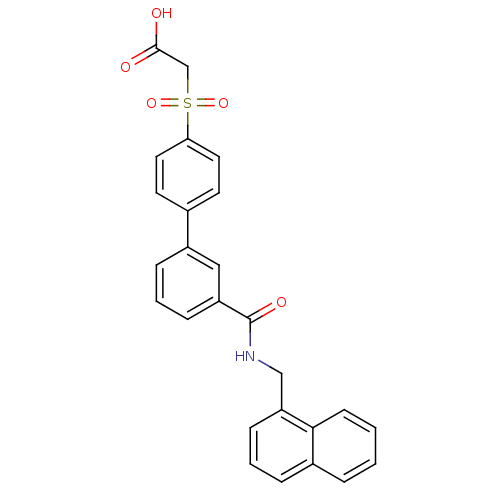 (CHEMBL434551 | [3'-(naphthalene-1-yl-methylcarbamo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137910 (CHEMBL434551 | [3'-(naphthalene-1-yl-methylcarbamo...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Binding affinity for human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137889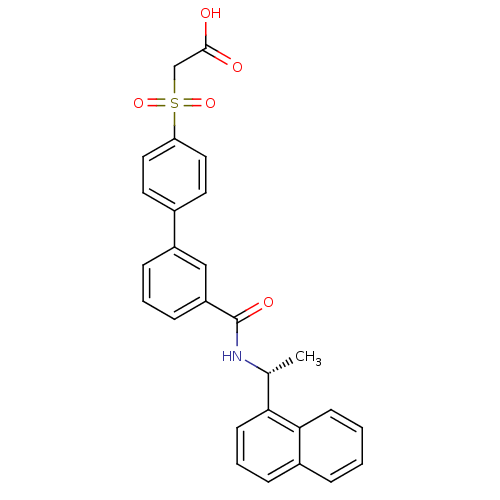 (CHEMBL176784 | [3'-(1-(R)-naphthalene-1-yl-ethylca...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137901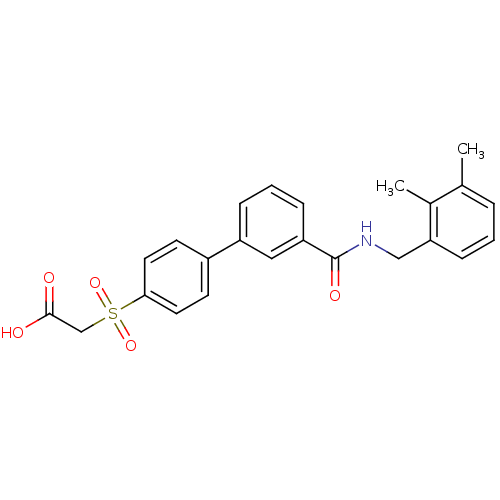 (CHEMBL175099 | [3'-(2,3-dimethyl-benzylcarbamoyl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137919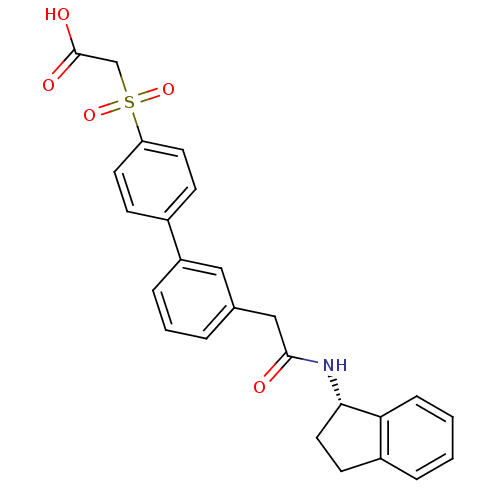 (CHEMBL174279 | [3'-((S)-Indan-1-ylcarbamoylmethyl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137899 (CHEMBL175280 | [3'-(3-methyl-benzylcarbamoyl)-biph...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137891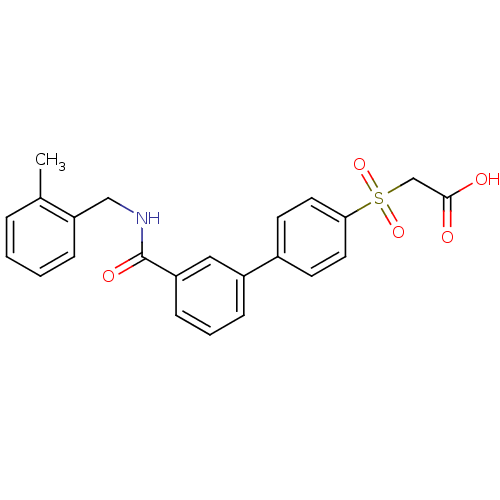 (CHEMBL368080 | [3'-(2-methyl-benzylcarbamoyl)-biph...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137925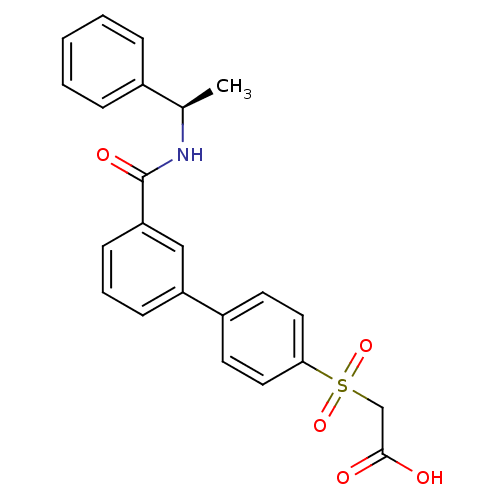 (CHEMBL368100 | [3'-((R)-1-Phenyl-ethylcarbamoyl)-b...