

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] SR141716 from rat recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267907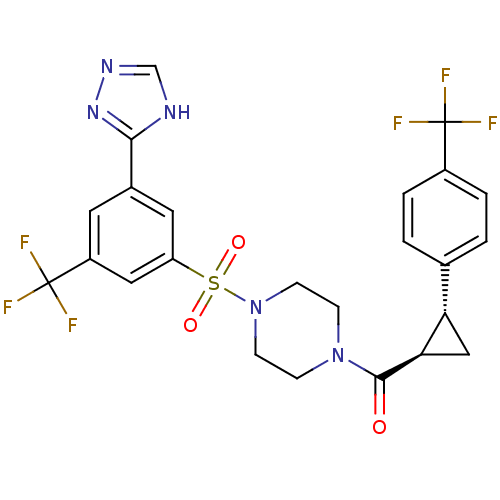 ((4-(3-(4H-1,2,4-triazol-3-yl)-5-(trifluoromethyl)p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267730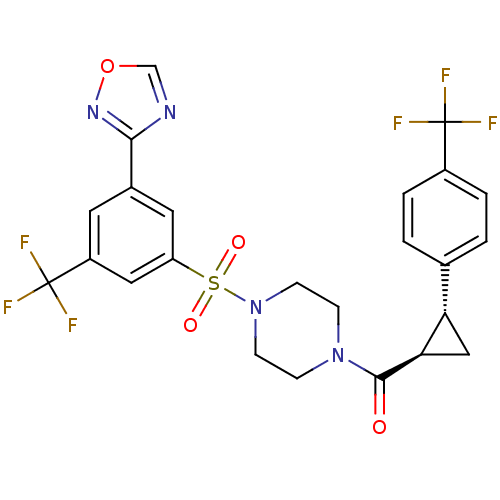 ((4-(3-(1,2,4-oxadiazol-3-yl)-5-(trifluoromethyl)ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267585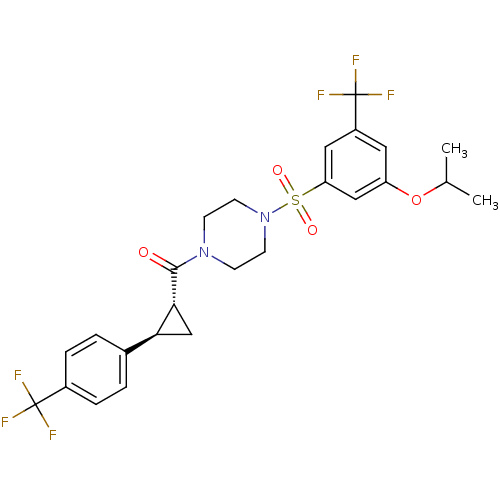 ((4-(3-isopropoxy-5-(trifluoromethyl)phenylsulfonyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267775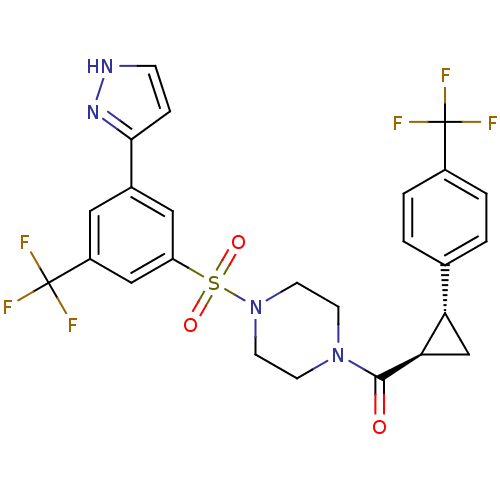 ((4-(3-(1H-pyrazol-5-yl)-5-(trifluoromethyl)phenyls...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267487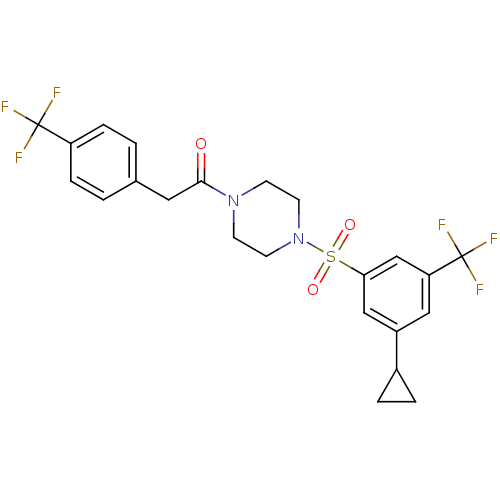 (1-(4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267584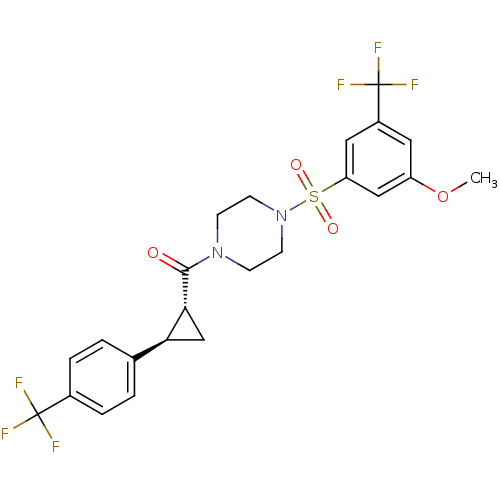 ((4-(3-methoxy-5-(trifluoromethyl)phenylsulfonyl)pi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267688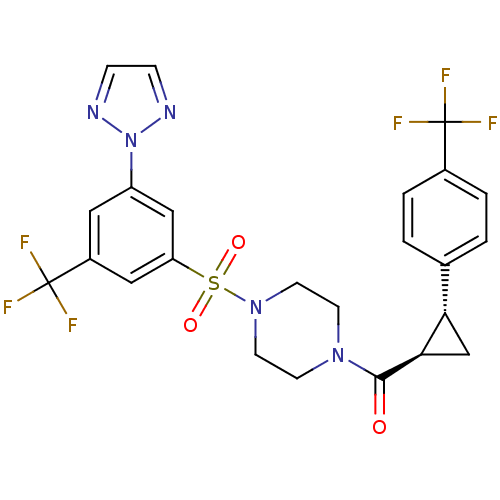 ((4-(3-(2H-1,2,3-triazol-2-yl)-5-(trifluoromethyl)p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267687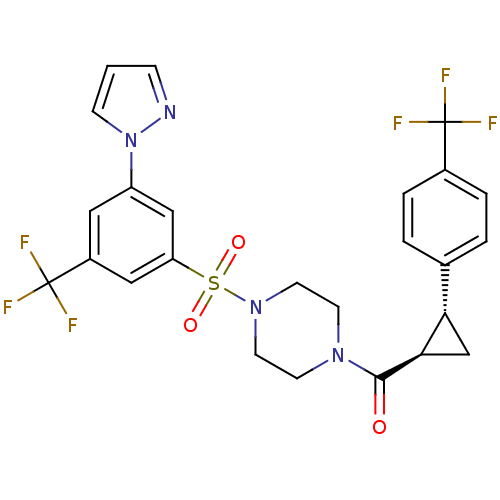 ((4-(3-(1H-pyrazol-1-yl)-5-(trifluoromethyl)phenyls...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267487 (1-(4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267686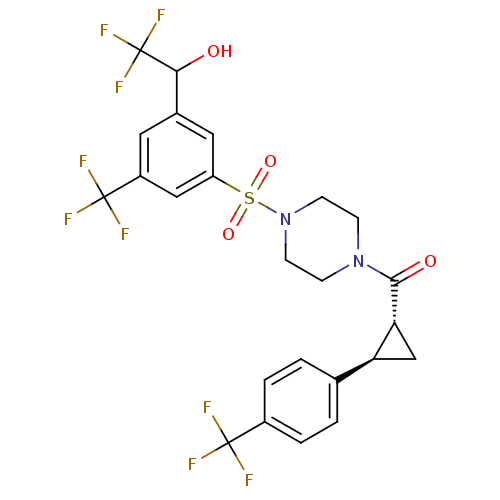 ((4-(3-(2,2,2-trifluoro-1-hydroxyethyl)-5-(trifluor...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267487 (1-(4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant CB1R expressed in CHO cells assessed as inhibition of methanandamide-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267617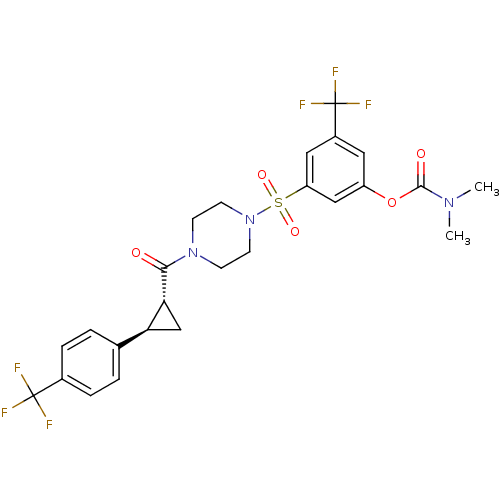 (3-(trifluoromethyl)-5-(4-((1R,2R)-2-(4-(trifluorom...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from rat recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267874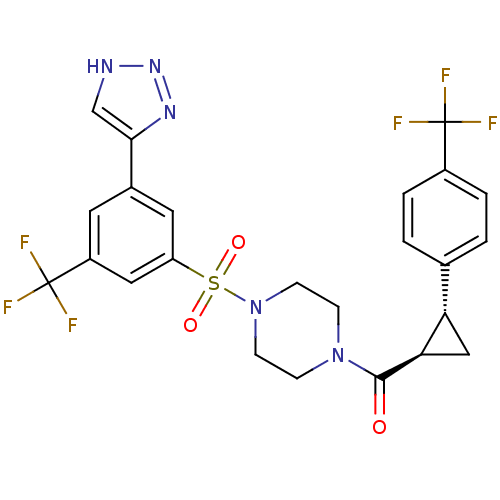 ((4-(3-(2H-1,2,3-triazol-4-yl)-5-(trifluoromethyl)p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267650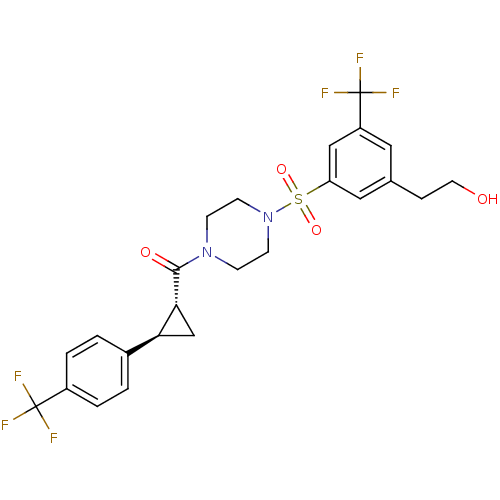 ((4-(3-(2-hydroxyethyl)-5-(trifluoromethyl)phenylsu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267484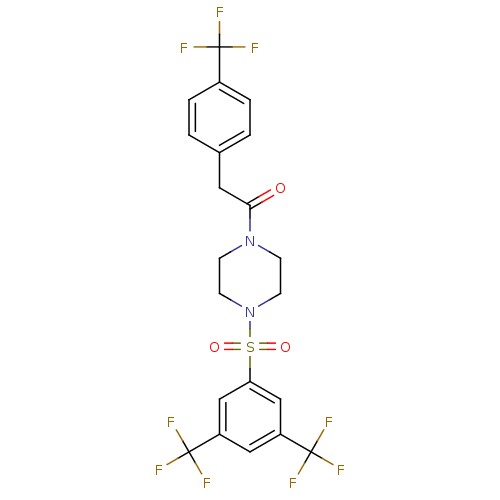 (1-(4-(3,5-bis(trifluoromethyl)phenylsulfonyl)piper...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267652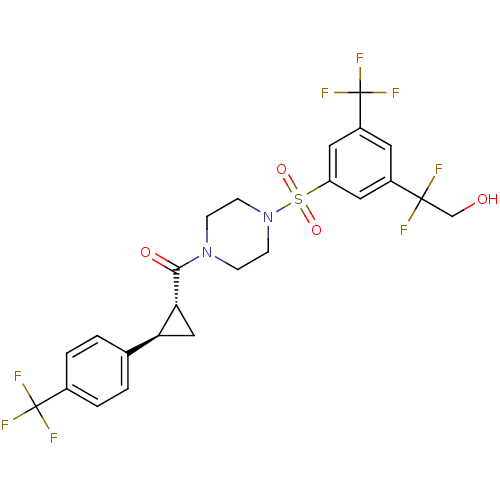 ((4-(3-(1,1-difluoro-2-hydroxyethyl)-5-(trifluorome...