

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50125474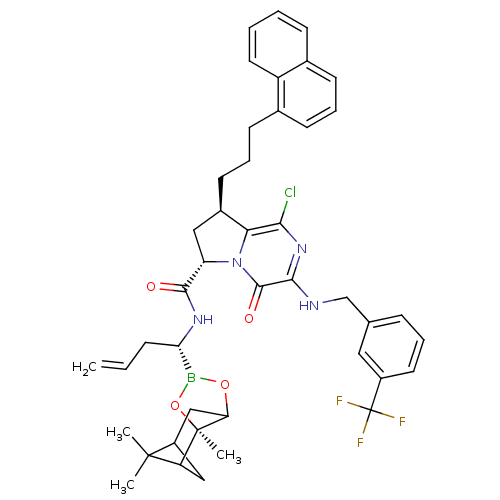 ((6S,8R)-1-Chloro-8-(3-naphthalen-1-yl-propyl)-4-ox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration against HCV NS3 protease. | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125491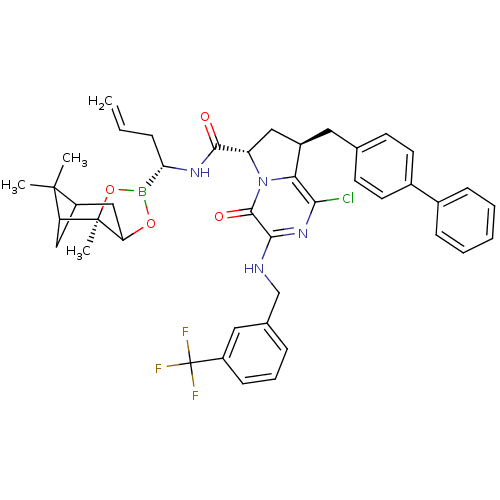 ((6S,8R)-8-Biphenyl-4-ylmethyl-1-chloro-4-oxo-3-(3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration against HCV NS3 protease. | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50109999 ((S)-4-[(S)-3-Carboxy-2-(3-carboxy-propionylamino)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Hepatitis C virus NS3 proteinase | Bioorg Med Chem Lett 12: 641-3 (2002) BindingDB Entry DOI: 10.7270/Q2SN089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50137962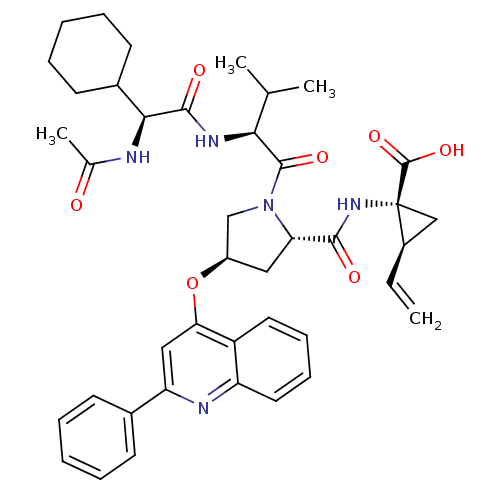 ((1R,2S)-1-((3R,5S)-1-((S)-2-((S)-2-acetamido-2-cyc...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3 protease | J Med Chem 47: 123-32 (2003) Article DOI: 10.1021/jm0303002 BindingDB Entry DOI: 10.7270/Q2Q52P2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50093024 (1-{[1-(2-{2-[2-(2-Acetylamino-3-carboxy-propionyla...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibitory activity against NS3 protease complexed with NS4A cofactor peptide (NS3-4A pep) | Bioorg Med Chem Lett 10: 2267-70 (2001) BindingDB Entry DOI: 10.7270/Q2S1831T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158847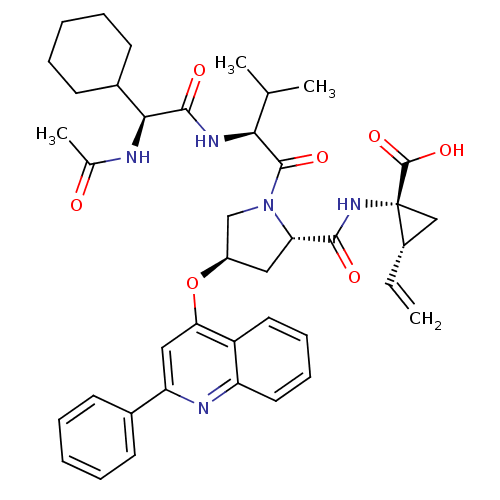 ((R)-1-{[(2S,4R)-1-[(S)-2-((S)-2-Acetylamino-2-cycl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50110000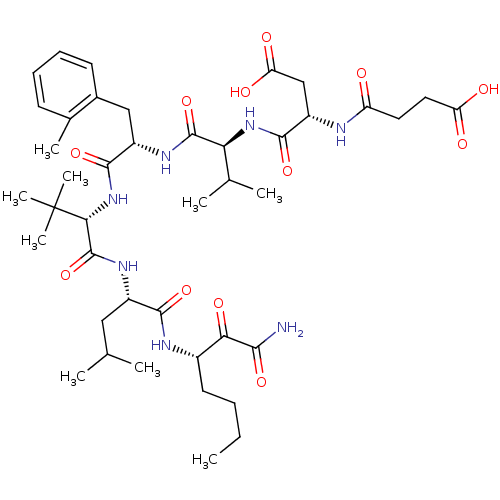 ((S)-N-[(S)-1-((S)-1-{(S)-1-[(S)-1-((S)-1-Aminooxal...