 Found 85 hits Enz. Inhib. hit(s) with all data for entry = 50041281
Found 85 hits Enz. Inhib. hit(s) with all data for entry = 50041281 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408979

(CHEMBL103400)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@H]1[C@H](O)[C@H](O)c2ccccc12 Show InChI InChI=1S/C33H40N2O6/c1-33(2,3)41-32(40)34-26(19-22-14-8-5-9-15-22)27(36)20-23(18-21-12-6-4-7-13-21)31(39)35-28-24-16-10-11-17-25(24)29(37)30(28)38/h4-17,23,26-30,36-38H,18-20H2,1-3H3,(H,34,40)(H,35,39)/t23-,26+,27+,28-,29-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408987
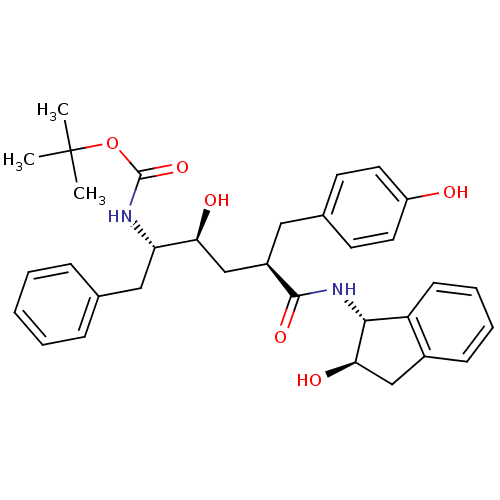
(CHEMBL318531)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C33H40N2O6/c1-33(2,3)41-32(40)34-27(18-21-9-5-4-6-10-21)28(37)20-24(17-22-13-15-25(36)16-14-22)31(39)35-30-26-12-8-7-11-23(26)19-29(30)38/h4-16,24,27-30,36-38H,17-20H2,1-3H3,(H,34,40)(H,35,39)/t24-,27+,28+,29-,30-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408995
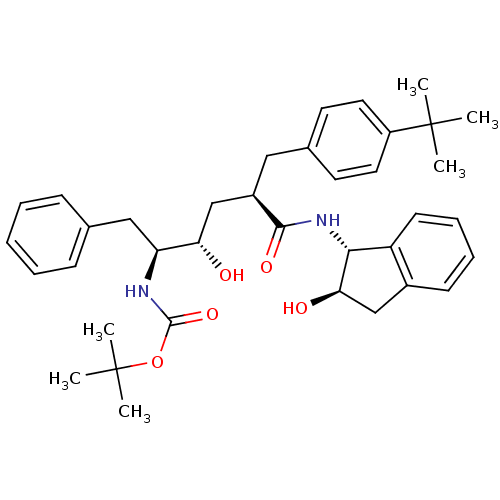
(CHEMBL319417)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccc(cc1)C(C)(C)C)C(=O)N[C@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C37H48N2O5/c1-36(2,3)28-18-16-25(17-19-28)20-27(34(42)39-33-29-15-11-10-14-26(29)22-32(33)41)23-31(40)30(21-24-12-8-7-9-13-24)38-35(43)44-37(4,5)6/h7-19,27,30-33,40-41H,20-23H2,1-6H3,(H,38,43)(H,39,42)/t27-,30+,31+,32-,33-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408943
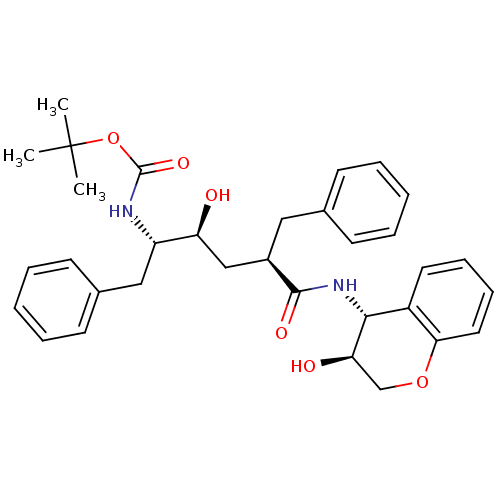
(CHEMBL103213)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@H]1[C@H](O)COc2ccccc12 Show InChI InChI=1S/C33H40N2O6/c1-33(2,3)41-32(39)34-26(19-23-14-8-5-9-15-23)27(36)20-24(18-22-12-6-4-7-13-22)31(38)35-30-25-16-10-11-17-29(25)40-21-28(30)37/h4-17,24,26-28,30,36-37H,18-21H2,1-3H3,(H,34,39)(H,35,38)/t24-,26+,27+,28-,30-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50005358

(CHEMBL430875)Show SMILES [H][C@](O)(C[C@@]([H])(CCCc1ccccc1)C(=O)N[C@]1([H])c2ccccc2[C@@]([H])(O)[C@@]1([H])O)[C@]([H])(Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C35H44N2O6/c1-35(2,3)43-34(42)36-28(21-24-15-8-5-9-16-24)29(38)22-25(18-12-17-23-13-6-4-7-14-23)33(41)37-30-26-19-10-11-20-27(26)31(39)32(30)40/h4-11,13-16,19-20,25,28-32,38-40H,12,17-18,21-22H2,1-3H3,(H,36,42)(H,37,41)/t25-,28+,29+,30-,31-,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50005357
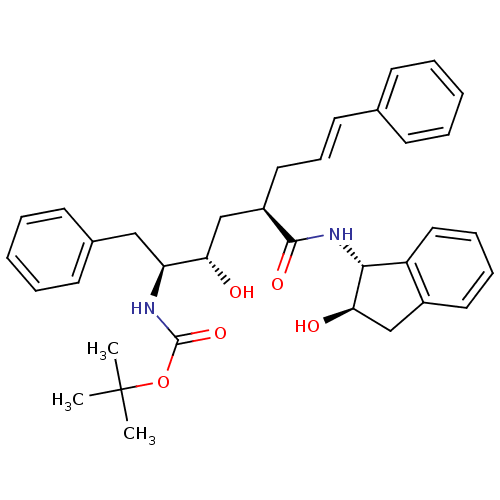