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137921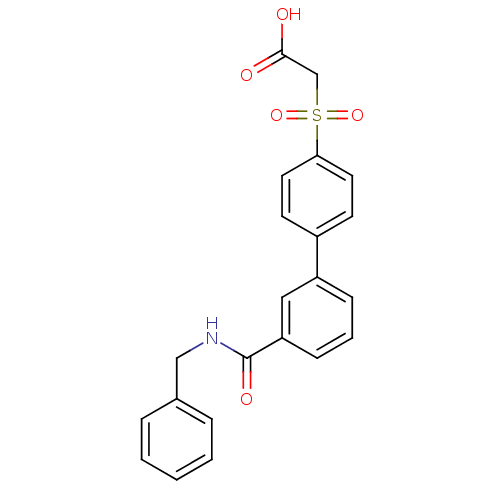 ((3'-Benzylcarbamoyl-biphenyl-4-sulfonyl)-acetic ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137928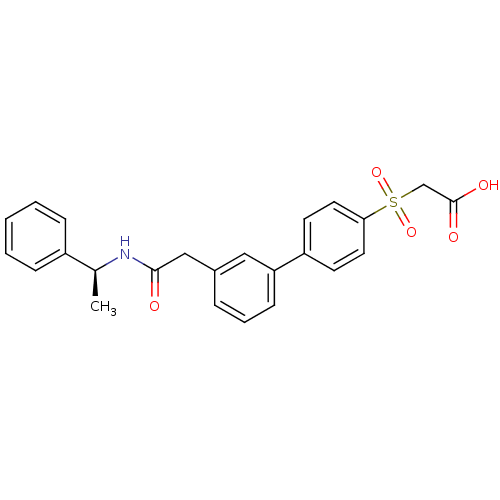 (CHEMBL172893 | {3'-[((S)-1-Phenyl-ethylcarbamoyl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137897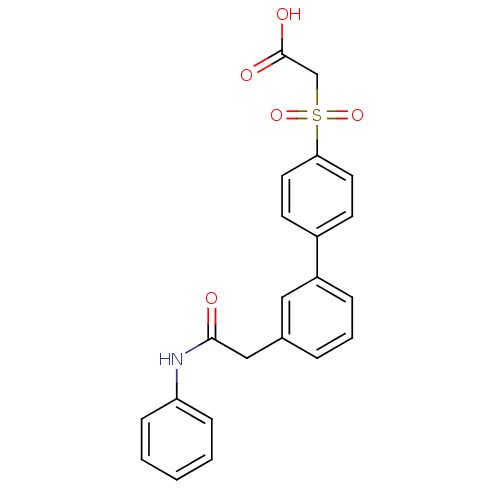 ((3'-Phenylcarbamoylmethyl-biphenyl-4-sulfonyl)-ace...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137917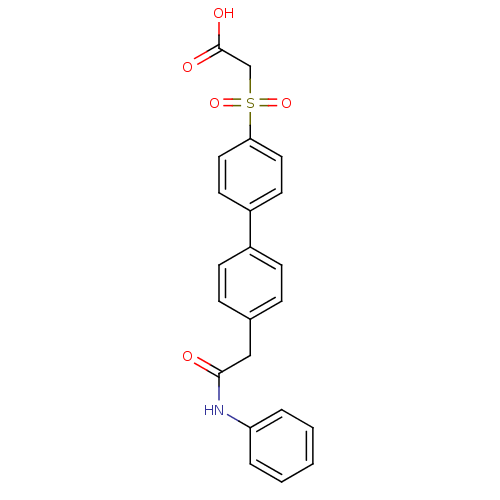 ((4'-Phenylcarbamoylmethyl-biphenyl-4-sulfonyl)-ace...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137883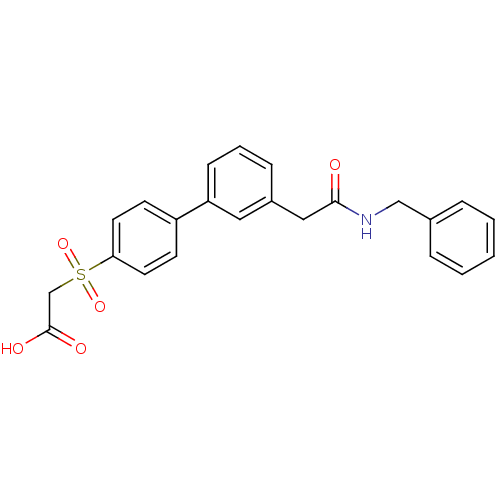 (CHEMBL368567 | [3'-(Benzylcarbamoyl-methyl)-biphen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137886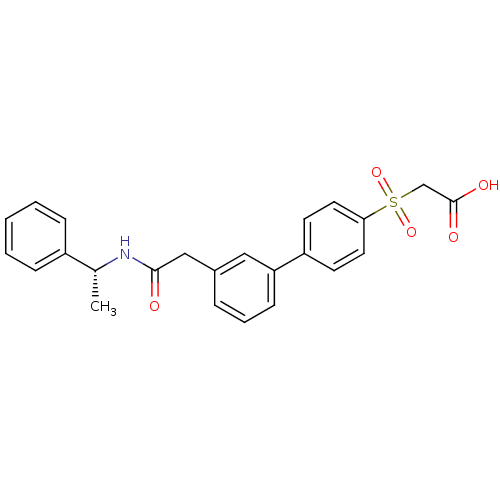 (CHEMBL175036 | {3'-[((R)-1-Phenyl-ethylcarbamoyl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137898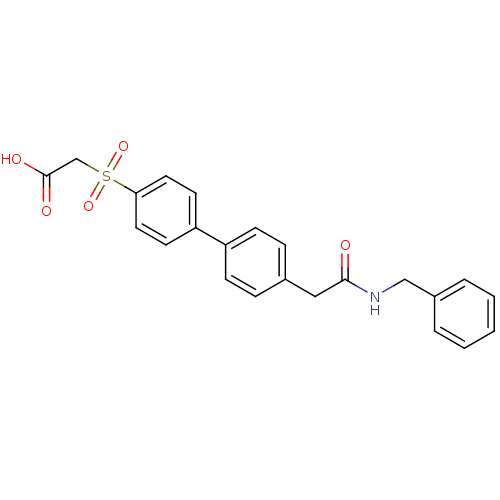 ((4'-Benzylcarbamoylmethyl-biphenyl-4-sulfonyl)-ace...