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Hepatitis C virus NS3 proteinase | Bioorg Med Chem Lett 12: 641-3 (2002) BindingDB Entry DOI: 10.7270/Q2SN089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125478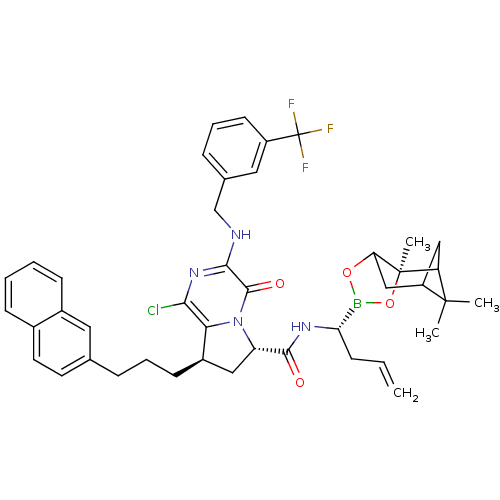 ((6S,8R)-1-Chloro-8-(3-naphthalen-2-yl-propyl)-4-ox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration against HCV NS3 protease. | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125481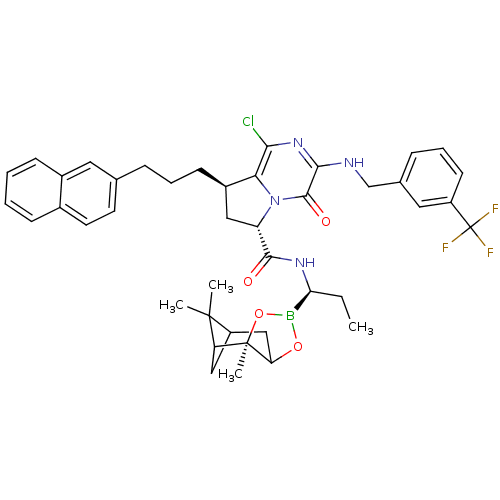 ((6S,8R)-1-Chloro-8-(3-naphthalen-2-yl-propyl)-4-ox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration against HCV NS3 protease. | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50169413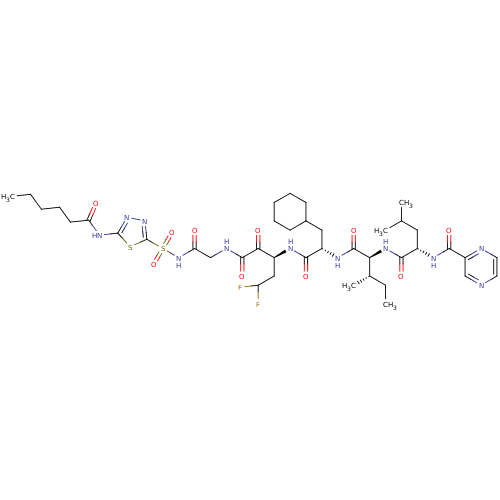 (CHEMBL188984 | Pyrazine-2-carboxylic acid {(S)-1-[...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against glycine alpha-ketoamide HCV NS3 protease | Bioorg Med Chem Lett 15: 3487-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.003 BindingDB Entry DOI: 10.7270/Q2V12495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50169419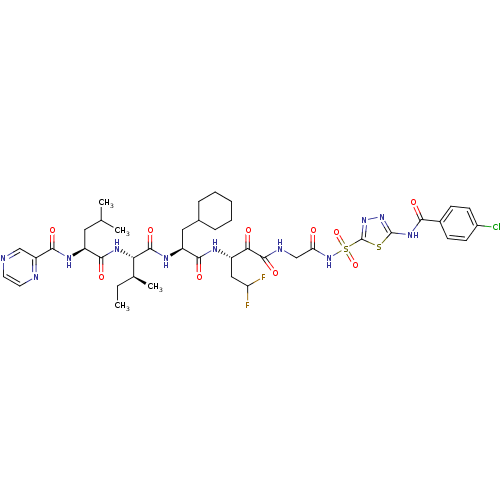 (CHEMBL189038 | Pyrazine-2-carboxylic acid ((S)-1-{...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against glycine alpha-ketoamide HCV NS3 protease | Bioorg Med Chem Lett 15: 3487-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.003 BindingDB Entry DOI: 10.7270/Q2V12495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50131393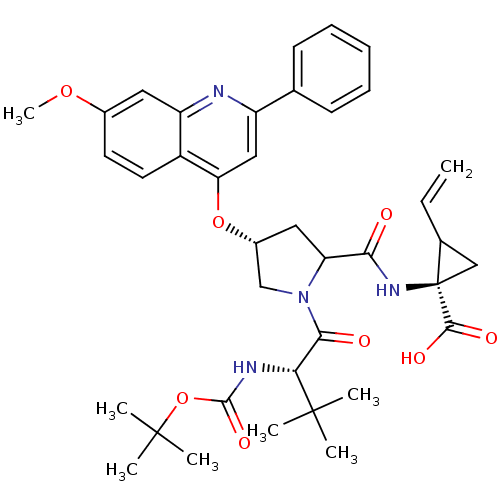 ((R)-1-{[(R)-1-((S)-2-tert-Butoxycarbonylamino-3,3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against hepatitis C virus (HCV) NS3 protease | Bioorg Med Chem Lett 13: 2745-8 (2003) BindingDB Entry DOI: 10.7270/Q2DZ07Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50093010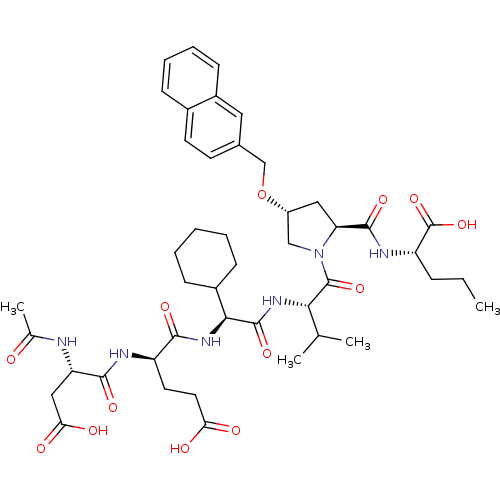 ((S)-2-{[(2S,4R)-1-((S)-2-{(S)-2-[(R)-2-((S)-2-Acet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibitory activity against NS3 protease complexed with NS4A cofactor peptide (NS3-4A pep) | Bioorg Med Chem Lett 10: 2267-70 (2001) BindingDB Entry DOI: 10.7270/Q2S1831T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50109998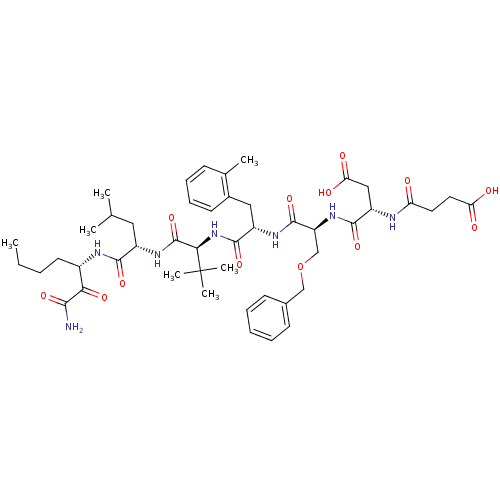 ((S)-N-[(S)-1-((S)-1-{(S)-1-[(S)-1-((S)-1-Aminooxal...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Hepatitis C virus NS3 proteinase | Bioorg Med Chem Lett 12: 641-3 (2002) BindingDB Entry DOI: 10.7270/Q2SN089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158823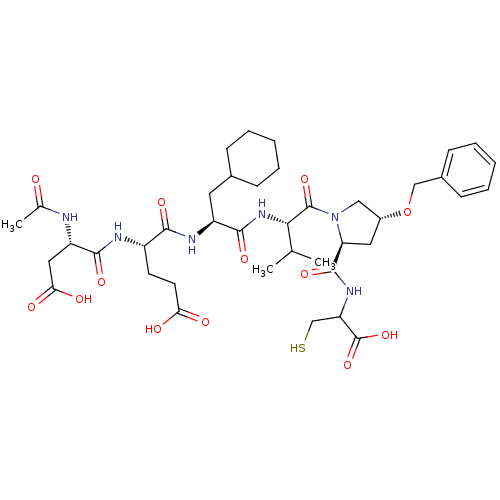 (AcAsp-Glu-Cha-Val-Prb-Cys | CHEMBL179963) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125486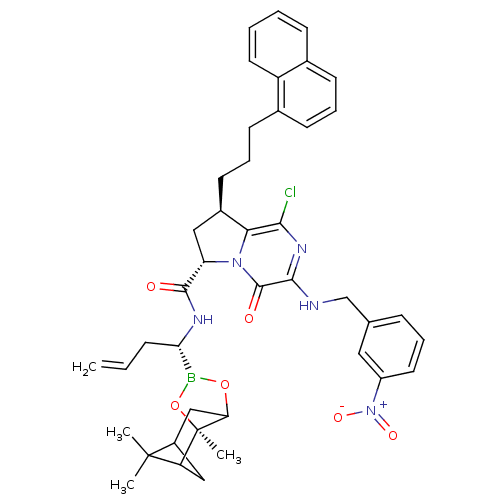 ((6S,8R)-1-Chloro-8-(3-naphthalen-1-yl-propyl)-3-(3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration against HCV NS3 protease. | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50169403 (CHEMBL183074 | Pyrazine-2-carboxylic acid [(S)-1-(...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against glycine alpha-ketoamide HCV NS3 protease | Bioorg Med Chem Lett 15: 3487-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.003 BindingDB Entry DOI: 10.7270/Q2V12495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50110003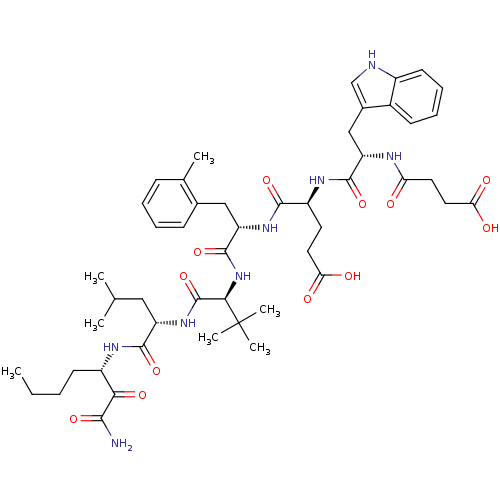 ((S)-4-((S)-1-{(S)-1-[(S)-1-((S)-1-Aminooxalyl-pent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Hepatitis C virus NS3 proteinase | Bioorg Med Chem Lett 12: 641-3 (2002) BindingDB Entry DOI: 10.7270/Q2SN089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50110002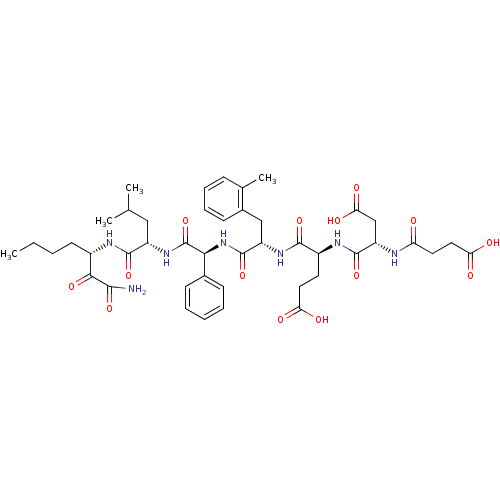 ((S)-4-[(S)-1-({[(S)-1-((S)-1-Aminooxalyl-pentylcar...