(CHEMBL418986)Show SMILES [H][C@](O)(C[C@@]([H])(C\C=C\c1ccccc1)C(=O)N[C@]1([H])c2ccccc2C[C@@]1([H])O)[C@]([H])(Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C35H42N2O5/c1-35(2,3)42-34(41)36-29(21-25-15-8-5-9-16-25)30(38)23-27(19-12-17-24-13-6-4-7-14-24)33(40)37-32-28-20-11-10-18-26(28)22-31(32)39/h4-18,20,27,29-32,38-39H,19,21-23H2,1-3H3,(H,36,41)(H,37,40)/b17-12+/t27-,29+,30+,31-,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408986

(CHEMBL318003)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C33H40N2O5/c1-33(2,3)40-32(39)34-27(19-23-14-8-5-9-15-23)28(36)21-25(18-22-12-6-4-7-13-22)31(38)35-30-26-17-11-10-16-24(26)20-29(30)37/h4-17,25,27-30,36-37H,18-21H2,1-3H3,(H,34,39)(H,35,38)/t25-,27+,28+,29-,30-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.250 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50005385
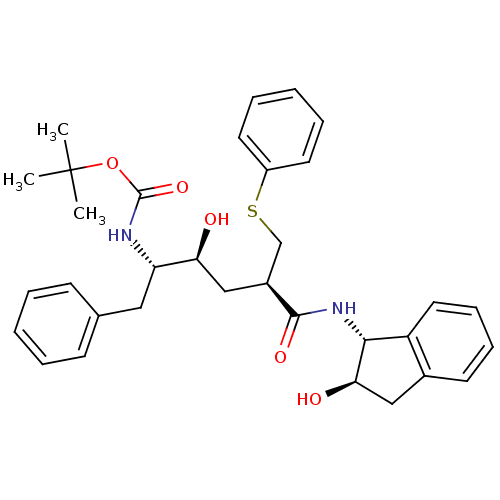
(CHEMBL105319)Show SMILES [H][C@](O)(C[C@@]([H])(CSc1ccccc1)C(=O)N[C@]1([H])c2ccccc2C[C@@]1([H])O)[C@]([H])(Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C33H40N2O5S/c1-33(2,3)40-32(39)34-27(18-22-12-6-4-7-13-22)28(36)20-24(21-41-25-15-8-5-9-16-25)31(38)35-30-26-17-11-10-14-23(26)19-29(30)37/h4-17,24,27-30,36-37H,18-21H2,1-3H3,(H,34,39)(H,35,38)/t24-,27-,28-,29+,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.250 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408985
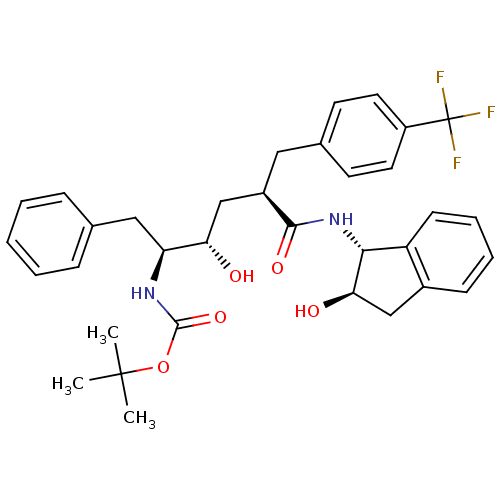
(CHEMBL263938)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccc(cc1)C(F)(F)F)C(=O)N[C@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C34H39F3N2O5/c1-33(2,3)44-32(43)38-27(18-21-9-5-4-6-10-21)28(40)20-24(17-22-13-15-25(16-14-22)34(35,36)37)31(42)39-30-26-12-8-7-11-23(26)19-29(30)41/h4-16,24,27-30,40-41H,17-20H2,1-3H3,(H,38,43)(H,39,42)/t24-,27+,28+,29-,30-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408983
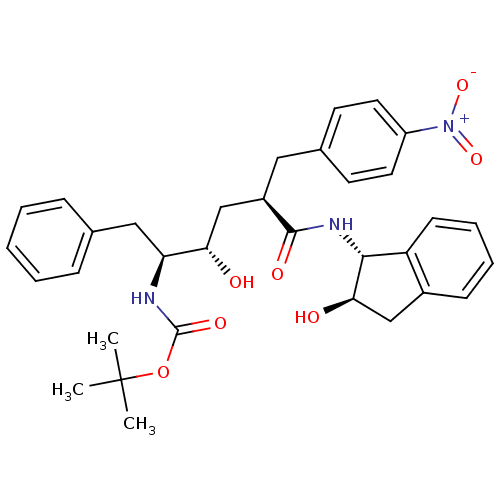
(CHEMBL322897)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccc(cc1)[N+]([O-])=O)C(=O)N[C@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C33H39N3O7/c1-33(2,3)43-32(40)34-27(18-21-9-5-4-6-10-21)28(37)20-24(17-22-13-15-25(16-14-22)36(41)42)31(39)35-30-26-12-8-7-11-23(26)19-29(30)38/h4-16,24,27-30,37-38H,17-20H2,1-3H3,(H,34,40)(H,35,39)/t24-,27+,28+,29-,30-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408952