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137924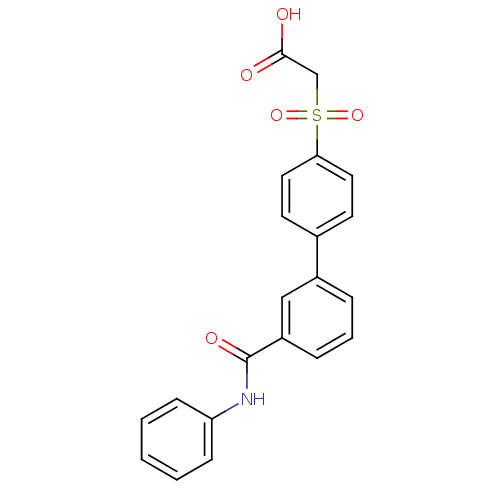 ((3'-Phenylcarbamoyl-biphenyl-4-sulfonyl)-acetic ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137915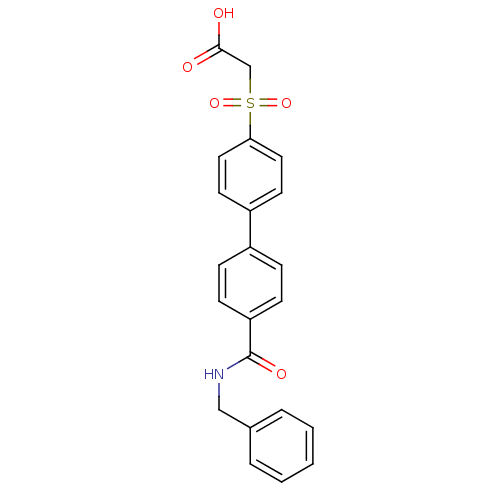 ((4'-Benzylcarbamoyl-biphenyl-4-sulfonyl)-acetic ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137895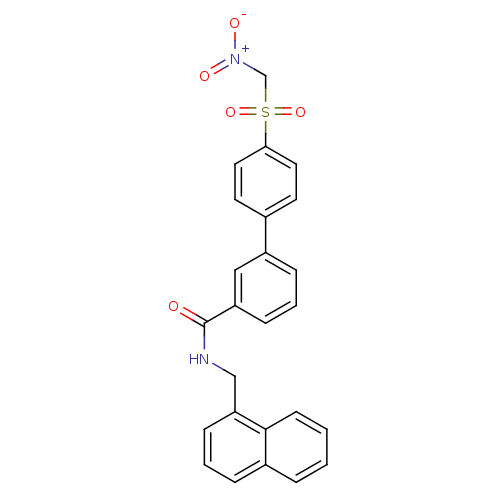 (4'-Nitromethanesulfonyl-biphenyl-3-carboxylic acid...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50326056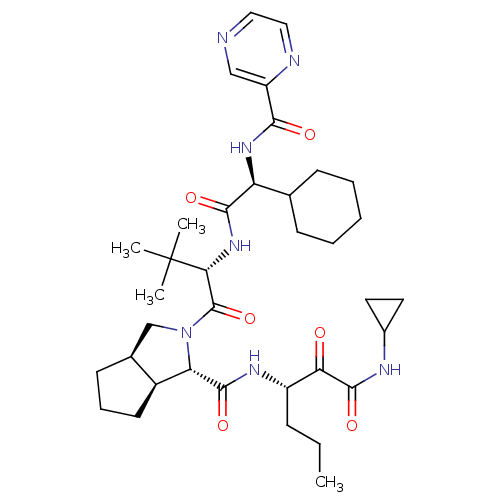 ((1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase after 60 mins fluorescence assay | Antimicrob Agents Chemother 54: 4611-8 (2010) Article DOI: 10.1128/AAC.00787-10 BindingDB Entry DOI: 10.7270/Q2NP24Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137884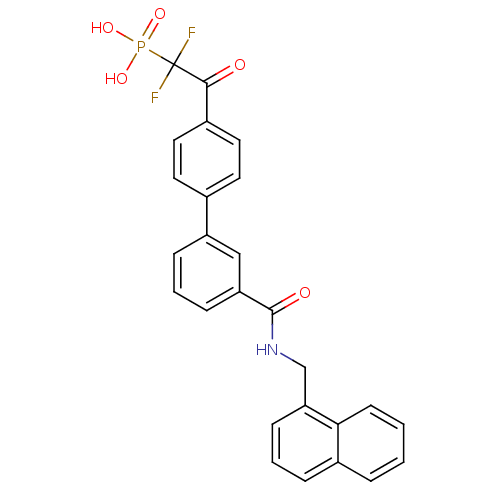 ((1,1-Difluoro-2-{3'-[(naphthalen-1-ylmethyl)-carba...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137927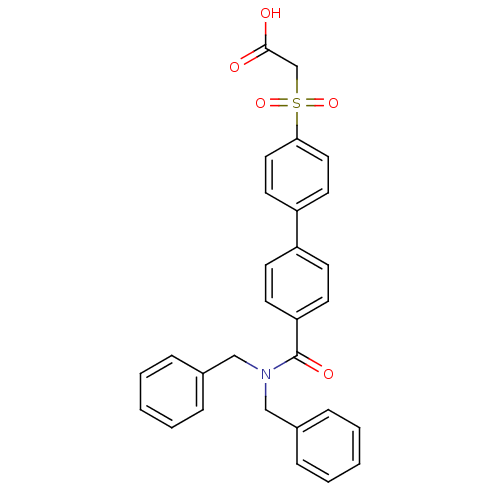 ((4'-Dibenzylcarbamoyl-biphenyl-4-sulfonyl)-acetic ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137890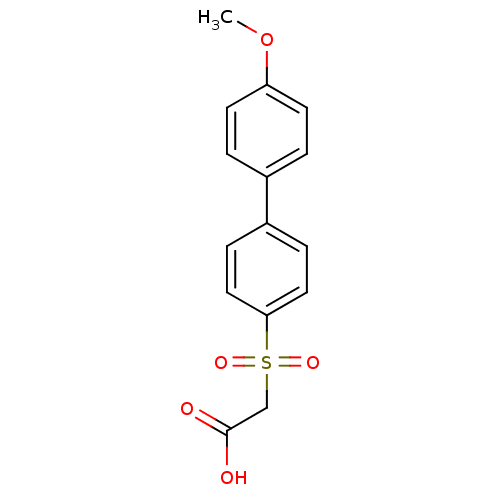 ((4'-Methoxy-biphenyl-4-sulfonyl)-acetic acid | CHE...