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Hepatitis C virus NS3 proteinase | Bioorg Med Chem Lett 12: 641-3 (2002) BindingDB Entry DOI: 10.7270/Q2SN089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50169421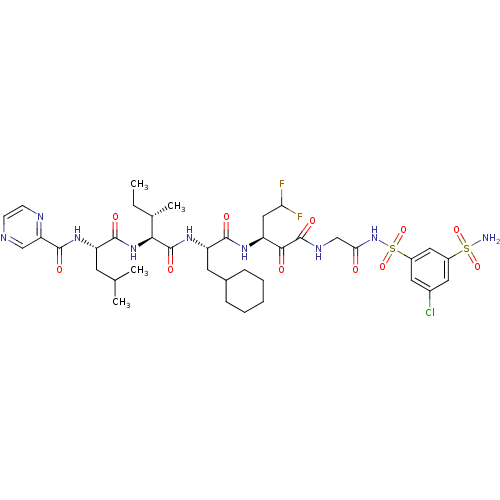 (CHEMBL424999 | Pyrazine-2-carboxylic acid {(S)-1-[...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against glycine alpha-ketoamide HCV NS3 protease | Bioorg Med Chem Lett 15: 3487-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.003 BindingDB Entry DOI: 10.7270/Q2V12495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125489 ((6S,8R)-1-Chloro-8-(4-methoxy-benzyl)-4-oxo-3-(3-t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration against HCV NS3 protease. | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50158813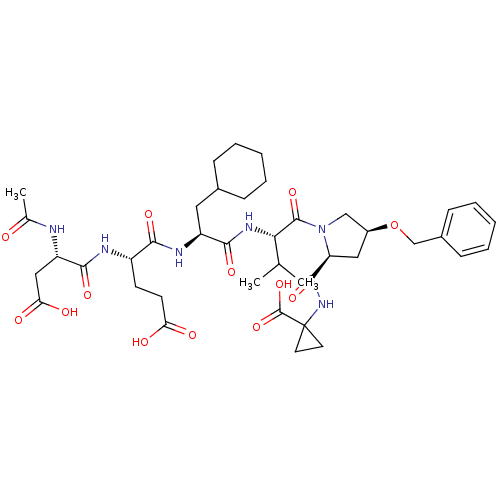 (AcAsp-Glu-Cha-Val-Prb-Cpg | CHEMBL360983) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50131396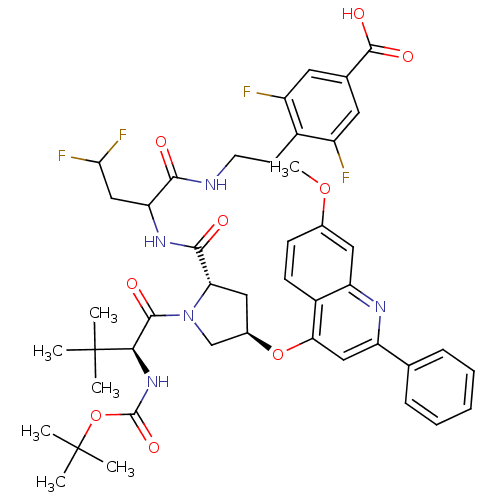 (4-[2-(2-{[(2S,4R)-1-((S)-2-tert-Butoxycarbonylamin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against hepatitis C virus (HCV) NS3 protease | Bioorg Med Chem Lett 13: 2745-8 (2003) BindingDB Entry DOI: 10.7270/Q2DZ07Q9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125376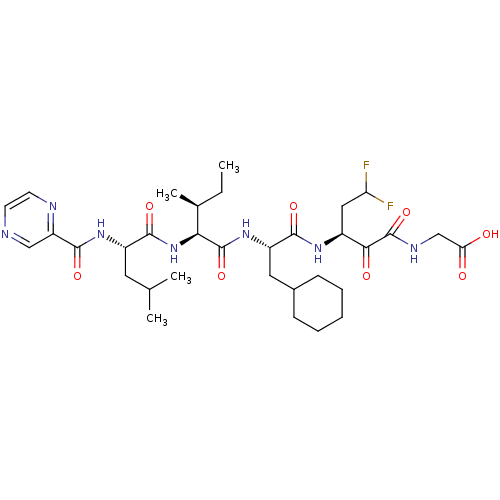 (CHEMBL275290 | {(S)-3-[(S)-3-Cyclohexyl-2-((2S,3S)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against glycine alpha-ketoamide HCV NS3 protease | Bioorg Med Chem Lett 15: 3487-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.003 BindingDB Entry DOI: 10.7270/Q2V12495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50169406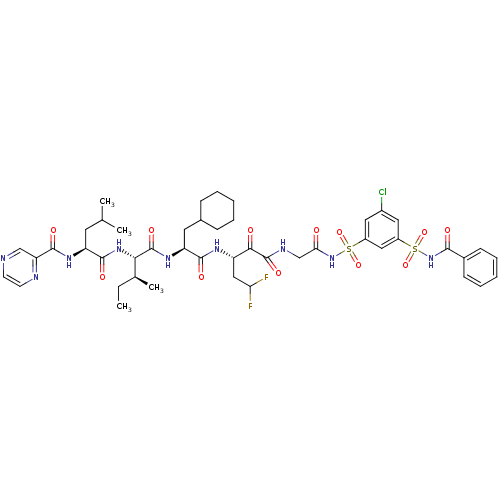 (CHEMBL407699 | Pyrazine-2-carboxylic acid {(S)-1-[...