(CHEMBL103821)Show SMILES Cc1ccc(C[C@H](C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)C(=O)N[C@H]2[C@H](O)Cc3ccccc23)cc1 Show InChI InChI=1S/C34H42N2O5/c1-22-14-16-24(17-15-22)18-26(32(39)36-31-27-13-9-8-12-25(27)20-30(31)38)21-29(37)28(19-23-10-6-5-7-11-23)35-33(40)41-34(2,3)4/h5-17,26,28-31,37-38H,18-21H2,1-4H3,(H,35,40)(H,36,39)/t26-,28+,29+,30-,31-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408945

(CHEMBL317868)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccc(N)cc1)C(=O)N[C@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C33H41N3O5/c1-33(2,3)41-32(40)35-27(18-21-9-5-4-6-10-21)28(37)20-24(17-22-13-15-25(34)16-14-22)31(39)36-30-26-12-8-7-11-23(26)19-29(30)38/h4-16,24,27-30,37-38H,17-20,34H2,1-3H3,(H,35,40)(H,36,39)/t24-,27+,28+,29-,30-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408967
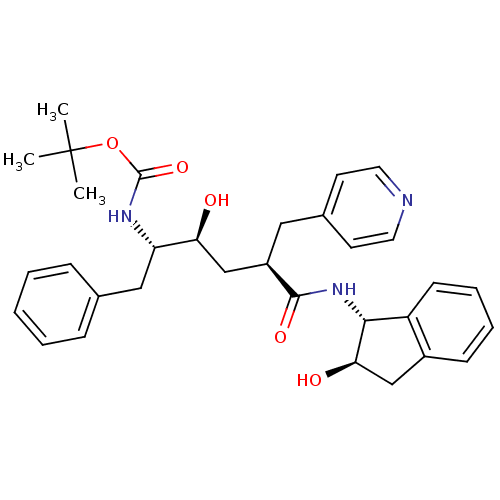
(CHEMBL322214)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccncc1)C(=O)N[C@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C32H39N3O5/c1-32(2,3)40-31(39)34-26(18-21-9-5-4-6-10-21)27(36)20-24(17-22-13-15-33-16-14-22)30(38)35-29-25-12-8-7-11-23(25)19-28(29)37/h4-16,24,26-29,36-37H,17-20H2,1-3H3,(H,34,39)(H,35,38)/t24-,26+,27+,28-,29-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408963
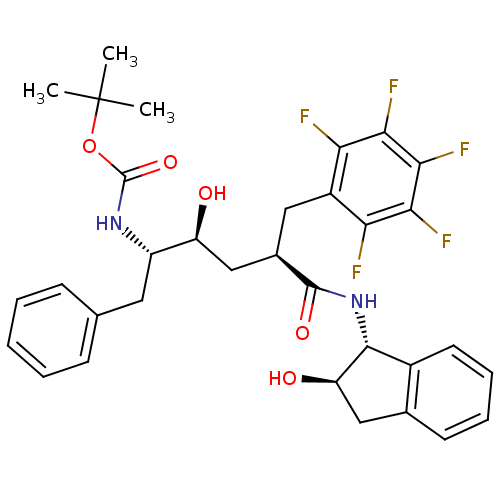
(CHEMBL322634)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1c(F)c(F)c(F)c(F)c1F)C(=O)N[C@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C33H35F5N2O5/c1-33(2,3)45-32(44)39-22(13-17-9-5-4-6-10-17)23(41)16-19(14-21-25(34)27(36)29(38)28(37)26(21)35)31(43)40-30-20-12-8-7-11-18(20)15-24(30)42/h4-12,19,22-24,30,41-42H,13-16H2,1-3H3,(H,39,44)(H,40,43)/t19-,22+,23+,24-,30-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408974

(CHEMBL317446)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@H]1[C@H](O)CCc2ccccc12 Show InChI InChI=1S/C34H42N2O5/c1-34(2,3)41-33(40)35-28(21-24-14-8-5-9-15-24)30(38)22-26(20-23-12-6-4-7-13-23)32(39)36-31-27-17-11-10-16-25(27)18-19-29(31)37/h4-17,26,28-31,37-38H,18-22H2,1-3H3,(H,35,40)(H,36,39)/t26-,28+,29-,30+,31-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408951
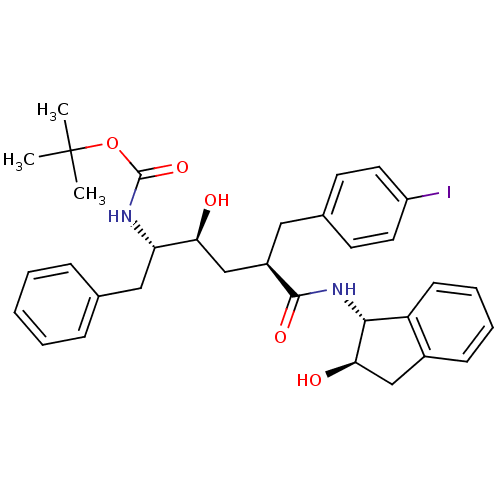
(CHEMBL420466)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccc(I)cc1)C(=O)N[C@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C33H39IN2O5/c1-33(2,3)41-32(40)35-27(18-21-9-5-4-6-10-21)28(37)20-24(17-22-13-15-25(34)16-14-22)31(39)36-30-26-12-8-7-11-23(26)19-29(30)38/h4-16,24,27-30,37-38H,17-20H2,1-3H3,(H,35,40)(H,36,39)/t24-,27+,28+,29-,30-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.719 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50005367