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137916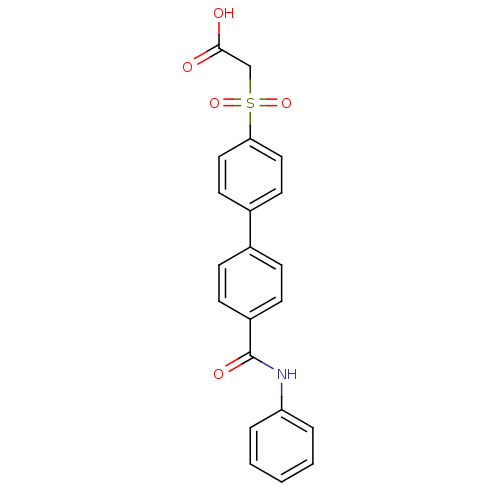 ((4'-Phenylcarbamoyl-biphenyl-4-sulfonyl)-acetic ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137908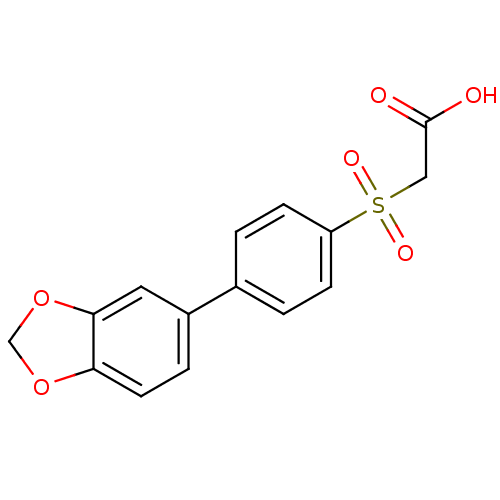 ((4-Benzo[1,3]dioxol-5-yl-benzenesulfonyl)-acetic a...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137904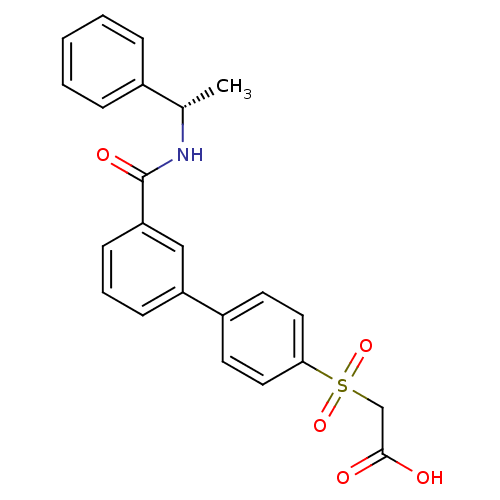 (CHEMBL367131 | [3'-((S)-1-Phenyl-ethylcarbamoyl)-b...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137909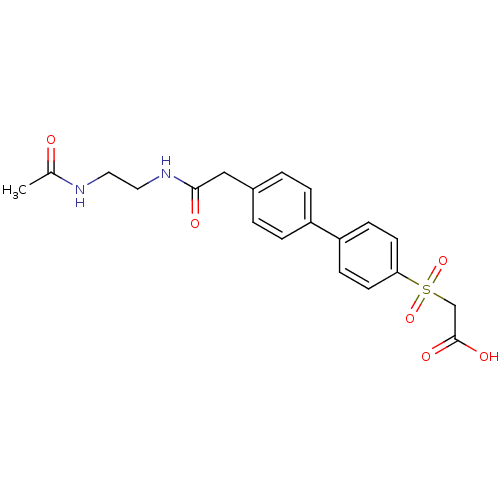 (CHEMBL439656 | {4'-[(2-Acetylamino-ethylcarbamoyl)...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137912 ((Biphenyl-4-sulfinyl)-acetic acid | (Biphenyl-4-su...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50326056 ((1S,3aR,6aS)-2-((S)-2-((S)-2-cyclohexyl-2-(pyrazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of human CatB after 60 mins fluorescence assay | Antimicrob Agents Chemother 54: 4611-8 (2010) Article DOI: 