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267486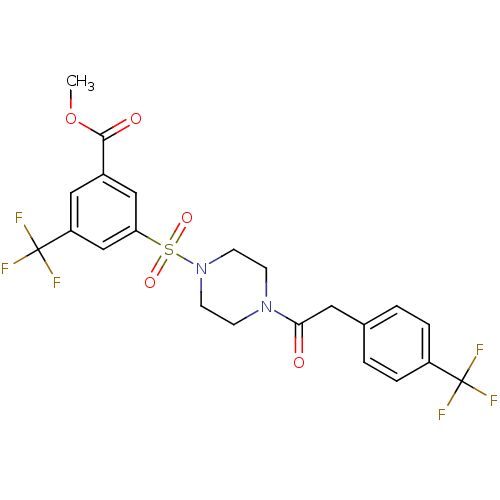 (CHEMBL478696 | methyl 3-(trifluoromethyl)-5-(4-(2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267583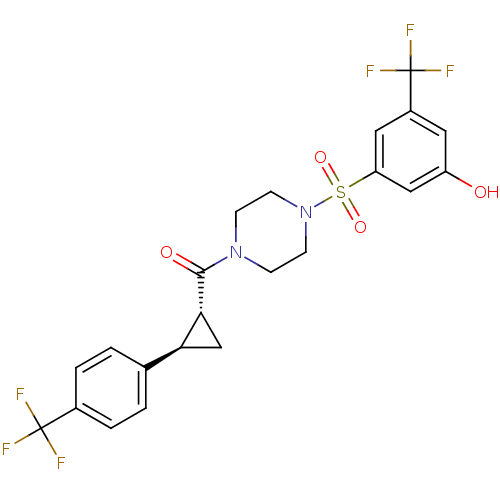 ((4-(3-hydroxy-5-(trifluoromethyl)phenylsulfonyl)pi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267485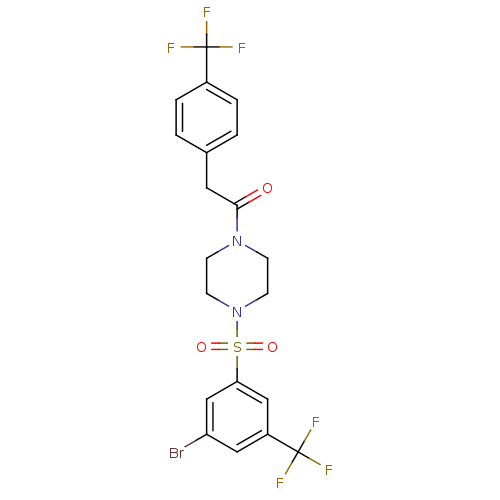 (1-(4-(3-bromo-5-(trifluoromethyl)phenylsulfonyl)pi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267728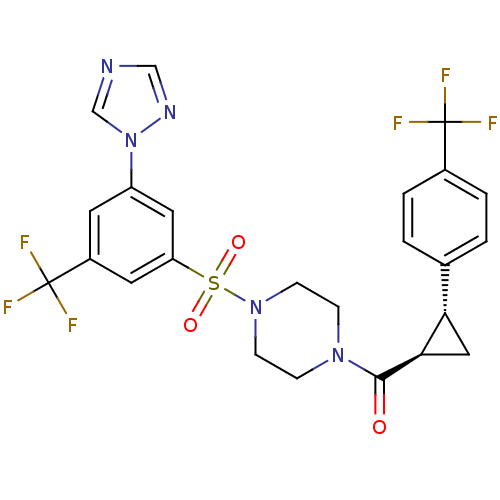 ((4-(3-(1H-1,2,4-triazol-1-yl)-5-(trifluoromethyl)p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267651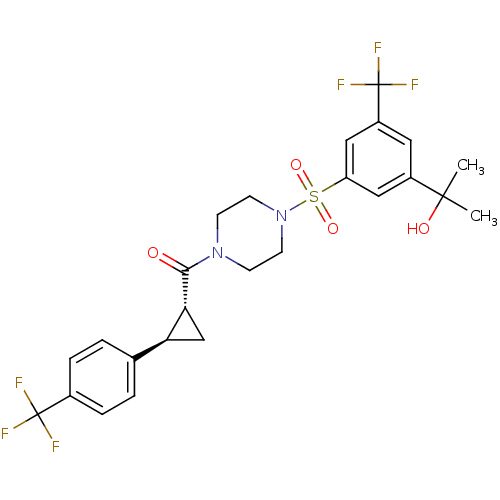 ((4-(3-(2-hydroxypropan-2-yl)-5-(trifluoromethyl)ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267616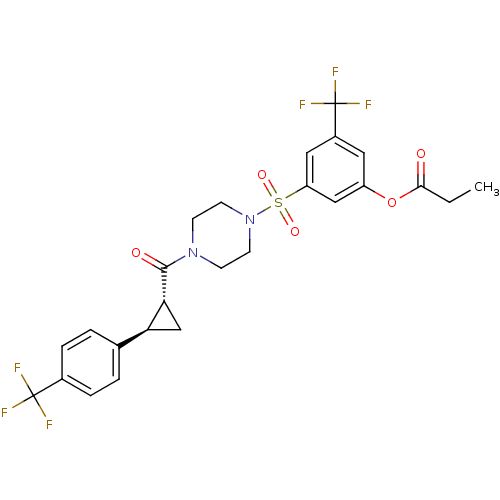 (3-(trifluoromethyl)-5-(4-((1R,2R)-2-(4-(trifluorom...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267615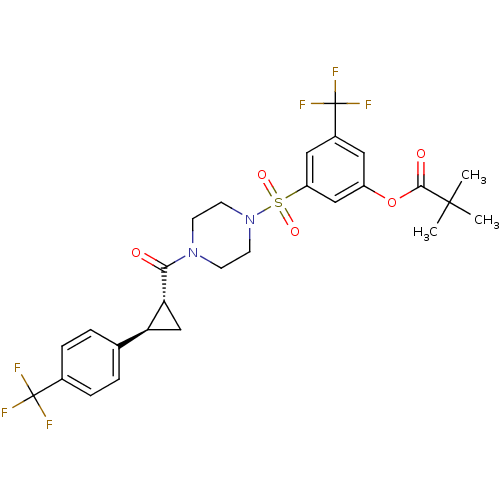 (3-(trifluoromethyl)-5-(4-((1R,2R)-2-(4-(trifluorom...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267776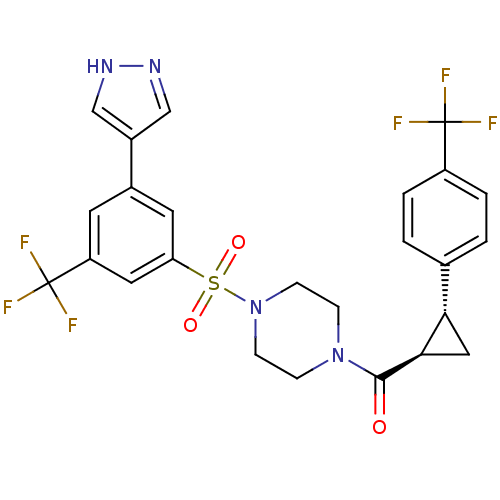 ((4-(3-(1H-pyrazol-4-yl)-5-(trifluoromethyl)phenyls...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant CB1R expressed in CHO cells assessed as inhibition of methanandamide-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267908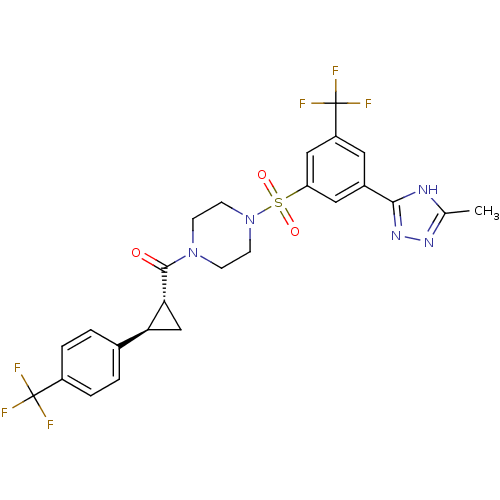 ((4-(3-(5-methyl-4H-1,2,4-triazol-3-yl)-5-(trifluor...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267873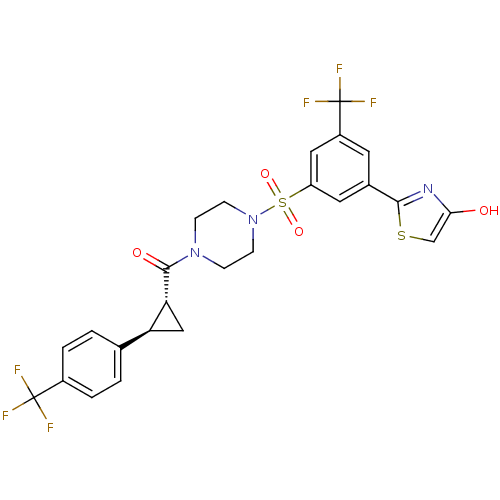 (2-(3-(trifluoromethyl)-5-(4-((1R,2R)-2-(4-(trifluo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267487 (1-(4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] SR141716 from rat recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267258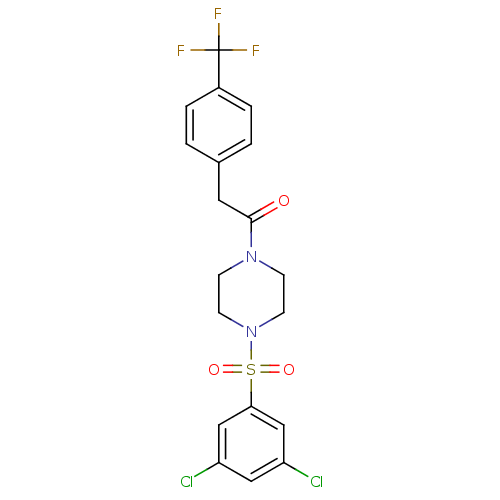 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267777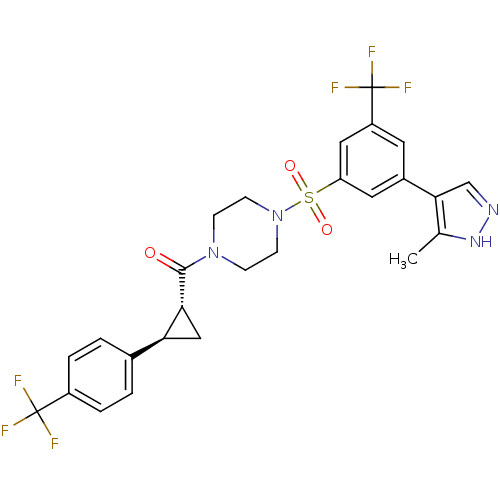 ((4-(3-(3-methyl-1H-pyrazol-4-yl)-5-(trifluoromethy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267543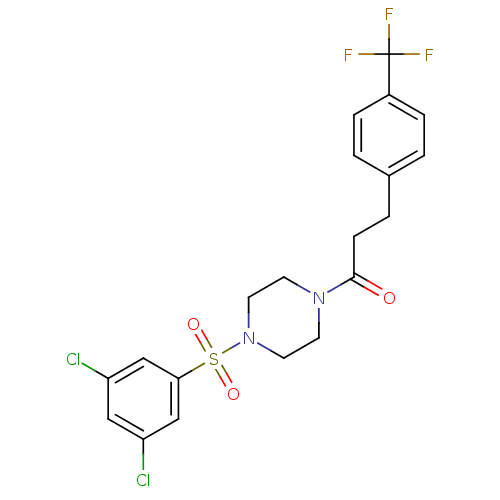 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267487 (1-(4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from rat recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50267777 ((4-(3-(3-methyl-1H-pyrazol-4-yl)-5-(trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG by MK0499 binding assay | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267582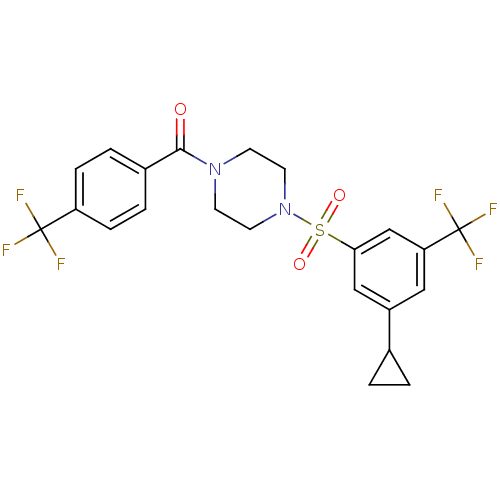 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267729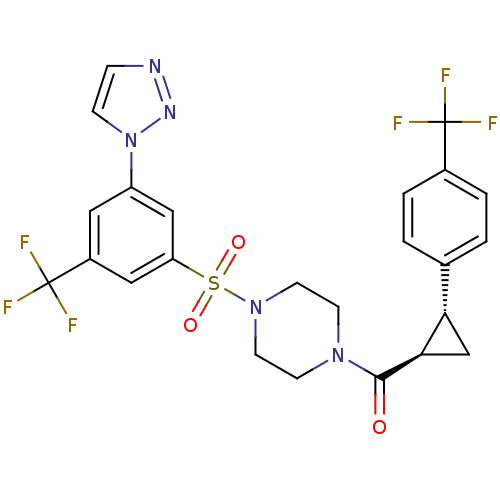 ((4-(3-(1H-1,2,3-triazol-1-yl)-5-(trifluoromethyl)p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267582 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50267686 ((4-(3-(2,2,2-trifluoro-1-hydroxyethyl)-5-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG by MK0499 binding assay | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267582 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at rat recombinant CB1R expressed in CHO cells assessed as inhibition of methanandamide-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267774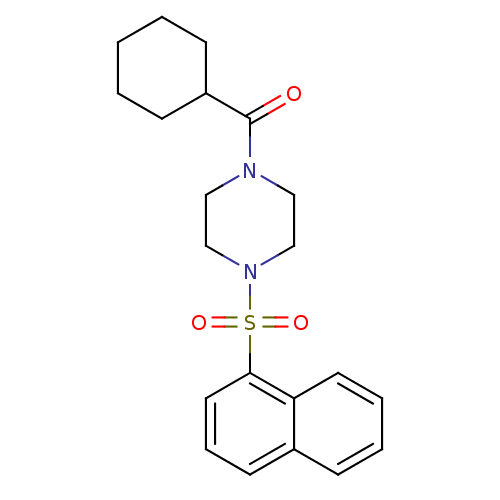 (CHEMBL523100 | cyclohexyl(4-(naphthalen-1-ylsulfon...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267824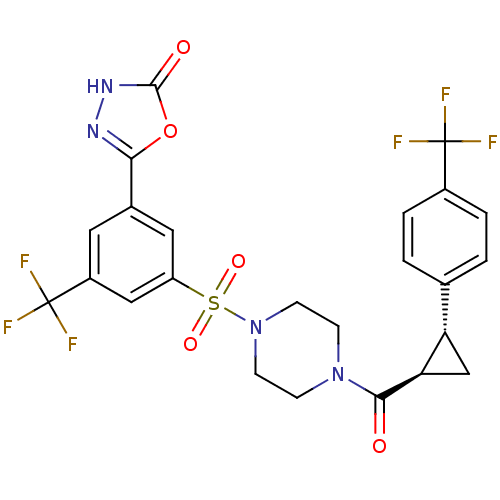 (5-(3-(trifluoromethyl)-5-(4-((1R,2R)-2-(4-(trifluo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267259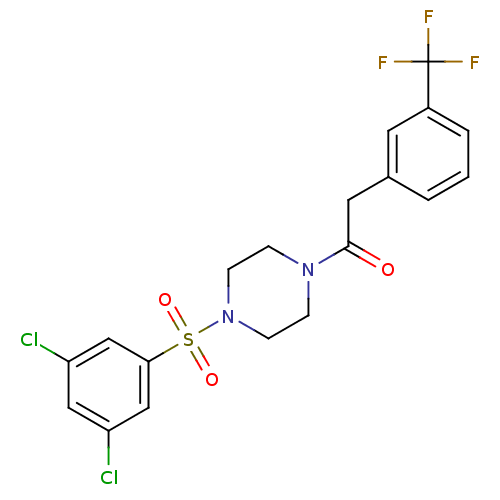 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267257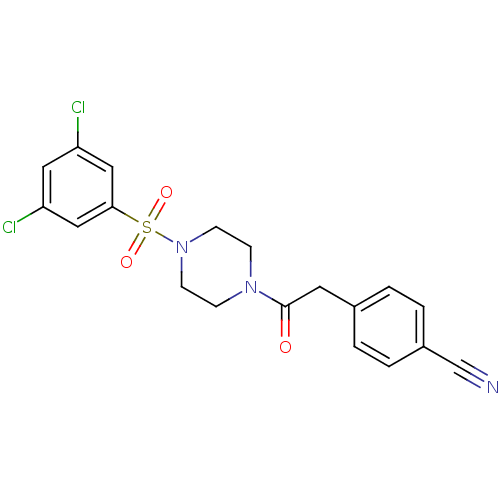 (4-(2-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267582 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 175 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from rat recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267823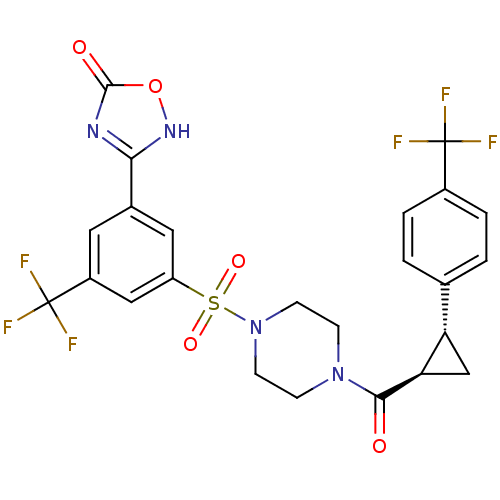 (3-(3-(trifluoromethyl)-5-(4-((1R,2R)-2-(4-(trifluo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267820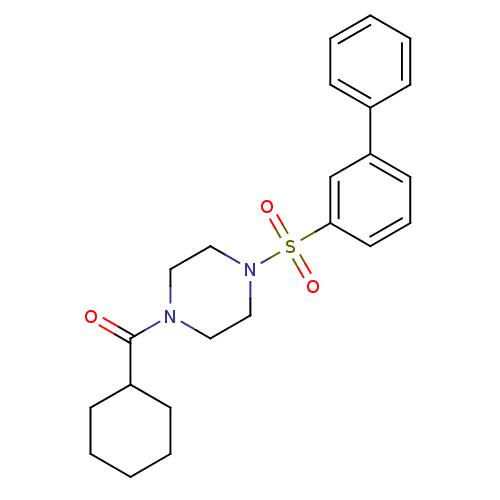 ((4-(biphenyl-3-ylsulfonyl)piperazin-1-yl)(cyclohex...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267942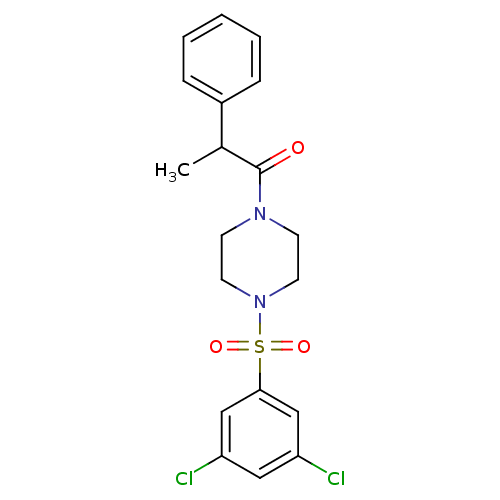 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267582 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H] SR141716 from rat recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267943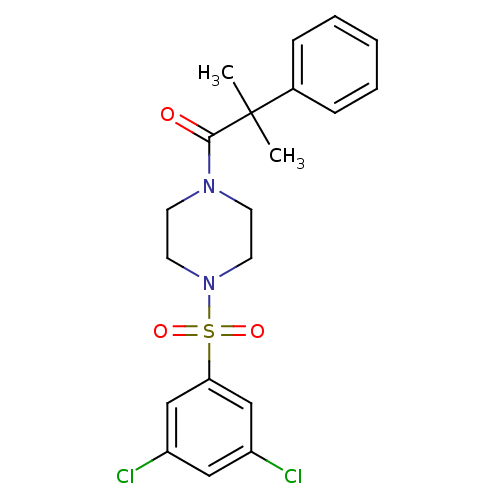 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267771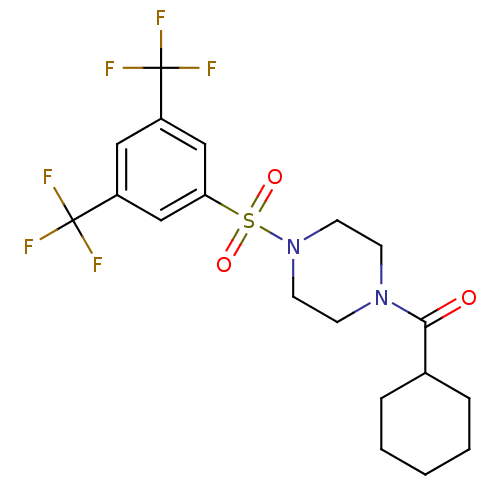 ((4-(3,5-bis(trifluoromethyl)phenylsulfonyl)piperaz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267871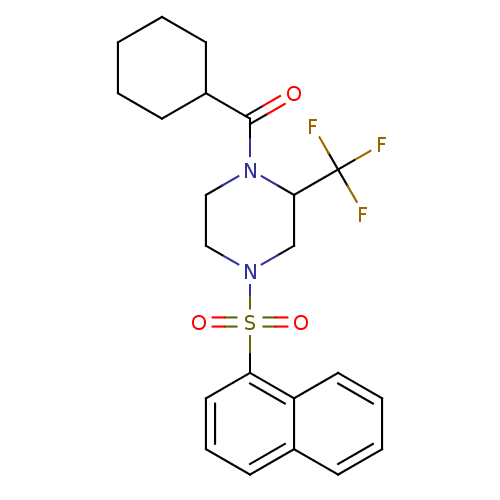 (CHEMBL489852 | cyclohexyl(4-(naphthalen-1-ylsulfon...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267869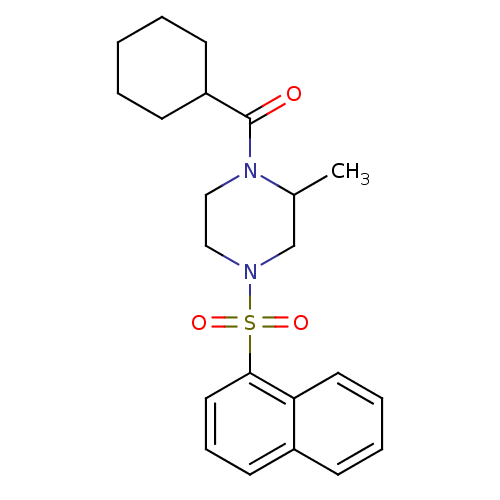 (CHEMBL522262 | cyclohexyl(2-methyl-4-(naphthalen-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267544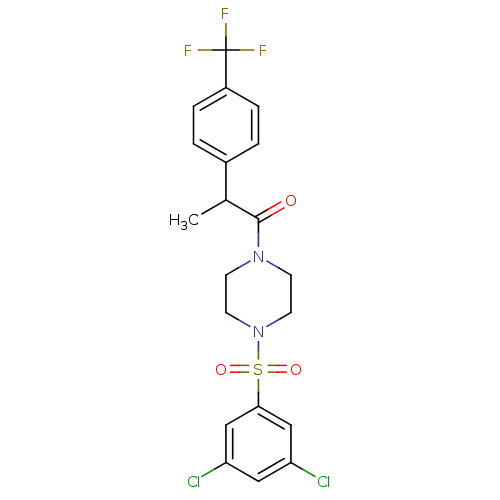 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267772 (CHEMBL522930 | cyclohexyl(4-(3,5-dimethylphenylsul...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267825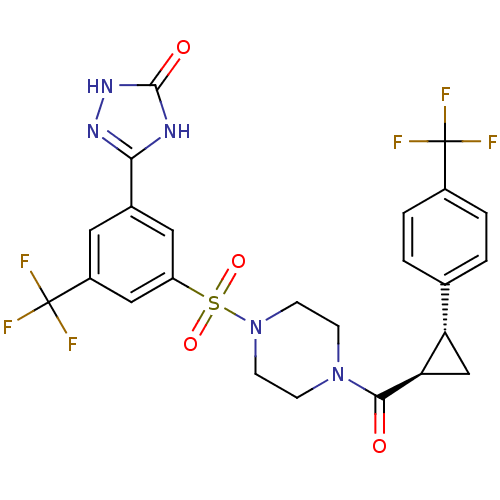 (3-(3-(trifluoromethyl)-5-(4-((1R,2R)-2-(4-(trifluo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267544 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267872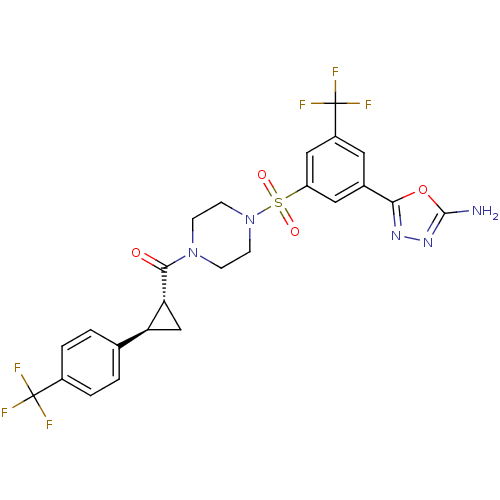 ((4-(3-(5-amino-1,3,4-oxadiazol-2-yl)-5-(trifluorom...