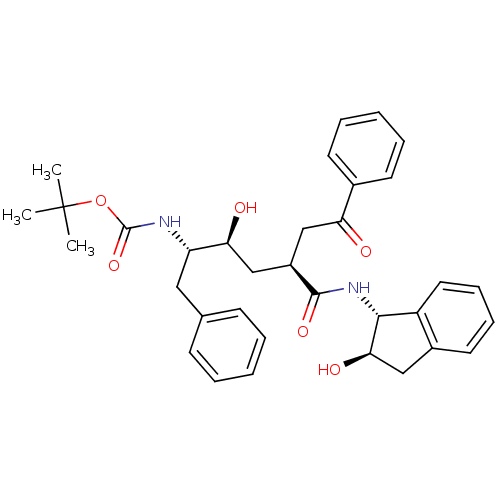
(CHEMBL318319)Show SMILES [H][C@](O)(C[C@@]([H])(CC(=O)c1ccccc1)C(=O)N[C@]1([H])c2ccccc2C[C@@]1([H])O)[C@]([H])(Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C34H40N2O6/c1-34(2,3)42-33(41)35-27(18-22-12-6-4-7-13-22)29(38)21-25(20-28(37)23-14-8-5-9-15-23)32(40)36-31-26-17-11-10-16-24(26)19-30(31)39/h4-17,25,27,29-31,38-39H,18-21H2,1-3H3,(H,35,41)(H,36,40)/t25-,27+,29+,30-,31-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408975
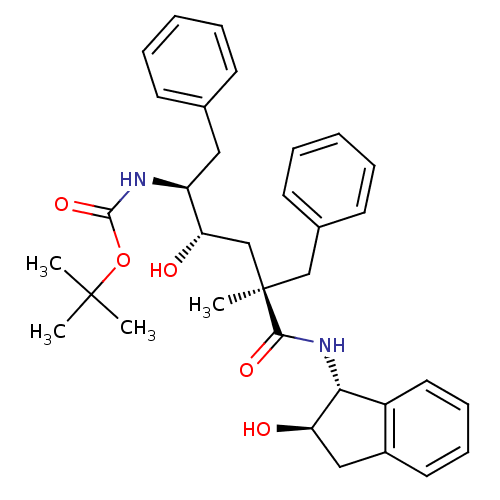
(CHEMBL320150)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@](C)(Cc1ccccc1)C(=O)N[C@H]1[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C34H42N2O5/c1-33(2,3)41-32(40)35-27(19-23-13-7-5-8-14-23)29(38)22-34(4,21-24-15-9-6-10-16-24)31(39)36-30-26-18-12-11-17-25(26)20-28(30)37/h5-18,27-30,37-38H,19-22H2,1-4H3,(H,35,40)(H,36,39)/t27-,28+,29-,30+,34+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408980

(CHEMBL421019)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1C=CC[C@H]1O |c:33| Show InChI InChI=1S/C29H38N2O5/c1-29(2,3)36-28(35)31-24(18-21-13-8-5-9-14-21)26(33)19-22(17-20-11-6-4-7-12-20)27(34)30-23-15-10-16-25(23)32/h4-15,22-26,32-33H,16-19H2,1-3H3,(H,30,34)(H,31,35)/t22-,23-,24+,25-,26+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50005391
(CHEMBL321503)Show SMILES [H][C@](O)(C[C@@]([H])(CC=C)C(=O)N[C@]1([H])c2ccccc2C[C@@]1([H])O)[C@]([H])(Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C29H38N2O5/c1-5-11-21(27(34)31-26-22-15-10-9-14-20(22)17-25(26)33)18-24(32)23(16-19-12-7-6-8-13-19)30-28(35)36-29(2,3)4/h5-10,12-15,21,23-26,32-33H,1,11,16-18H2,2-4H3,(H,30,35)(H,31,34)/t21-,23+,24+,25-,26-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50005389

(CHEMBL322221)Show SMILES [H][C@](CC(O)[C@]([H])(Cc1ccccc1)NC(=O)OC(C)(C)C)(Cc1ccccc1)C(=O)N[C@@]1([H])CCc2ccccc12 Show InChI InChI=1S/C33H40N2O4/c1-33(2,3)39-32(38)35-29(21-24-14-8-5-9-15-24)30(36)22-26(20-23-12-6-4-7-13-23)31(37)34-28-19-18-25-16-10-11-17-27(25)28/h4-17,26,28-30,36H,18-22H2,1-3H3,(H,34,37)(H,35,38)/t26-,28+,29+,30?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50455440