10.1128/AAC.00787-10 BindingDB Entry DOI: 10.7270/Q2NP24Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Rattus norvegicus) | BDBM50336545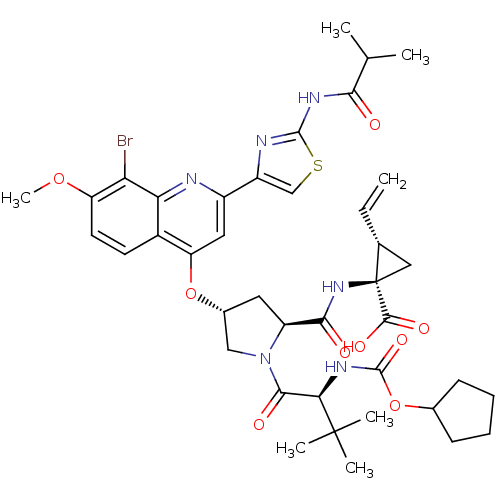 ((1R,2S)-1-((2S,4R)-4-(8-bromo-2-(2-isobutyramidoth...) | KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of rat hapatic, acyl coA cholesterol acetyltransferase | Antimicrob Agents Chemother 54: 4611-8 (2010) Article DOI: 10.1128/AAC.00787-10 BindingDB Entry DOI: 10.7270/Q2NP24Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137882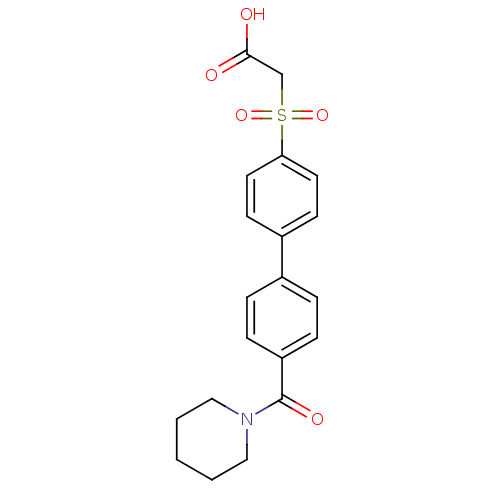 (CHEMBL368466 | [4'-(Piperidine-1-carbonyl)-bipheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137922 ((3'-Methoxy-biphenyl-4-sulfonyl)-acetic acid | CHE...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137923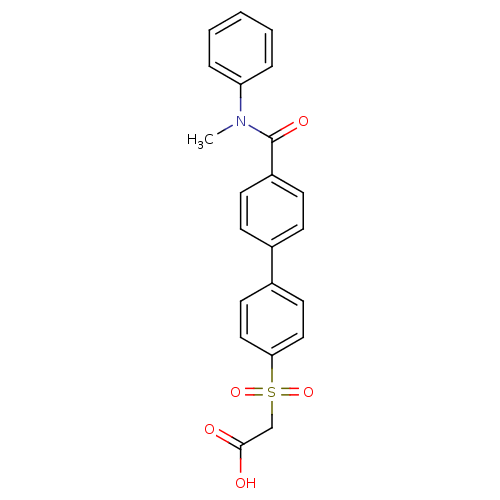 ((4'-Methyl-phenylcarbamoyl-biphenyl-4-sulfonyl)-ac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137931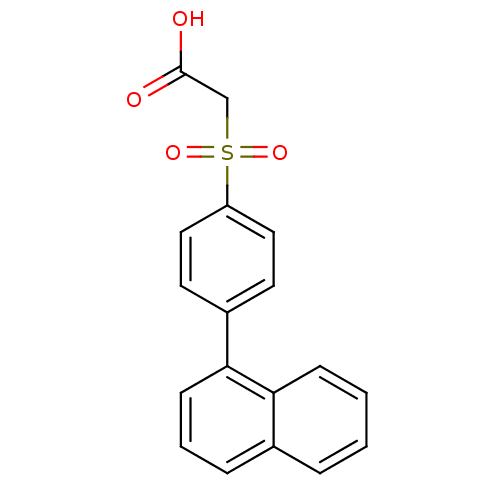 ((4-Naphthalen-1-yl-benzenesulfonyl)-acetic acid | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM12311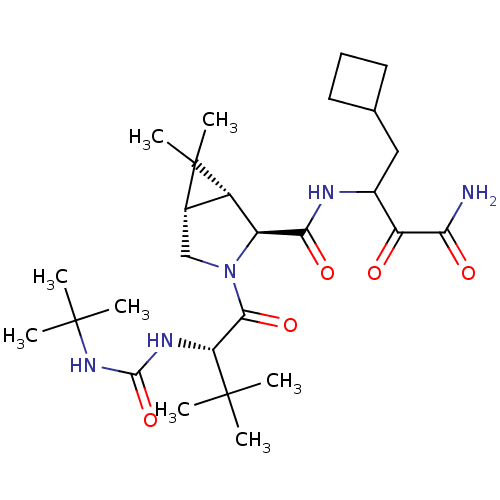 ((1R,5S)-N-[3-Amino-1-(cyclobutylmethyl)-2,3-dioxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of human CatB after 60 mins fluorescence assay | Antimicrob Agents Chemother 54: 4611-8 (2010) Article DOI: 10.1128/AAC.00787-10 BindingDB Entry DOI: 10.7270/Q2NP24Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137911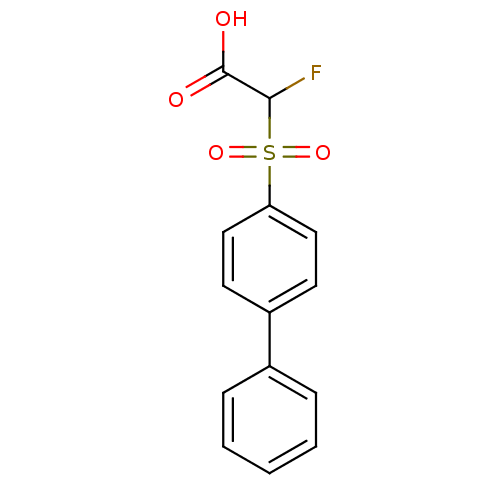 ((Biphenyl-4-sulfonyl)-fluoro-acetic acid | CHEMBL1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase 2B catalytic subunit beta isoform (Homo sapiens (Human)) | BDBM50336545 ((1R,2S)-1-((2S,4R)-4-(8-bromo-2-(2-isobutyramidoth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of human PP2B | Antimicrob Agents Chemother 54: 4611-8 (2010) Article DOI: 10.1128/AAC.00787-10 BindingDB Entry DOI: 10.7270/Q2NP24Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137905 (2-(Biphenyl-4-sulfonyl)-propionic acid | CHEMBL175...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM12311 ((1R,5S)-N-[3-Amino-1-(cyclobutylmethyl)-2,3-dioxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase after 60 mins fluorescence