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against glycine alpha-ketoamide HCV NS3 protease | Bioorg Med Chem Lett 15: 3487-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.003 BindingDB Entry DOI: 10.7270/Q2V12495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50169415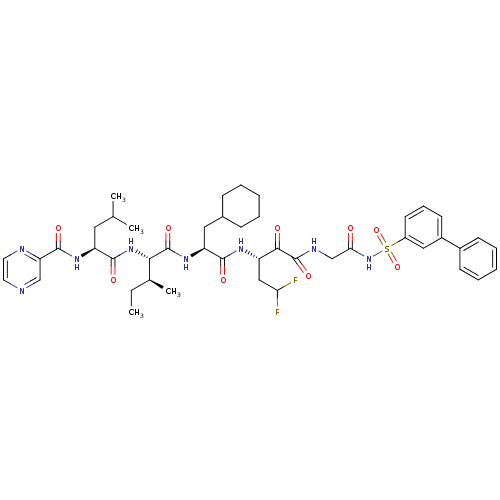 (CHEMBL188449 | Pyrazine-2-carboxylic acid {(S)-1-[...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against glycine alpha-ketoamide HCV NS3 protease | Bioorg Med Chem Lett 15: 3487-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.003 BindingDB Entry DOI: 10.7270/Q2V12495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125483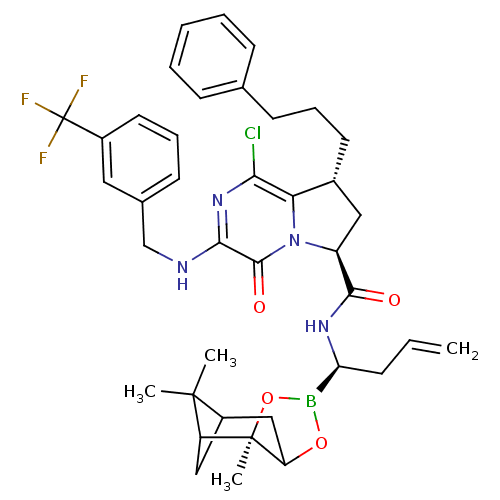 ((6S,8R)-1-Chloro-4-oxo-8-(3-phenyl-propyl)-3-(3-tr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration against HCV NS3 protease. | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125476 ((6S,8R)-1-Chloro-8-(4-methyl-benzyl)-4-oxo-3-(3-tr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration against HCV NS3 protease. | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125475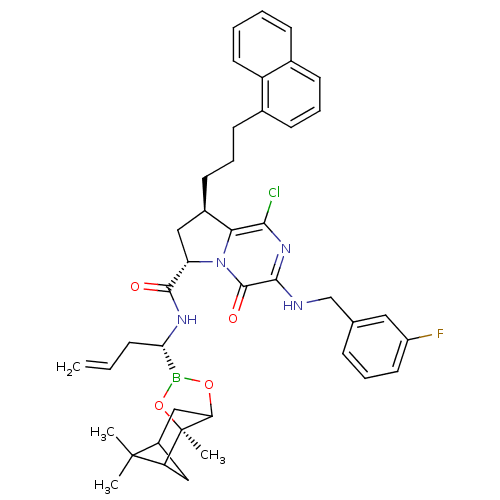 ((6S,8R)-1-Chloro-3-(3-fluoro-benzylamino)-8-(3-nap...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration against HCV NS3 protease. | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125485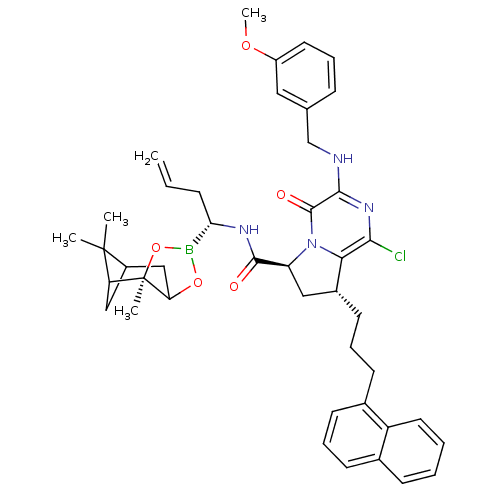 ((6S,8R)-1-Chloro-3-(3-methoxy-benzylamino)-8-(3-na...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration against HCV NS3 protease. | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124048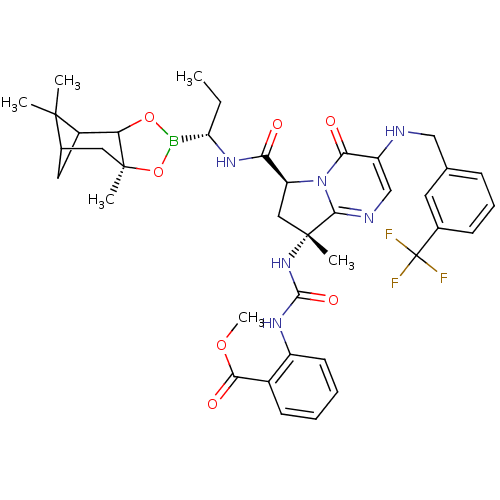 (2-((R)-3-{(S)-8-Methyl-4-oxo-3-(3-trifluoromethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50110001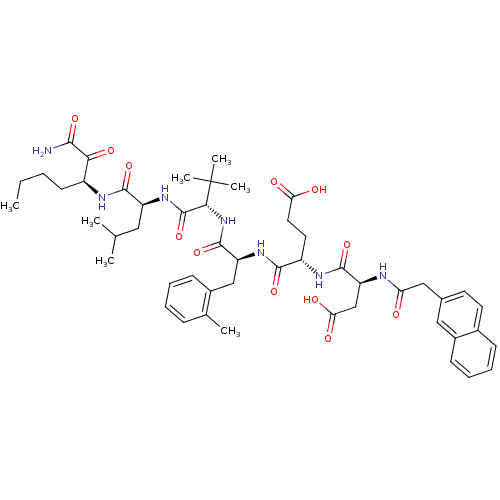 ((S)-4-((S)-1-{(S)-1-[(S)-1-((S)-1-Aminooxalyl-pent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Hepatitis C virus NS3 proteinase | Bioorg Med Chem Lett 12: 641-3 (2002) BindingDB Entry DOI: 10.7270/Q2SN089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124044 ((S)-8-Methyl-8-[(3-methylsulfanyl-phenylcarbamoyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125490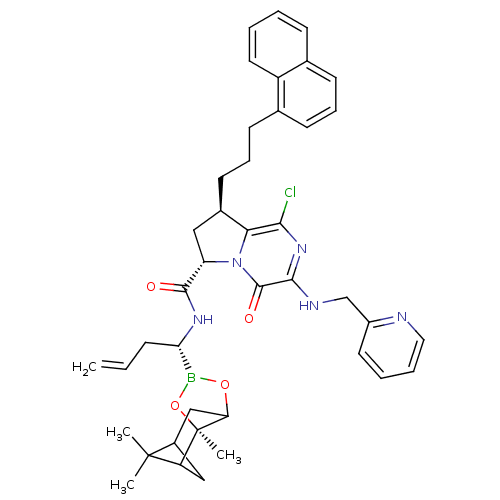 ((6S,8R)-1-Chloro-8-(3-naphthalen-1-yl-propyl)-4-ox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration against HCV NS3 protease. | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein [1658-1692] (Hepatitis C virus) | BDBM92629 ((3S)-3-{[(1R)-1-(4-chloro-2-fluoro-3-phenoxyphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | 62 | n/a | n/a | n/a | n/a | 25 |
Astex Pharmaceuticals | Assay Description The protease activity of the full-length NS3-NS4a and the protease domain were measured using a FRET-based assay using a peptide substrate derived fr... | Nat Chem Biol 8: 920-5 (2012) Article DOI: 10.1038/nchembio.1081 BindingDB Entry DOI: 10.7270/Q26Q1VVN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124056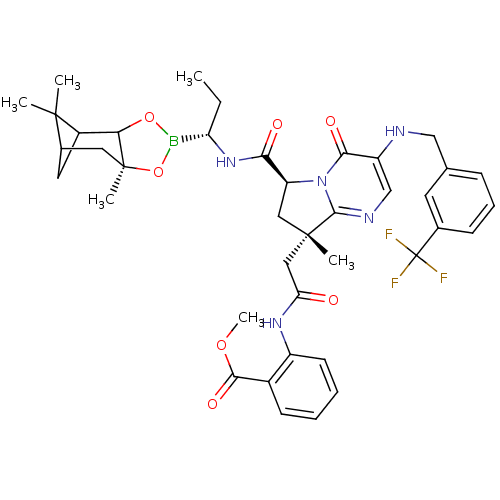 (2-((S)-2-{(S)-8-Methyl-4-oxo-3-(3-trifluoromethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50370548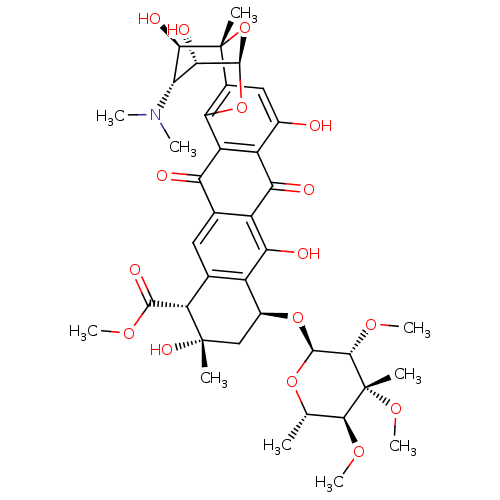 (NOGALAMYCIN) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus helicase | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50370545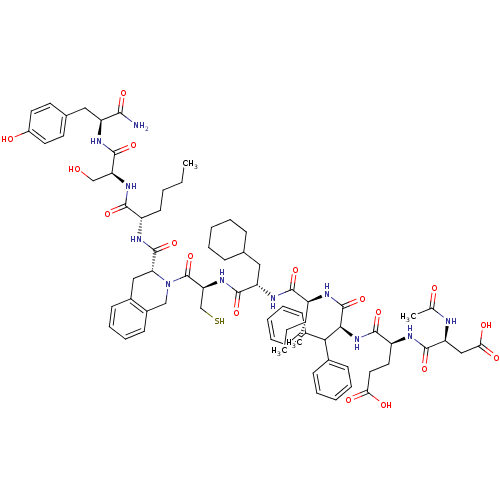 (CHEMBL1791289) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Inhibitory concentration against hepatitis C virus NS3 protease | J Med Chem 48: 1-20 (2005) Article DOI: 10.1021/jm0400101 BindingDB Entry DOI: 