(CHEMBL2367850)Show SMILES [H][C@](O)(C[C@@]([H])(Cc1ccccc1)C(=O)N[C@H](CO)c1ccccc1)[C@]([H])(Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C32H40N2O5/c1-32(2,3)39-31(38)34-27(20-24-15-9-5-10-16-24)29(36)21-26(19-23-13-7-4-8-14-23)30(37)33-28(22-35)25-17-11-6-12-18-25/h4-18,26-29,35-36H,19-22H2,1-3H3,(H,33,37)(H,34,38)/t26-,27+,28-,29+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408966

(CHEMBL99949)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@@H](O)COc2ccccc12 Show InChI InChI=1S/C33H40N2O6/c1-33(2,3)41-32(39)34-26(19-23-14-8-5-9-15-23)27(36)20-24(18-22-12-6-4-7-13-22)31(38)35-30-25-16-10-11-17-29(25)40-21-28(30)37/h4-17,24,26-28,30,36-37H,18-21H2,1-3H3,(H,34,39)(H,35,38)/t24-,26+,27+,28+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408970
(CHEMBL321551)Show SMILES COC(=O)[C@@H]1Cc2ccccc2[C@H]1NC(=O)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C35H42N2O6/c1-35(2,3)43-34(41)36-29(20-24-15-9-6-10-16-24)30(38)22-26(19-23-13-7-5-8-14-23)32(39)37-31-27-18-12-11-17-25(27)21-28(31)33(40)42-4/h5-18,26,28-31,38H,19-22H2,1-4H3,(H,36,41)(H,37,39)/t26-,28-,29+,30+,31-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50408947
(CHEMBL103211)Show SMILES CCOC(=O)c1c(C)[nH]c(\C=C2\C(=O)Nc3ccccc23)c1C Show InChI InChI=1S/C18H18N2O3/c1-4-23-18(22)16-10(2)15(19-11(16)3)9-13-12-7-5-6-8-14(12)20-17(13)21/h5-9,19H,4H2,1-3H3,(H,20,21)/b13-9+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM758

(CHEMBL277034 | Hydroxyethylene deriv. 9 | tert-but...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)NCc1ccccc1 |r| Show InChI InChI=1S/C31H38N2O4/c1-31(2,3)37-30(36)33-27(20-24-15-9-5-10-16-24)28(34)21-26(19-23-13-7-4-8-14-23)29(35)32-22-25-17-11-6-12-18-25/h4-18,26-28,34H,19-22H2,1-3H3,(H,32,35)(H,33,36)/t26-,27+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50408957
(CHEMBL437643)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1CCCC[C@H]1O Show InChI InChI=1S/C30H42N2O5/c1-30(2,3)37-29(36)32-25(19-22-14-8-5-9-15-22)27(34)20-23(18-21-12-6-4-7-13-21)28(35)31-24-16-10-11-17-26(24)33/h4-9,12-15,23-27,33-34H,10-11,16-20H2,1-3H3,(H,31,35)(H,32,36)/t23-,24-,25+,26-,27+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM833
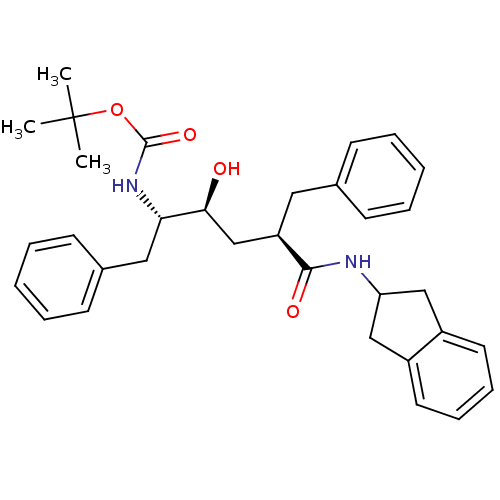
(Benzocycloalkyl Amines deriv. 3 | Benzocycloalkyl ...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)NC1Cc2ccccc2C1 |r| Show InChI InChI=1S/C33H40N2O4/c1-33(2,3)39-32(38)35-29(19-24-14-8-5-9-15-24)30(36)22-27(18-23-12-6-4-7-13-23)31(37)34-28-20-25-16-10-11-17-26(25)21-28/h4-17,27-30,36H,18-22H2,1-3H3,(H,34,37)(H,35,38)/t27-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50408953