assay | Antimicrob Agents Chemother 54: 4611-8 (2010) Article DOI: 10.1128/AAC.00787-10 BindingDB Entry DOI: 10.7270/Q2NP24Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137900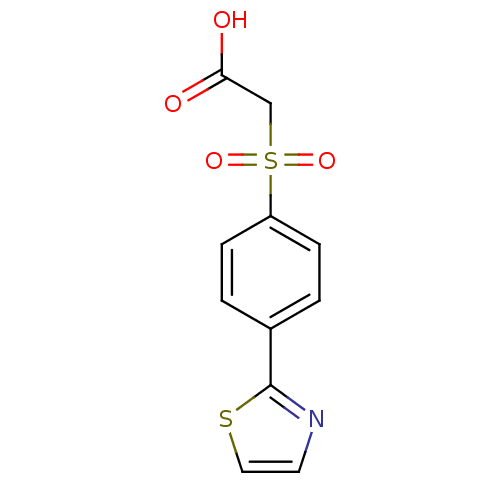 ((4-Thiazol-2-yl-benzenesulfonyl)-acetic acid | CHE...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137893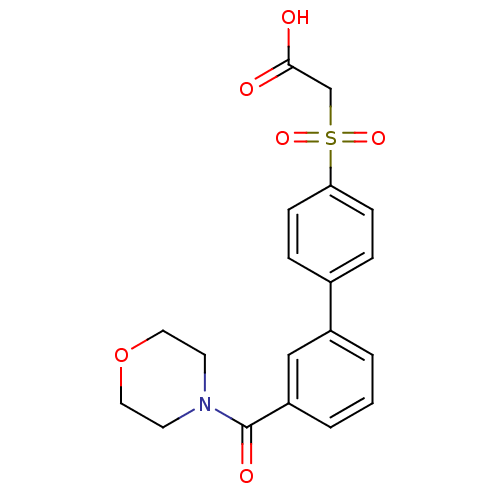 (CHEMBL174590 | [3'-(Morpholine-4-carbonyl)-bipheny...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50336545 ((1R,2S)-1-((2S,4R)-4-(8-bromo-2-(2-isobutyramidoth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of human CatB after 60 mins fluorescence assay | Antimicrob Agents Chemother 54: 4611-8 (2010) Article DOI: 10.1128/AAC.00787-10 BindingDB Entry DOI: 10.7270/Q2NP24Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50336545 ((1R,2S)-1-((2S,4R)-4-(8-bromo-2-(2-isobutyramidoth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase after 60 mins fluorescence assay | Antimicrob Agents Chemother 54: 4611-8 (2010) Article DOI: 10.1128/AAC.00787-10 BindingDB Entry DOI: 10.7270/Q2NP24Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137888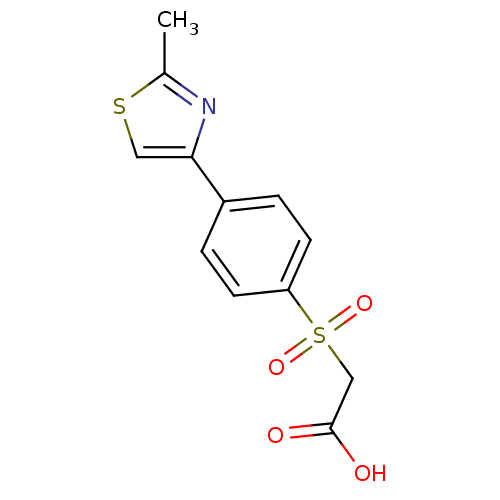 (CHEMBL368316 | [4-(2-Methyl-thiazol-4-yl)-benzenes...