10.7270/Q2XP75Q1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124063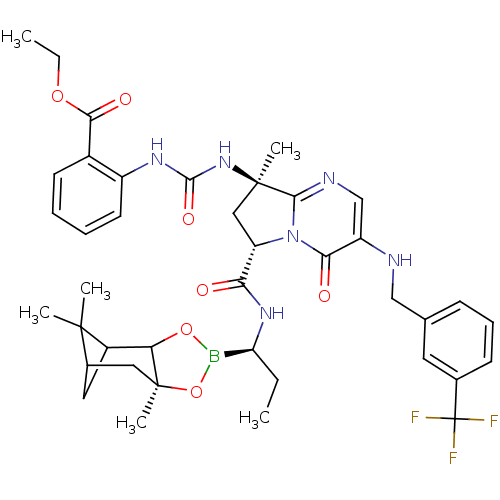 (2-((R)-3-{(S)-8-Methyl-4-oxo-3-(3-trifluoromethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50109997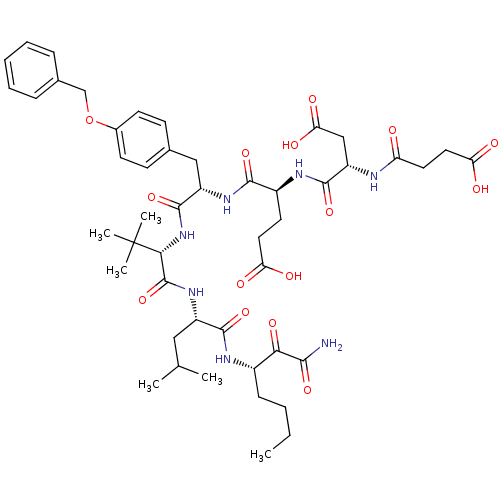 ((S)-4-[(S)-1-{(S)-1-[(S)-1-((S)-1-Aminooxalyl-pent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Hepatitis C virus NS3 proteinase | Bioorg Med Chem Lett 12: 641-3 (2002) BindingDB Entry DOI: 10.7270/Q2SN089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50169407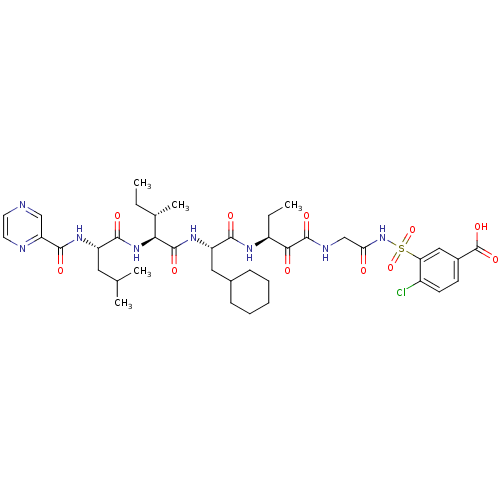 (4-Chloro-3-(2-{(S)-3-[(S)-3-cyclohexyl-2-((S)-3-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against glycine alpha-ketoamide HCV NS3 protease | Bioorg Med Chem Lett 15: 3487-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.003 BindingDB Entry DOI: 10.7270/Q2V12495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125487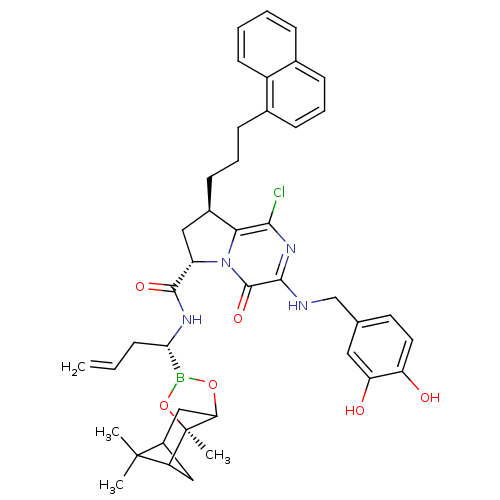 ((6S,8R)-1-Chloro-3-(3,4-dihydroxy-benzylamino)-8-(...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration against HCV NS3 protease. | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50137963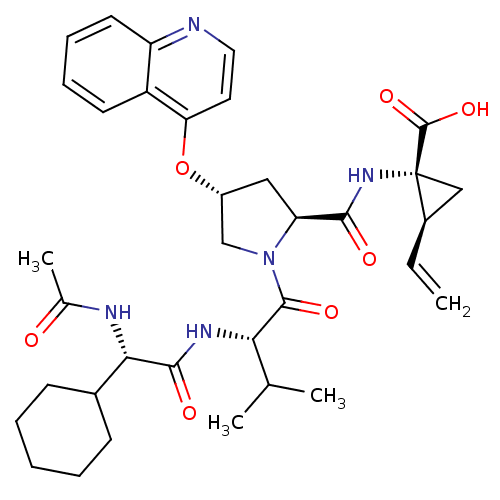 ((1R,2S)-1-((3R,5S)-1-((S)-2-((S)-2-acetamido-2-cyc...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3 protease | J Med Chem 47: 123-32 (2003) Article DOI: 10.1021/jm0303002 BindingDB Entry DOI: 10.7270/Q2Q52P2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50137959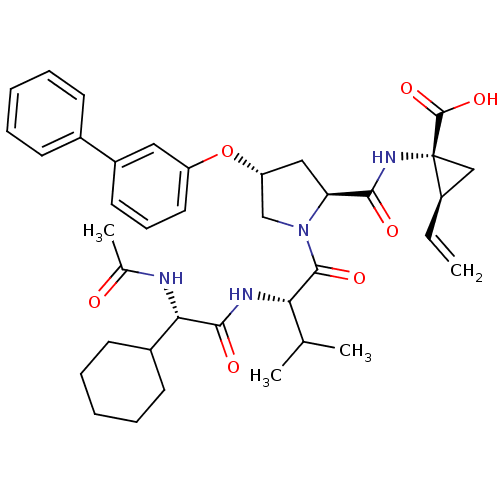 (1-{[(R)-(S)-1-[(S)-2-((S)-2-Acetylamino-2-cyclohex...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus NS3 protease | J Med Chem 47: 123-32 (2003) Article DOI: 10.1021/jm0303002 BindingDB Entry DOI: 10.7270/Q2Q52P2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50169404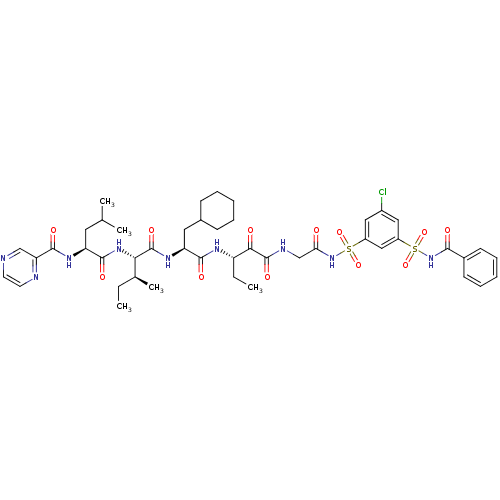 (CHEMBL412198 | Pyrazine-2-carboxylic acid {(S)-1-[...