

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50068039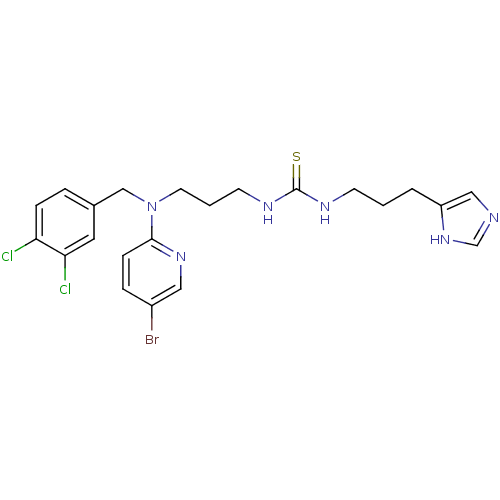 (1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Binding affinity to human SST4 receptor | Medchemcomm 3: 56-60 (2012) Article DOI: 10.1039/c1md00200g BindingDB Entry DOI: 10.7270/Q2XP77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50496732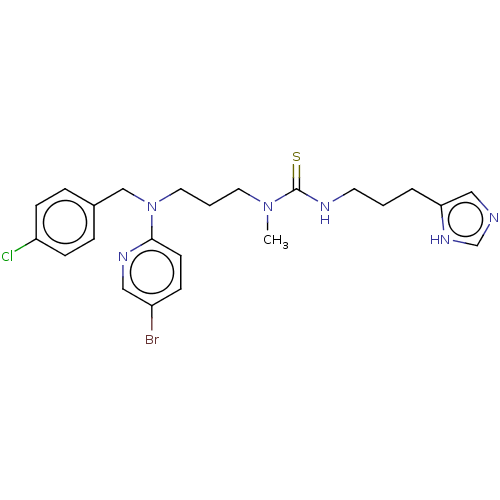 (CHEMBL1591268) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Binding affinity to human SST4 receptor | Medchemcomm 3: 56-60 (2012) Article DOI: 10.1039/c1md00200g BindingDB Entry DOI: 10.7270/Q2XP77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18428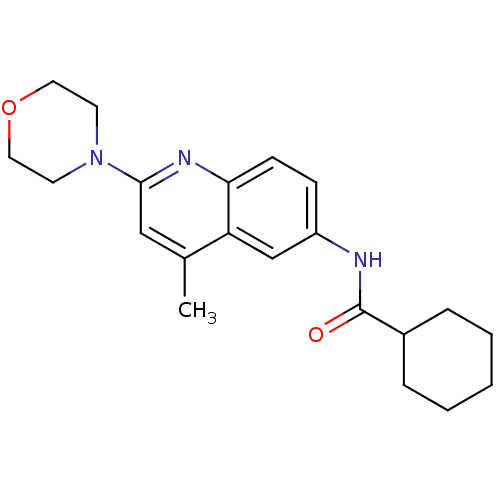 (Aminoquinoline compound, 1 | N-[4-methyl-2-(morpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 21 | -43.2 | 31 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50496735 (CHEMBL1355048) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Binding affinity to human SST4 receptor | Medchemcomm 3: 56-60 (2012) Article DOI: 10.1039/c1md00200g BindingDB Entry DOI: 10.7270/Q2XP77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18438 (4-benzenesulfonamido-N-(5-ethyl-1,3,4-thiadiazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 52 | -41.0 | 103 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18431 (Aminoquinoline compound, 16 | N-[4-methyl-2-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 55 | -40.9 | 133 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18430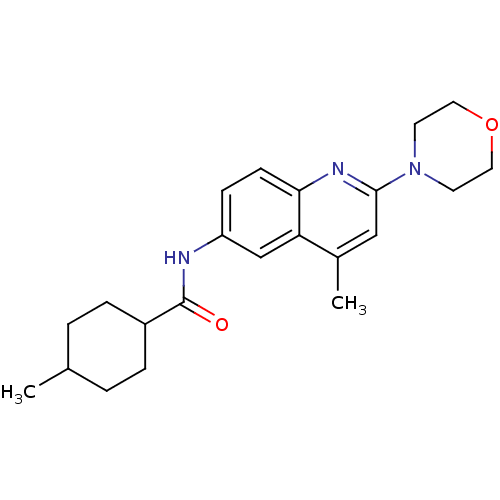 (4-methyl-N-[4-methyl-2-(morpholin-4-yl)quinolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56 | -40.8 | 63 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18439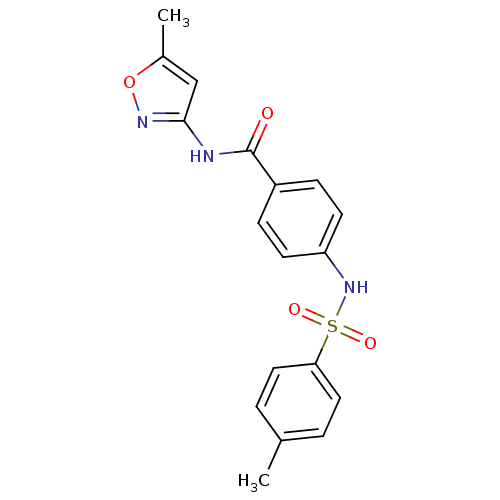 (N-(5-methyl-1,2-oxazol-3-yl)-4-[(4-methylbenzene)s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 102 | -39.4 | 168 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18433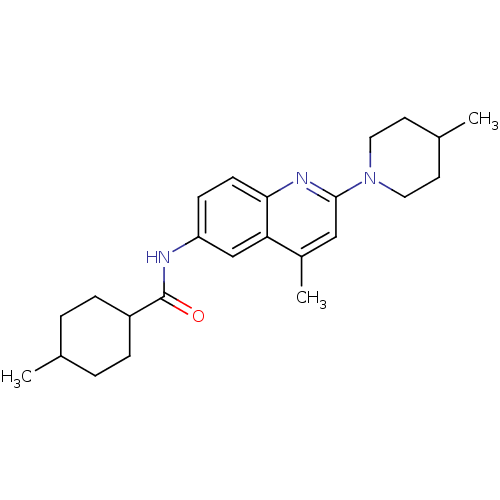 (4-methyl-N-[4-methyl-2-(4-methylpiperidin-1-yl)qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | -39.0 | 268 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18432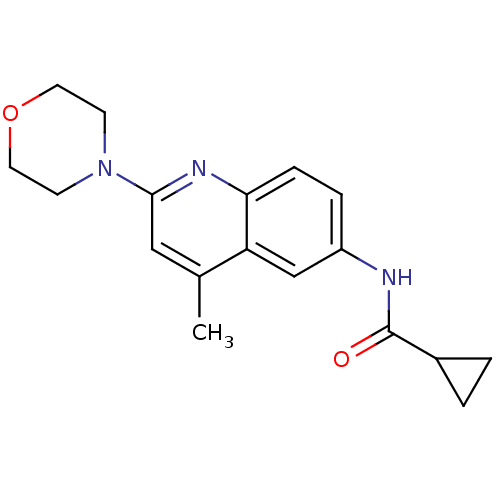 (Aminoquinoline compound, 17 | N-[4-methyl-2-(morph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 121 | -39.0 | 183 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50496733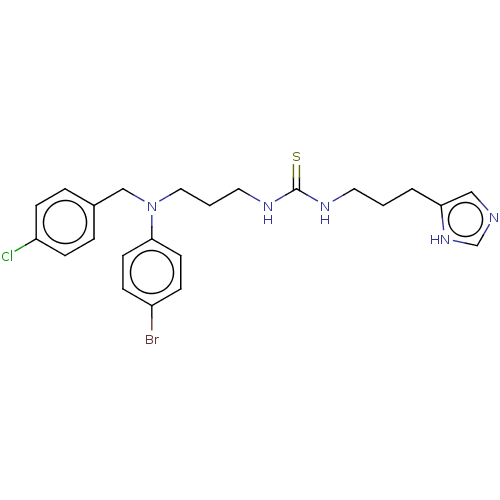 (CHEMBL1591395) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Binding affinity to human SST4 receptor | Medchemcomm 3: 56-60 (2012) Article DOI: 10.1039/c1md00200g BindingDB Entry DOI: 10.7270/Q2XP77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18434 (4-methyl-N-[4-methyl-2-(piperidin-1-yl)quinolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 184 | -37.9 | 452 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18447 (2-({4-[(5-chloro-2-methoxyphenyl)amino]-6-(pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 320 | -36.6 | 430 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18435 (Aminoquinoline compound, 20 | N-[2-(diethylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 514 | -35.4 | 1.06E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18443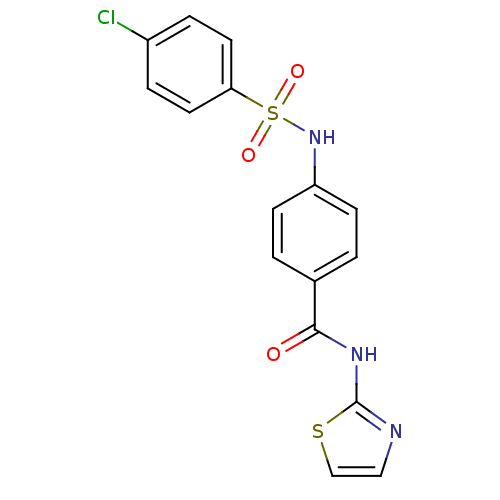 (4-[(4-chlorobenzene)sulfonamido]-N-(1,3-thiazol-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 556 | -35.2 | 1.29E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 4 (Homo sapiens (Human)) | BDBM50496734 (CHEMBL1590268) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute Curated by ChEMBL | Assay Description Binding affinity to human SST4 receptor | Medchemcomm 3: 56-60 (2012) Article DOI: 10.1039/c1md00200g BindingDB Entry DOI: 10.7270/Q2XP77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18436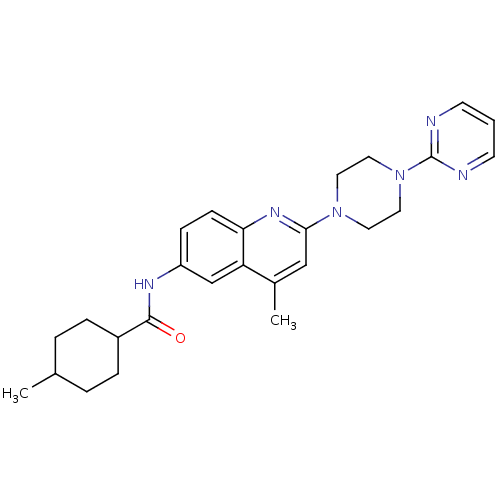 (4-methyl-N-{4-methyl-2-[4-(pyrimidin-2-yl)piperazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 975 | -33.8 | 2.45E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18448 (2-({4-[(3-methylphenyl)amino]-6-(pyrrolidin-1-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.78E+3 | -31.3 | 4.31E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18449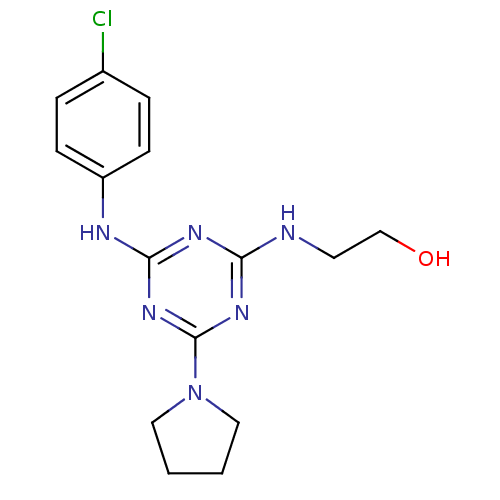 (2-({4-[(4-chlorophenyl)amino]-6-(pyrrolidin-1-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 4.23E+3 | -30.3 | 7.73E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18440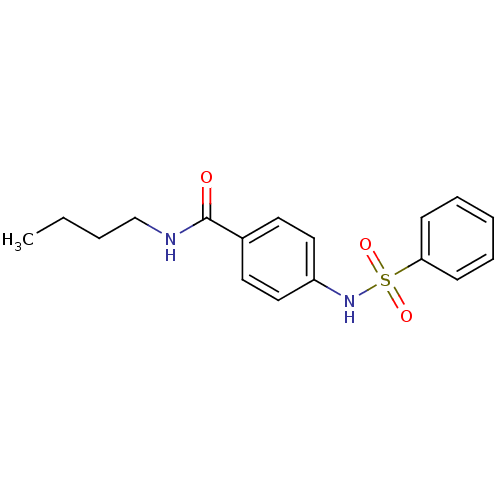 (4-benzenesulfonamido-N-butylbenzamide | Sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 7.15E+3 | -29.0 | 2.46E+4 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18441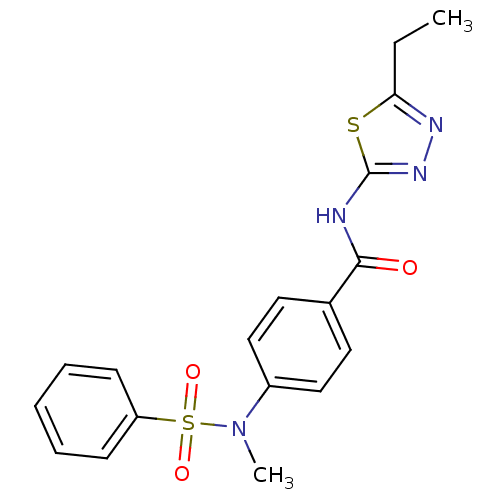 (4-[benzene(methyl)sulfonamido]-N-(5-ethyl-1,3,4-th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 8.44E+3 | -28.6 | 2.96E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18445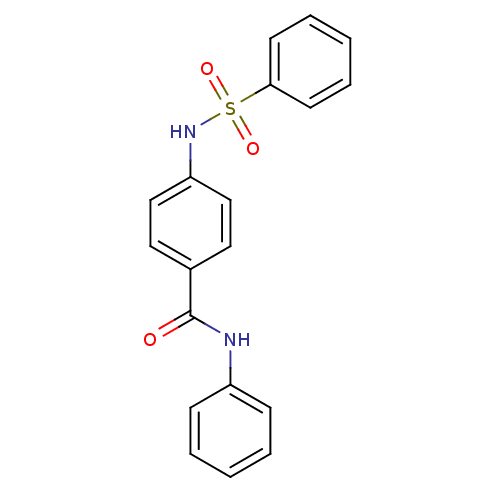 (4-benzenesulfonamido-N-phenylbenzamide | Sulfonami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.34E+4 | -27.4 | 6.46E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18442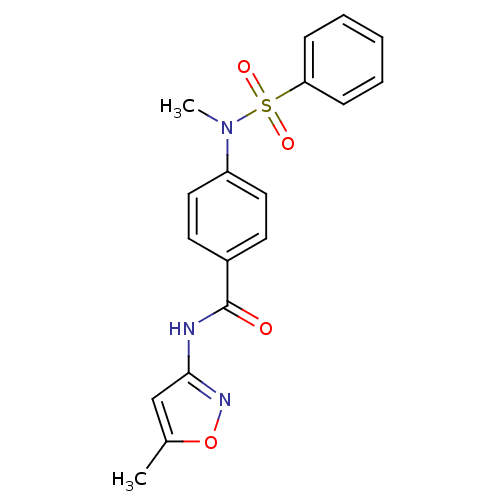 (4-[benzene(methyl)sulfonamido]-N-(5-methyl-1,2-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.92E+4 | -26.6 | 2.52E+4 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18444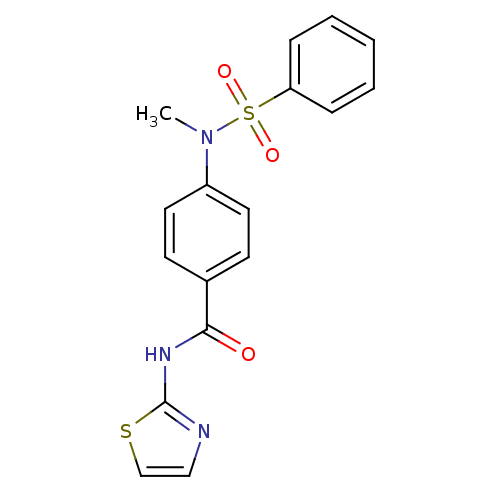 (4-[benzene(methyl)sulfonamido]-N-(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.34E+4 | -26.1 | 3.44E+4 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18446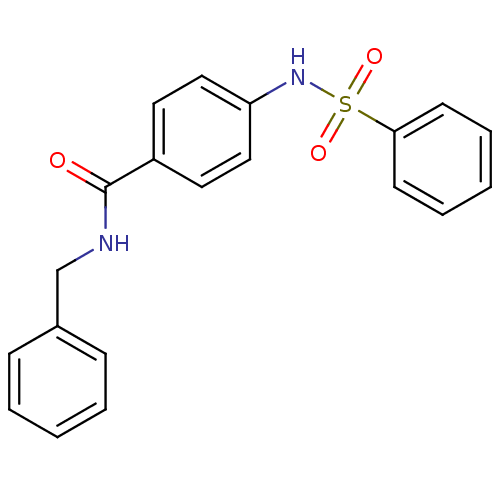 (4-benzenesulfonamido-N-benzylbenzamide | Sulfonami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.06E+4 | -24.2 | >1.00E+5 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18437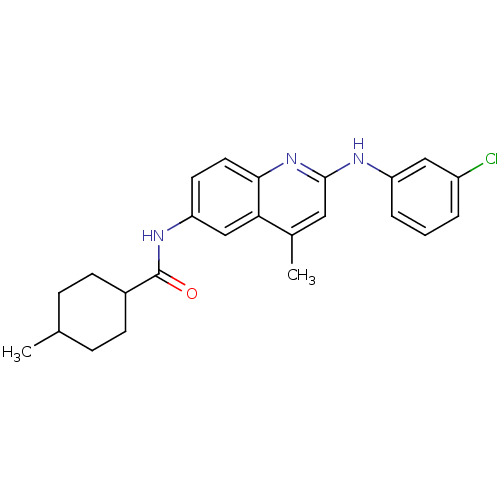 (Aminoquinoline compound, 22 | N-{2-[(3-chloropheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.22E+5 | -22.0 | n/a | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Proc Natl Acad Sci U S A 104: 13192-7 (2007) Article DOI: 10.1073/pnas.0705637104 BindingDB Entry DOI: 10.7270/Q2V1232M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50182801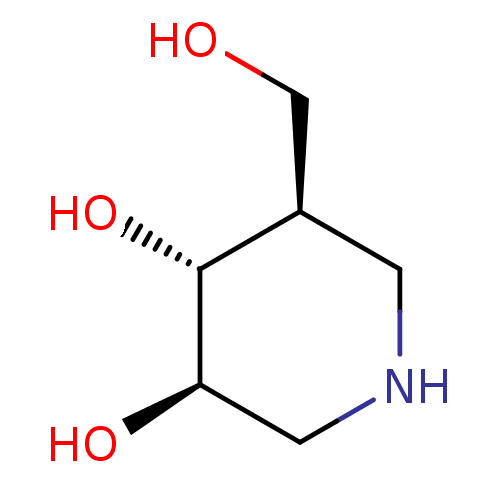 ((3R,4R,5R)-5-(Hydroxymethyl)piperidine-3,4-diol, 8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to substrate addition measured aft... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18428 (Aminoquinoline compound, 1 | N-[4-methyl-2-(morpho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to substrate addition measured aft... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18438 (4-benzenesulfonamido-N-(5-ethyl-1,3,4-thiadiazol-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to substrate addition measured aft... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50396168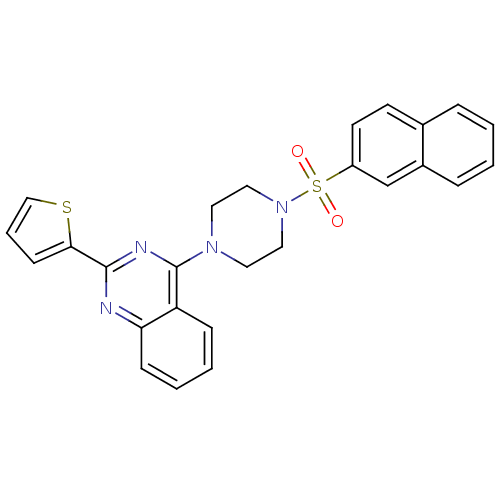 (CHEMBL1361379) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase in human spleen homogenate using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to subs... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18471 (2-[(4-{[2-(tert-butoxy)phenyl]amino}-6-(pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Bioorg Med Chem Lett 17: 5783-9 (2007) Article DOI: 10.1016/j.bmcl.2007.08.050 BindingDB Entry DOI: 10.7270/Q2Q81BBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50068039 (1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase in human spleen homogenate using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to subs... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18480 (2-({4-[(2-hydroxyethyl)amino]-6-(pyrrolidin-1-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Bioorg Med Chem Lett 17: 5783-9 (2007) Article DOI: 10.1016/j.bmcl.2007.08.050 BindingDB Entry DOI: 10.7270/Q2Q81BBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18447 (2-({4-[(5-chloro-2-methoxyphenyl)amino]-6-(pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to substrate addition measured aft... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18447 (2-({4-[(5-chloro-2-methoxyphenyl)amino]-6-(pyrroli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Bioorg Med Chem Lett 17: 5783-9 (2007) Article DOI: 10.1016/j.bmcl.2007.08.050 BindingDB Entry DOI: 10.7270/Q2Q81BBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50068039 (1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translation Sciences Curated by ChEMBL | Assay Description Inhibition of wild type glucocerebrosidase using 4-methylumbellifereno-Glc as substrate incubated for 5 mins prior to substrate addition measured aft... | J Med Chem 55: 5734-48 (2012) Article DOI: 10.1021/jm300063b BindingDB Entry DOI: 10.7270/Q2H70GZ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18479 (4-chloro-2-({4-[(2-hydroxyethyl)amino]-6-(pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Bioorg Med Chem Lett 17: 5783-9 (2007) Article DOI: 10.1016/j.bmcl.2007.08.050 BindingDB Entry DOI: 10.7270/Q2Q81BBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398032 (CHEMBL1603014) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using blue-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18469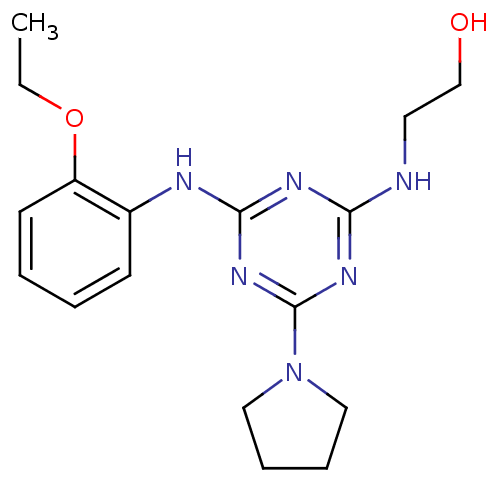 (2-({4-[(2-ethoxyphenyl)amino]-6-(pyrrolidin-1-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Bioorg Med Chem Lett 17: 5783-9 (2007) Article DOI: 10.1016/j.bmcl.2007.08.050 BindingDB Entry DOI: 10.7270/Q2Q81BBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398032 (CHEMBL1603014) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using red-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18481 (2-({4-[(5-chloro-2-ethoxyphenyl)amino]-6-(pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Bioorg Med Chem Lett 17: 5783-9 (2007) Article DOI: 10.1016/j.bmcl.2007.08.050 BindingDB Entry DOI: 10.7270/Q2Q81BBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18483 (2-[(4-{[2-(tert-butoxy)-5-chlorophenyl]amino}-6-(p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Bioorg Med Chem Lett 17: 5783-9 (2007) Article DOI: 10.1016/j.bmcl.2007.08.050 BindingDB Entry DOI: 10.7270/Q2Q81BBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18465 (2-({4-[(2-methoxyphenyl)amino]-6-(pyrrolidin-1-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Bioorg Med Chem Lett 17: 5783-9 (2007) Article DOI: 10.1016/j.bmcl.2007.08.050 BindingDB Entry DOI: 10.7270/Q2Q81BBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398022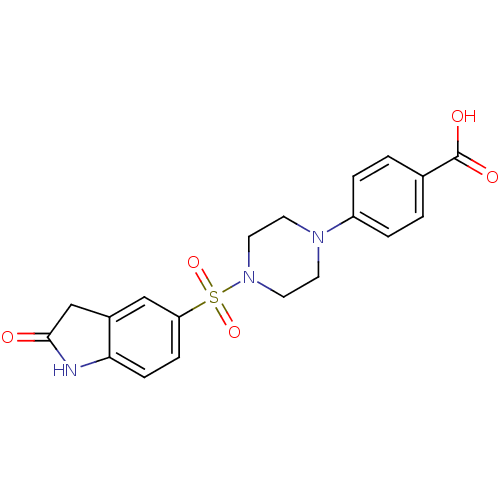 (CHEMBL2181097) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using blue-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398022 (CHEMBL2181097) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using red-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18475 (2-({4-[(2,5-dimethoxyphenyl)amino]-6-(pyrrolidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Bioorg Med Chem Lett 17: 5783-9 (2007) Article DOI: 10.1016/j.bmcl.2007.08.050 BindingDB Entry DOI: 10.7270/Q2Q81BBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398020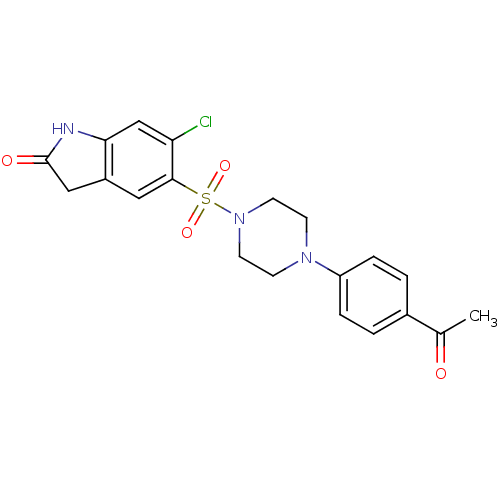 (CHEMBL2181103) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using red-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398020 (CHEMBL2181103) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using blue-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50398029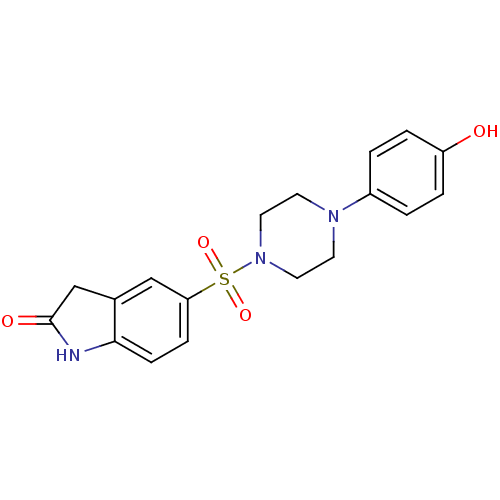 (CHEMBL2180814) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Center for Advancing Translational Sciences Curated by ChEMBL | Assay Description Inhibition of wild type recombinant GAA preincubated for 5 mins measured after 45 mins using blue-shifted dye by fluorescence assay | J Med Chem 55: 7546-59 (2012) Article DOI: 10.1021/jm3005543 BindingDB Entry DOI: 10.7270/Q27945SS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18468 (2-({4-[(2-hydroxyethyl)amino]-6-(pyrrolidin-1-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 5.9 | 21 |
NIH | Assay Description Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss... | Bioorg Med Chem Lett 17: 5783-9 (2007) Article DOI: 10.1016/j.bmcl.2007.08.050 BindingDB Entry DOI: 10.7270/Q2Q81BBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 105 total ) | Next | Last >> |