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267773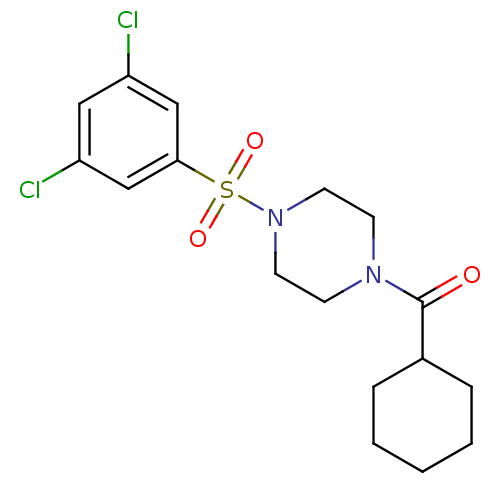 (CHEMBL489429 | cyclohexyl(4-(3,5-dichlorophenylsul...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267260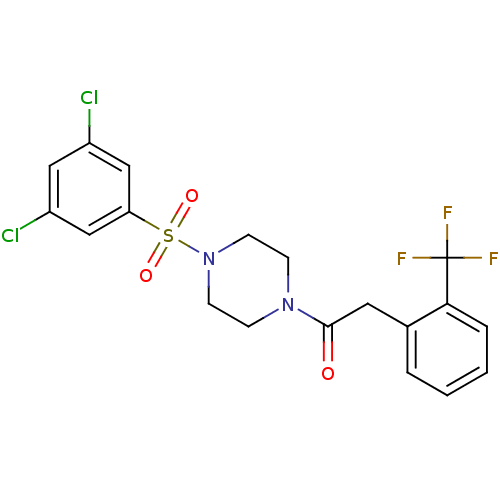 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50267775 ((4-(3-(1H-pyrazol-5-yl)-5-(trifluoromethyl)phenyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG by MK0499 binding assay | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50267776 ((4-(3-(1H-pyrazol-4-yl)-5-(trifluoromethyl)phenyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG by MK0499 binding assay | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267903 (CHEMBL525204 | cyclohexyl(2,2-dimethyl-4-(naphthal...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267821 (CHEMBL445204 | cyclopropyl(4-(3,5-dichlorophenylsu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267941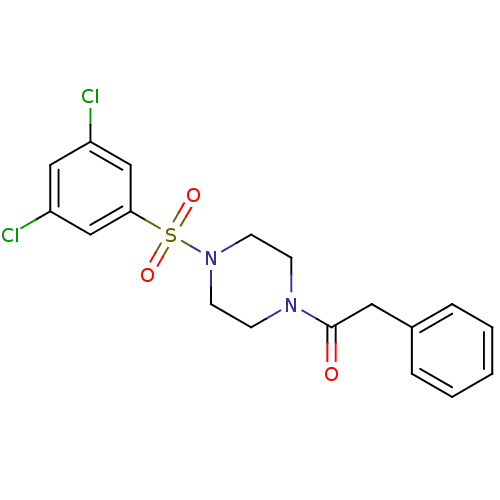 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50267650 ((4-(3-(2-hydroxyethyl)-5-(trifluoromethyl)phenylsu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG by MK0499 binding assay | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50267907 ((4-(3-(4H-1,2,4-triazol-3-yl)-5-(trifluoromethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG by MK0499 binding assay | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50267651 ((4-(3-(2-hydroxypropan-2-yl)-5-(trifluoromethyl)ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG by MK0499 binding assay | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50267730 ((4-(3-(1,2,4-oxadiazol-3-yl)-5-(trifluoromethyl)ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG by MK0499 binding assay | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50267908 ((4-(3-(5-methyl-4H-1,2,4-triazol-3-yl)-5-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG by MK0499 binding assay | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50267652 ((4-(3-(1,1-difluoro-2-hydroxyethyl)-5-(trifluorome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG by MK0499 binding assay | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267904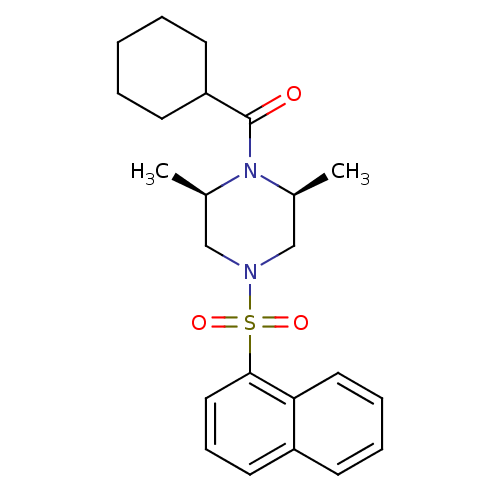 (CHEMBL504182 | cyclohexyl((2R,6S)-2,6-dimethyl-4-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267256 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267906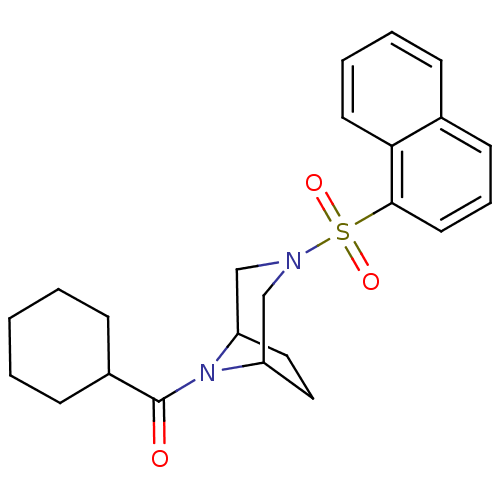 (CHEMBL489450 | cyclohexyl(3-(naphthalen-1-ylsulfon...