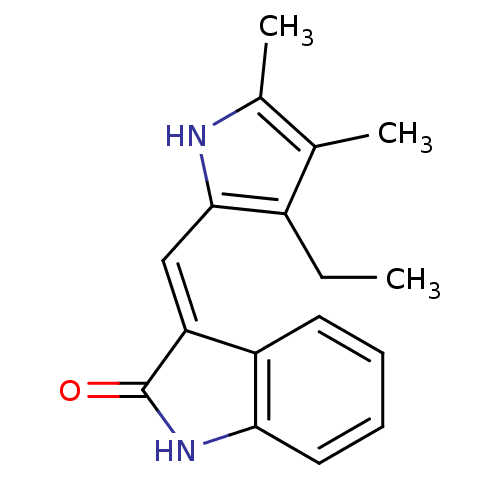
(CHEMBL320186)Show InChI InChI=1S/C17H18N2O/c1-4-12-10(2)11(3)18-16(12)9-14-13-7-5-6-8-15(13)19-17(14)20/h5-9,18H,4H2,1-3H3,(H,19,20)/b14-9+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50455439
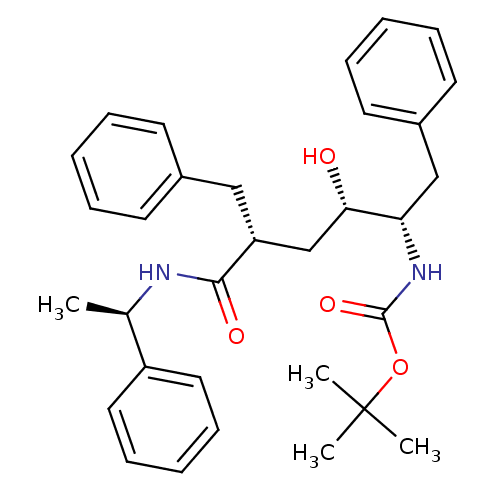
(CHEMBL2367852)Show SMILES [H][C@](C)(NC(=O)[C@]([H])(Cc1ccccc1)C[C@]([H])(O)[C@]([H])(Cc1ccccc1)NC(=O)OC(C)(C)C)c1ccccc1 Show InChI InChI=1S/C32H40N2O4/c1-23(26-18-12-7-13-19-26)33-30(36)27(20-24-14-8-5-9-15-24)22-29(35)28(21-25-16-10-6-11-17-25)34-31(37)38-32(2,3)4/h5-19,23,27-29,35H,20-22H2,1-4H3,(H,33,36)(H,34,37)/t23-,27-,28+,29+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50455442
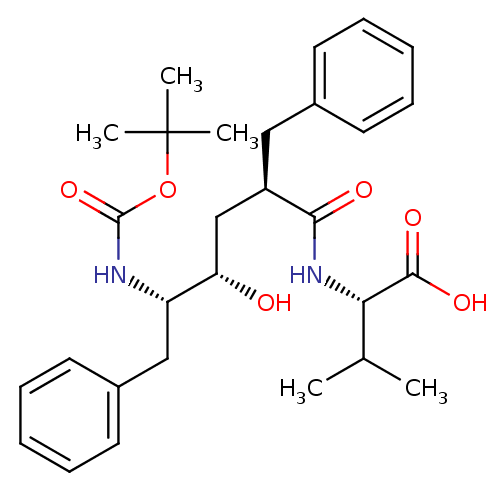
(CHEMBL2367848)Show SMILES [H][C@](O)(C[C@@]([H])(Cc1ccccc1)C(=O)N[C@@]([H])(C(C)C)C(O)=O)[C@]([H])(Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C29H40N2O6/c1-19(2)25(27(34)35)31-26(33)22(16-20-12-8-6-9-13-20)18-24(32)23(17-21-14-10-7-11-15-21)30-28(36)37-29(3,4)5/h6-15,19,22-25,32H,16-18H2,1-5H3,(H,30,36)(H,31,33)(H,34,35)/t22-,23+,24+,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50408949
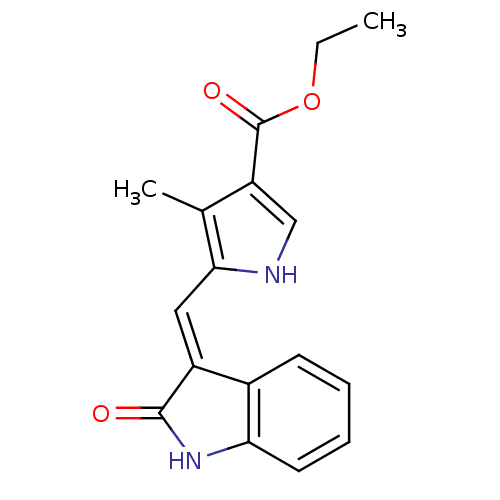
(CHEMBL323523)Show InChI InChI=1S/C17H16N2O3/c1-3-22-17(21)13-9-18-15(10(13)2)8-12-11-6-4-5-7-14(11)19-16(12)20/h4-9,18H,3H2,1-2H3,(H,19,20)/b12-8+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 182 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50035629
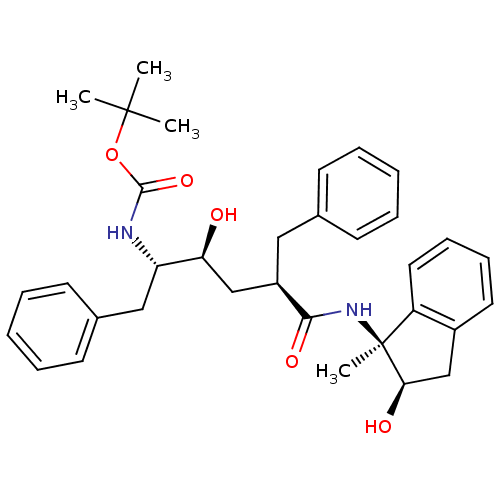
(CHEMBL105299 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-4-((...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@]1(C)[C@H](O)Cc2ccccc12 Show InChI InChI=1S/C34H42N2O5/c1-33(2,3)41-32(40)35-28(20-24-15-9-6-10-16-24)29(37)21-26(19-23-13-7-5-8-14-23)31(39)36-34(4)27-18-12-11-17-25(27)22-30(34)38/h5-18,26,28-30,37-38H,19-22H2,1-4H3,(H,35,40)(H,36,39)/t26-,28+,29+,30-,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50065292
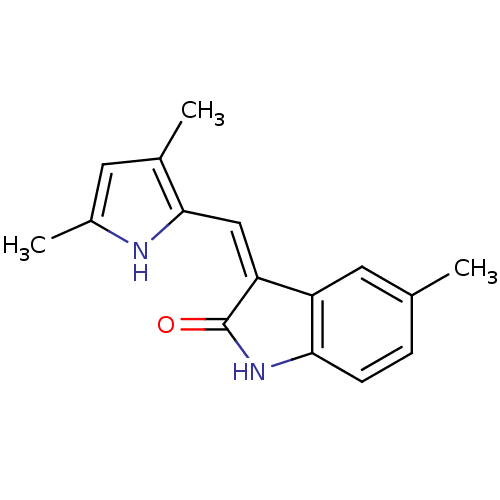
((Z)-3-(3,5-Dimethyl-1H-pyrrol-2-ylmethylene)-5-met...)Show InChI InChI=1S/C16H16N2O/c1-9-4-5-14-12(6-9)13(16(19)18-14)8-15-10(2)7-11(3)17-15/h4-8,17H,1-3H3,(H,18,19)/b13-8- | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 302 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50375638
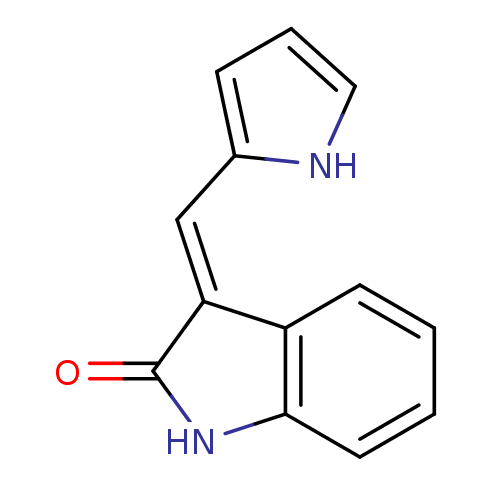