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Mus musculus) | BDBM50336545 ((1R,2S)-1-((2S,4R)-4-(8-bromo-2-(2-isobutyramidoth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant cathepsin E by fluorimetry | Antimicrob Agents Chemother 54: 4611-8 (2010) Article DOI: 10.1128/AAC.00787-10 BindingDB Entry DOI: 10.7270/Q2NP24Q7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137929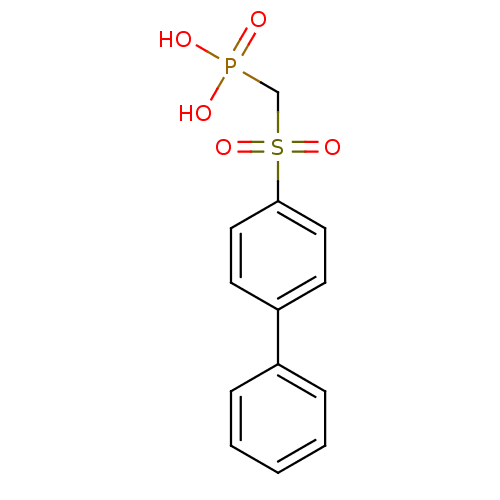 ((Biphenyl-4-sulfinyl)-acetic acid | (Biphenyl-4-su...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replication protein E1 (Human papillomavirus type 11) | BDBM50137894 ((Biphenyl-4-sulfonyl)-methanesulfonic acid | (Biph...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Ability to inhibit in vitro ATPase activity of human papillomavirus (HPV6) E1 helicase | J Med Chem 47: 18-21 (2003) Article DOI: 10.1021/jm034206x BindingDB Entry DOI: 10.7270/Q23F4P2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 66 total ) | Next | Last >> |