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267354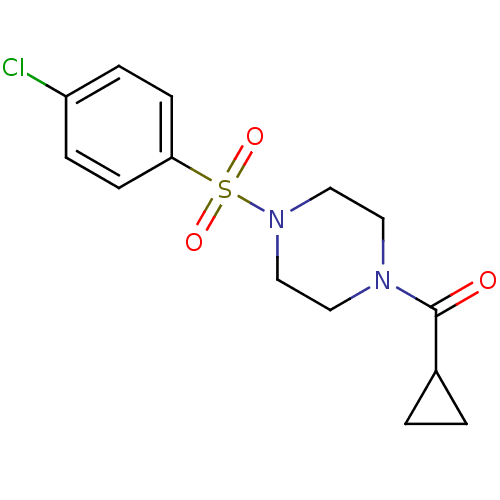 ((4-(4-chlorophenylsulfonyl)piperazin-1-yl)(cyclopr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50267874 ((4-(3-(2H-1,2,3-triazol-4-yl)-5-(trifluoromethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG by MK0499 binding assay | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50267873 (2-(3-(trifluoromethyl)-5-(4-((1R,2R)-2-(4-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human ERG by MK0499 binding assay | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267407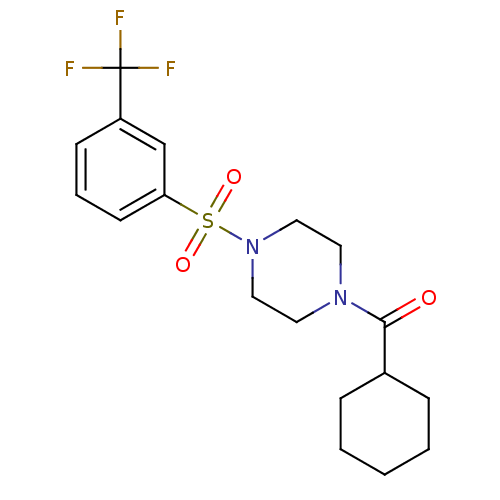 (CHEMBL477867 | cyclohexyl(4-(3-(trifluoromethyl)ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267870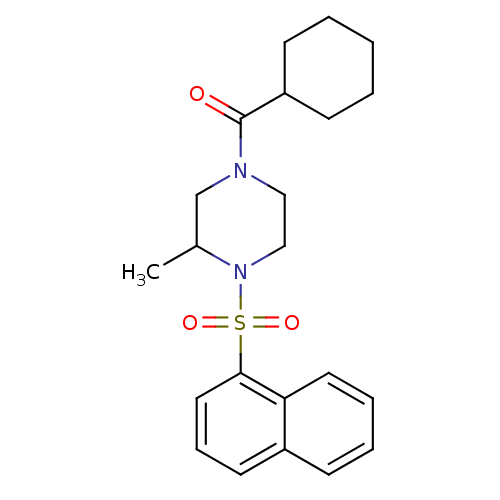 (CHEMBL489645 | cyclohexyl(3-methyl-4-(naphthalen-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50267907 ((4-(3-(4H-1,2,4-triazol-3-yl)-5-(trifluoromethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50267907 ((4-(3-(4H-1,2,4-triazol-3-yl)-5-(trifluoromethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267403 (CHEMBL477864 | cyclopropyl(4-(2,5-dichlorophenylsu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267905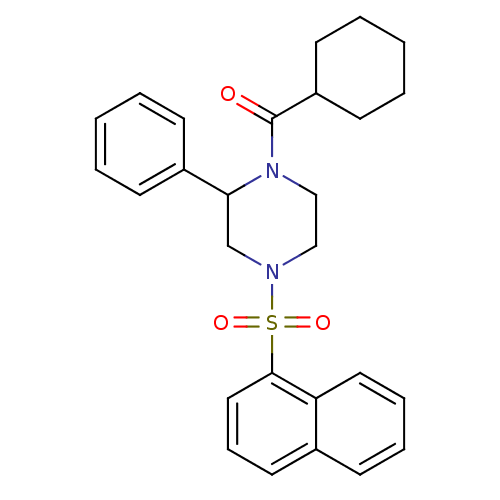 (CHEMBL523603 | cyclohexyl(4-(naphthalen-1-ylsulfon...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267405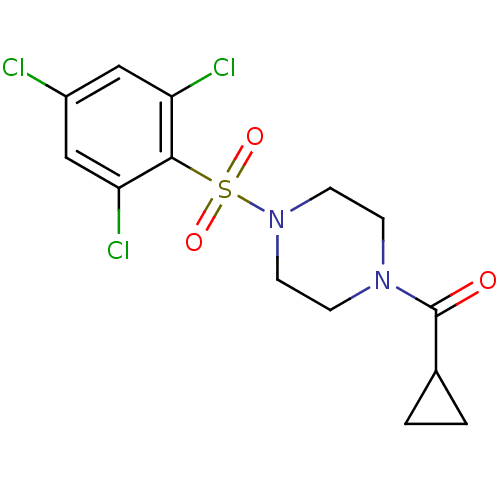 (CHEMBL478066 | cyclopropyl(4-(2,4,6-trichloropheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267406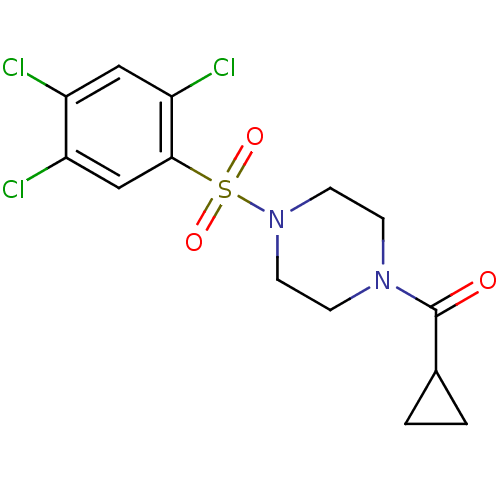 (CHEMBL517354 | cyclopropyl(4-(2,4,5-trichloropheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267404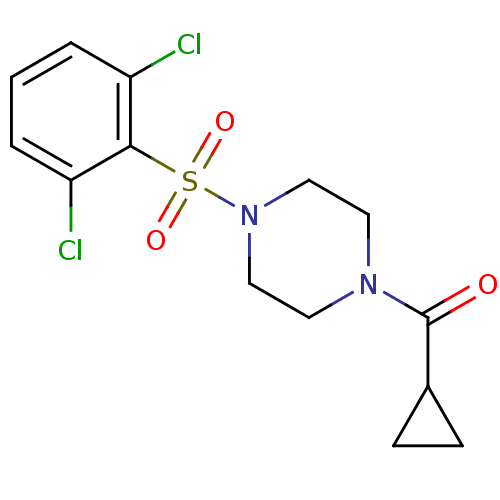 (CHEMBL477865 | cyclopropyl(4-(2,6-dichlorophenylsu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267774 (CHEMBL523100 | cyclohexyl(4-(naphthalen-1-ylsulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267729 ((4-(3-(1H-1,2,3-triazol-1-yl)-5-(trifluoromethyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267819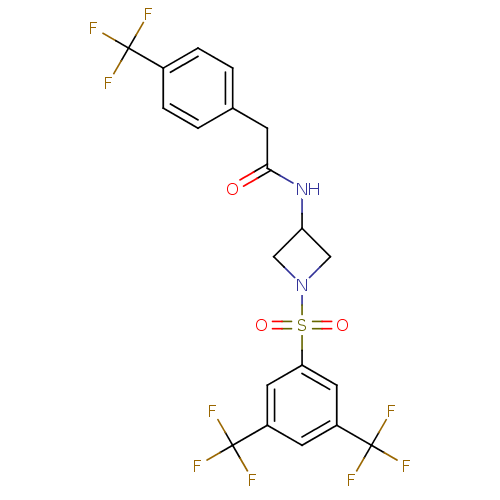 (CHEMBL522945 | N-(1-(3,5-bis(trifluoromethyl)pheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267486 (CHEMBL478696 | methyl 3-(trifluoromethyl)-5-(4-(2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267824 (5-(3-(trifluoromethyl)-5-(4-((1R,2R)-2-(4-(trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267773 (CHEMBL489429 | cyclohexyl(4-(3,5-dichlorophenylsul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267823 (3-(3-(trifluoromethyl)-5-(4-((1R,2R)-2-(4-(trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267907 ((4-(3-(4H-1,2,4-triazol-3-yl)-5-(trifluoromethyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267487 (1-(4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267820 ((4-(biphenyl-3-ylsulfonyl)piperazin-1-yl)(cyclohex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267776 ((4-(3-(1H-pyrazol-4-yl)-5-(trifluoromethyl)phenyls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267775 ((4-(3-(1H-pyrazol-5-yl)-5-(trifluoromethyl)phenyls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267822 (3,5-bis(trifluoromethyl)-N-(1-(2-(4-(trifluorometh...