(CHEMBL101797)Show InChI InChI=1S/C13H10N2O/c16-13-11(8-9-4-3-7-14-9)10-5-1-2-6-12(10)15-13/h1-8,14H,(H,15,16)/b11-8+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 389 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50408958

(CHEMBL320161)Show InChI InChI=1S/C17H18N2O3/c1-10-9-18-15(11(10)6-7-16(20)21)8-13-12-4-2-3-5-14(12)19-17(13)22/h2-5,8-9,16,18,20-21H,6-7H2,1H3,(H,19,22)/b13-8+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50455441
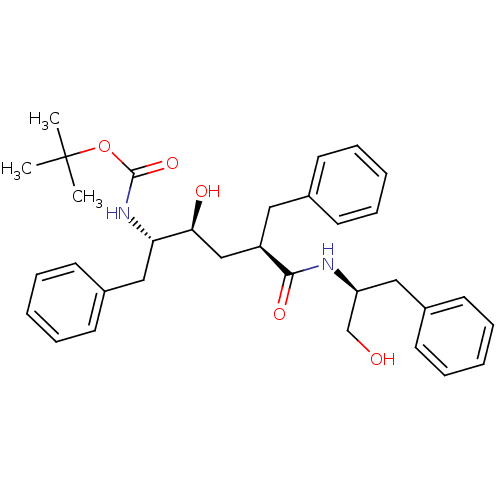
(CHEMBL2367851)Show SMILES [H][C@@](CO)(Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)C[C@]([H])(O)[C@]([H])(Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C33H42N2O5/c1-33(2,3)40-32(39)35-29(21-26-17-11-6-12-18-26)30(37)22-27(19-24-13-7-4-8-14-24)31(38)34-28(23-36)20-25-15-9-5-10-16-25/h4-18,27-30,36-37H,19-23H2,1-3H3,(H,34,38)(H,35,39)/t27-,28+,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50408982
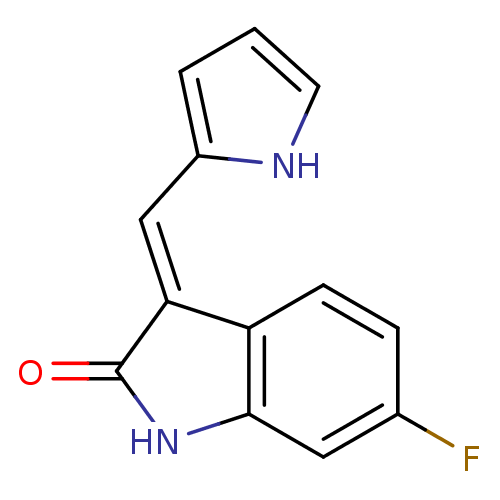
(CHEMBL323085)Show InChI InChI=1S/C13H9FN2O/c14-8-3-4-10-11(7-9-2-1-5-15-9)13(17)16-12(10)6-8/h1-7,15H,(H,16,17)/b11-7+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50408992
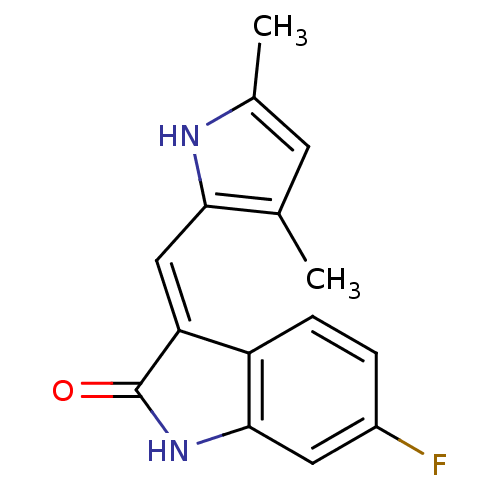
(CHEMBL104330)Show InChI InChI=1S/C15H13FN2O/c1-8-5-9(2)17-13(8)7-12-11-4-3-10(16)6-14(11)18-15(12)19/h3-7,17H,1-2H3,(H,18,19)/b12-7+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 708 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50111603
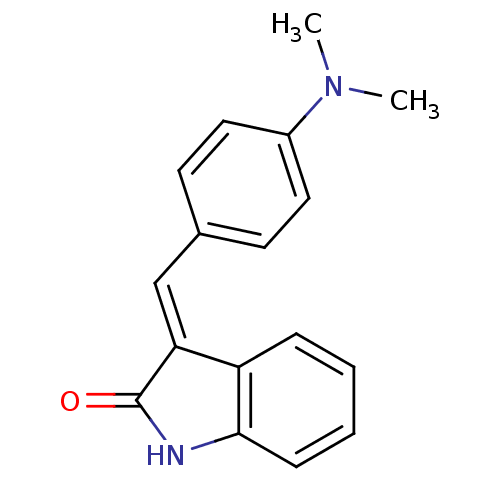
(3-(4-(dimethylamino)benzylidene)indolin-2-one | 3-...)Show InChI InChI=1S/C17H16N2O/c1-19(2)13-9-7-12(8-10-13)11-15-14-5-3-4-6-16(14)18-17(15)20/h3-11H,1-2H3,(H,18,20)/b15-11+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 794 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50065288
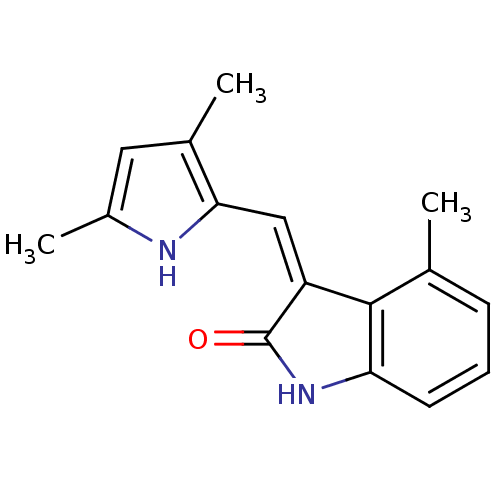
(3-(3,5-Dimethyl-1H-pyrrol-2-ylmethylene)-4-methyl-...)Show InChI InChI=1S/C16H16N2O/c1-9-5-4-6-13-15(9)12(16(19)18-13)8-14-10(2)7-11(3)17-14/h4-8,17H,1-3H3,(H,18,19)/b12-8- | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50408965

(CHEMBL105739)Show InChI InChI=1S/C15H14N2O/c1-9-7-10(2)16-14(9)8-12-11-5-3-4-6-13(11)17-15(12)18/h3-8,16H,1-2H3,(H,17,18)/b12-8+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50065312
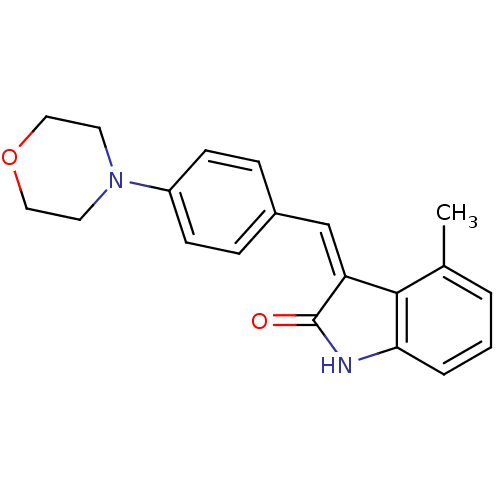