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against glycine alpha-ketoamide HCV NS3 protease | Bioorg Med Chem Lett 15: 3487-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.003 BindingDB Entry DOI: 10.7270/Q2V12495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50169410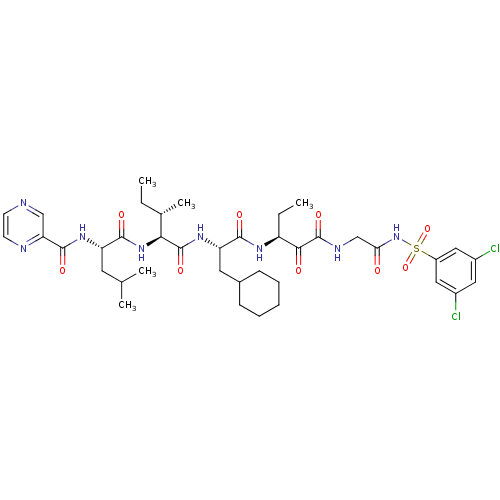 (CHEMBL187781 | Pyrazine-2-carboxylic acid {(S)-1-[...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against glycine alpha-ketoamide HCV NS3 protease | Bioorg Med Chem Lett 15: 3487-90 (2005) Article DOI: 10.1016/j.bmcl.2005.06.003 BindingDB Entry DOI: 10.7270/Q2V12495 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50109996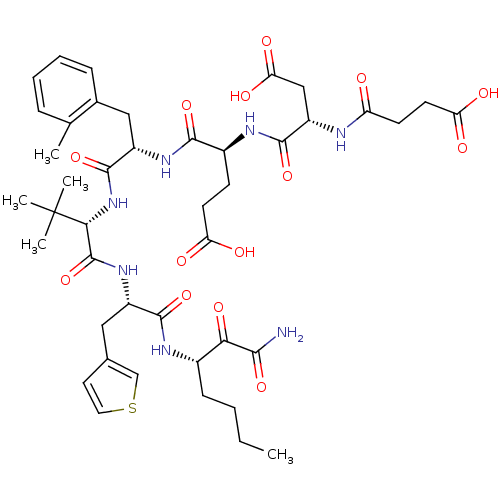 ((S)-4-((S)-1-{(S)-1-[(S)-1-((S)-1-Aminooxalyl-pent...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Discovery Welwyn Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against Hepatitis C virus NS3 proteinase | Bioorg Med Chem Lett 12: 641-3 (2002) BindingDB Entry DOI: 10.7270/Q2SN089N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124055 ((S)-8-Methyl-8-[(R)-3-(3-methylsulfanyl-phenyl)-ur...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124049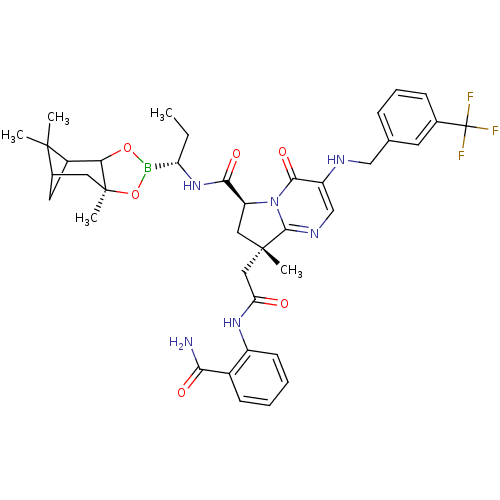 ((S)-8-[(2-Carbamoyl-phenylcarbamoyl)-methyl]-8-met...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50124039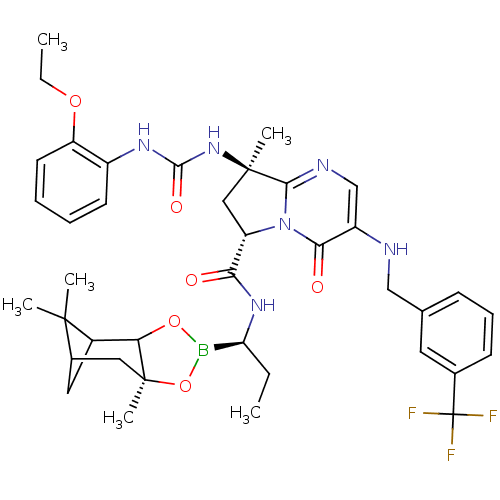 ((S)-8-[(R)-3-(2-Ethoxy-phenyl)-ureido]-8-methyl-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity hepatitis C virus NS3 protease. | Bioorg Med Chem Lett 13: 785-8 (2003) BindingDB Entry DOI: 10.7270/Q2JW8D84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 276 total ) | Next | Last >> |