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267777 ((4-(3-(3-methyl-1H-pyrazol-4-yl)-5-(trifluoromethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267872 ((4-(3-(5-amino-1,3,4-oxadiazol-2-yl)-5-(trifluorom...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267688 ((4-(3-(2H-1,2,3-triazol-2-yl)-5-(trifluoromethyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267687 ((4-(3-(1H-pyrazol-1-yl)-5-(trifluoromethyl)phenyls...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267868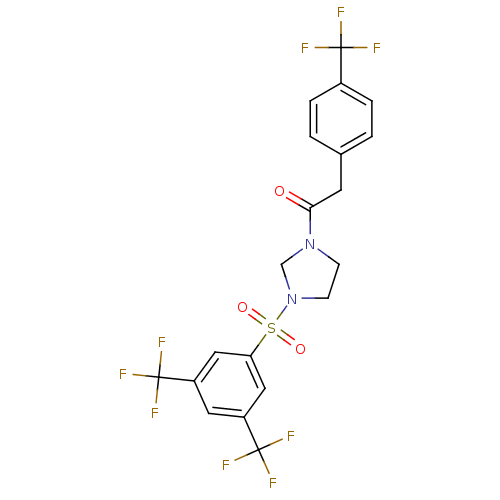 (1-(3-(3,5-bis(trifluoromethyl)phenylsulfonyl)imida...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267825 (3-(3-(trifluoromethyl)-5-(4-((1R,2R)-2-(4-(trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267873 (2-(3-(trifluoromethyl)-5-(4-((1R,2R)-2-(4-(trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267908 ((4-(3-(5-methyl-4H-1,2,4-triazol-3-yl)-5-(trifluor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267485 (1-(4-(3-bromo-5-(trifluoromethyl)phenylsulfonyl)pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267728 ((4-(3-(1H-1,2,4-triazol-1-yl)-5-(trifluoromethyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267650 ((4-(3-(2-hydroxyethyl)-5-(trifluoromethyl)phenylsu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267940 ((4-(3,5-bis(trifluoromethyl)phenylsulfonyl)piperaz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267484 (1-(4-(3,5-bis(trifluoromethyl)phenylsulfonyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267771 ((4-(3,5-bis(trifluoromethyl)phenylsulfonyl)piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267652 ((4-(3-(1,1-difluoro-2-hydroxyethyl)-5-(trifluorome...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267686 ((4-(3-(2,2,2-trifluoro-1-hydroxyethyl)-5-(trifluor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267939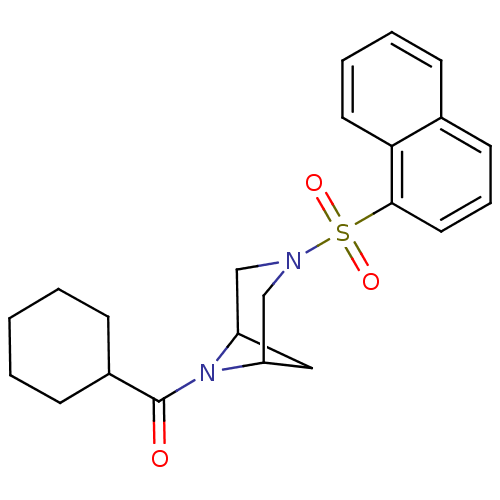 (CHEMBL484478 | cyclohexyl(3-(naphthalen-1-ylsulfon...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB1R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267772 (CHEMBL522930 | cyclohexyl(4-(3,5-dimethylphenylsul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267730 ((4-(3-(1,2,4-oxadiazol-3-yl)-5-(trifluoromethyl)ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267651 ((4-(3-(2-hydroxypropan-2-yl)-5-(trifluoromethyl)ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50267874 ((4-(3-(2H-1,2,3-triazol-4-yl)-5-(trifluoromethyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human