((Z)-4-methyl-3-(4-morpholinobenzylidene)indolin-2-...)Show InChI InChI=1S/C20H20N2O2/c1-14-3-2-4-18-19(14)17(20(23)21-18)13-15-5-7-16(8-6-15)22-9-11-24-12-10-22/h2-8,13H,9-12H2,1H3,(H,21,23)/b17-13- | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50408971
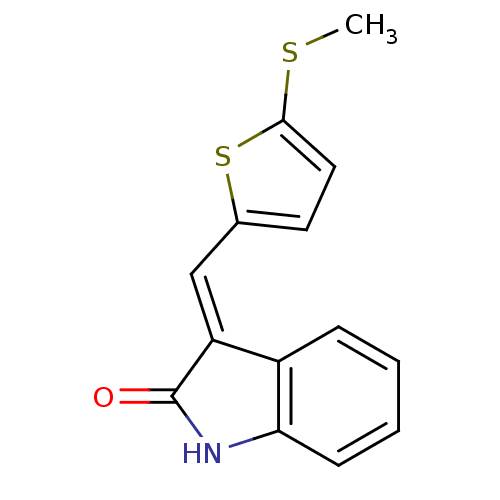
(CHEMBL441641)Show InChI InChI=1S/C14H11NOS2/c1-17-13-7-6-9(18-13)8-11-10-4-2-3-5-12(10)15-14(11)16/h2-8H,1H3,(H,15,16)/b11-8+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50408956
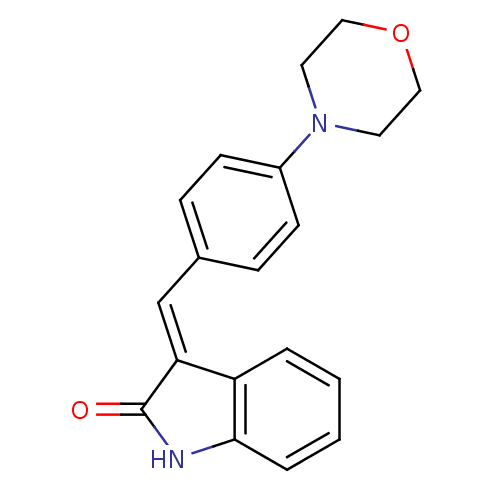
(CHEMBL321797)Show InChI InChI=1S/C19H18N2O2/c22-19-17(16-3-1-2-4-18(16)20-19)13-14-5-7-15(8-6-14)21-9-11-23-12-10-21/h1-8,13H,9-12H2,(H,20,22)/b17-13+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50065290
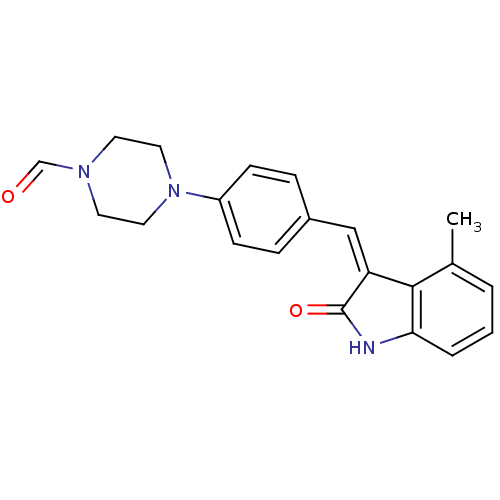
((Z)-4-(4-((4-methyl-2-oxoindolin-3-ylidene)methyl)...)Show SMILES Cc1cccc2NC(=O)\C(=C/c3ccc(cc3)N3CCN(CC3)C=O)c12 Show InChI InChI=1S/C21H21N3O2/c1-15-3-2-4-19-20(15)18(21(26)22-19)13-16-5-7-17(8-6-16)24-11-9-23(14-25)10-12-24/h2-8,13-14H,9-12H2,1H3,(H,22,26)/b18-13- | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50131995

((E)-3-(4-hydroxybenzylidene)indolin-2-one | 3-(4-H...)Show InChI InChI=1S/C15H11NO2/c17-11-7-5-10(6-8-11)9-13-12-3-1-2-4-14(12)16-15(13)18/h1-9,17H,(H,16,18)/b13-9+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50408978
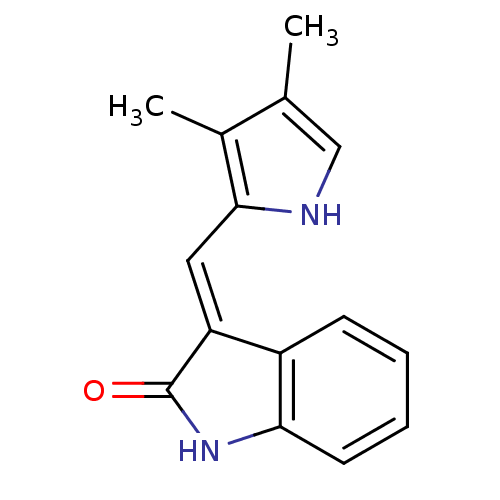
(CHEMBL104908)Show InChI InChI=1S/C15H14N2O/c1-9-8-16-14(10(9)2)7-12-11-5-3-4-6-13(11)17-15(12)18/h3-8,16H,1-2H3,(H,17,18)/b12-7+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50005383
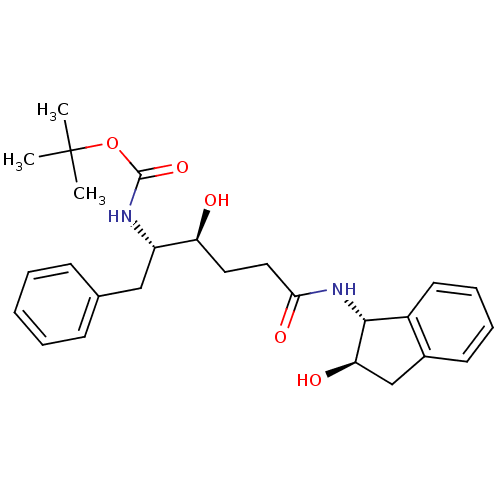
(CHEMBL319192)Show SMILES [H][C@](O)(CCC(=O)N[C@]1([H])c2ccccc2C[C@@]1([H])O)[C@]([H])(Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C26H34N2O5/c1-26(2,3)33-25(32)27-20(15-17-9-5-4-6-10-17)21(29)13-14-23(31)28-24-19-12-8-7-11-18(19)16-22(24)30/h4-12,20-22,24,29-30H,13-16H2,1-3H3,(H,27,32)(H,28,31)/t20-,21-,22+,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease. |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Mus musculus) | BDBM50408977
(CHEMBL102770)Show InChI InChI=1S/C19H18N2O/c22-19-17(16-5-1-2-6-18(16)20-19)13-14-7-9-15(10-8-14)21-11-3-4-12-21/h1-2,5-10,13H,3-4,11-12H2,(H,20,22)/b17-13+ | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Inhibitory activity against vascular endothelial growth factor receptor 2 (FLK1) |
J Med Chem 43: 3020-32 (2000)
BindingDB Entry DOI: 10.7270/Q2HM59P2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data