recombinant CB2R expressed in CHO cells | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50267907 ((4-(3-(4H-1,2,4-triazol-3-yl)-5-(trifluoromethyl)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50267907 ((4-(3-(4H-1,2,4-triazol-3-yl)-5-(trifluoromethyl)p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267869 (CHEMBL522262 | cyclohexyl(2-methyl-4-(naphthalen-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267871 (CHEMBL489852 | cyclohexyl(4-(naphthalen-1-ylsulfon...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267544 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at rat recombinant CB1R expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267943 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267942 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267582 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at rat recombinant CB1R expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267906 (CHEMBL489450 | cyclohexyl(3-(naphthalen-1-ylsulfon...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267771 ((4-(3,5-bis(trifluoromethyl)phenylsulfonyl)piperaz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50267487 (1-(4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at rat recombinant CB1R expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267939 (CHEMBL484478 | cyclohexyl(3-(naphthalen-1-ylsulfon...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267821 (CHEMBL445204 | cyclopropyl(4-(3,5-dichlorophenylsu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267941 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267772 (CHEMBL522930 | cyclohexyl(4-(3,5-dimethylphenylsul...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267485 (1-(4-(3-bromo-5-(trifluoromethyl)phenylsulfonyl)pi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267354 ((4-(4-chlorophenylsulfonyl)piperazin-1-yl)(cyclopr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267905 (CHEMBL523603 | cyclohexyl(4-(naphthalen-1-ylsulfon...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267487 (1-(4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267407 (CHEMBL477867 | cyclohexyl(4-(3-(trifluoromethyl)ph...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267868 (1-(3-(3,5-bis(trifluoromethyl)phenylsulfonyl)imida...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267406 (CHEMBL517354 | cyclopropyl(4-(2,4,5-trichloropheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267256 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267544 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267820 ((4-(biphenyl-3-ylsulfonyl)piperazin-1-yl)(cyclohex...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267486 (CHEMBL478696 | methyl 3-(trifluoromethyl)-5-(4-(2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267903 (CHEMBL525204 | cyclohexyl(2,2-dimethyl-4-(naphthal...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267258 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267257 (4-(2-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267545 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1R expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267582 ((4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfony...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1R expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267940 ((4-(3,5-bis(trifluoromethyl)phenylsulfonyl)piperaz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267822 (3,5-bis(trifluoromethyl)-N-(1-(2-(4-(trifluorometh...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267405 (CHEMBL478066 | cyclopropyl(4-(2,4,6-trichloropheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267404 (CHEMBL477865 | cyclopropyl(4-(2,6-dichlorophenylsu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267259 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267484 (1-(4-(3,5-bis(trifluoromethyl)phenylsulfonyl)piper...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267543 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267487 (1-(4-(3-cyclopropyl-5-(trifluoromethyl)phenylsulfo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at human recombinant CB1R expressed in CHO cells assessed as inhibition of forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267870 (CHEMBL489645 | cyclohexyl(3-methyl-4-(naphthalen-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267904 (CHEMBL504182 | cyclohexyl((2R,6S)-2,6-dimethyl-4-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267260 (1-(4-(3,5-dichlorophenylsulfonyl)piperazin-1-yl)-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267774 (CHEMBL523100 | cyclohexyl(4-(naphthalen-1-ylsulfon...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267773 (CHEMBL489429 | cyclohexyl(4-(3,5-dichlorophenylsul...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267819 (CHEMBL522945 | N-(1-(3,5-bis(trifluoromethyl)pheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50267403 (CHEMBL477864 | cyclopropyl(4-(2,5-dichlorophenylsu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inverse agonist activity at human recombinant CB1R expressed in CHO cells assessed as increase in forskolin-stimulated cAMP level | J Med Chem 52: 2550-8 (2009) Article DOI: 10.1021/jm900063x BindingDB Entry DOI: 10.7270/Q2028RF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||