 Found 2675 hits with Last Name = 'wong' and Initial = 'h'
Found 2675 hits with Last Name = 'wong' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50315177
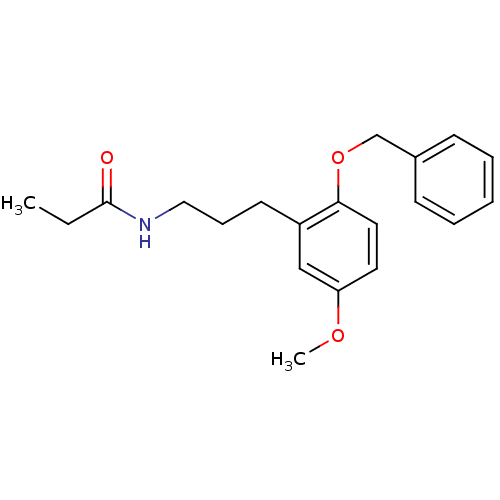
(CHEMBL1091161 | N-(3-(2-(benzyloxy)-5-methoxypheny...)Show InChI InChI=1S/C20H25NO3/c1-3-20(22)21-13-7-10-17-14-18(23-2)11-12-19(17)24-15-16-8-5-4-6-9-16/h4-6,8-9,11-12,14H,3,7,10,13,15H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2582-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.084
BindingDB Entry DOI: 10.7270/Q2S75GG4 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50315171
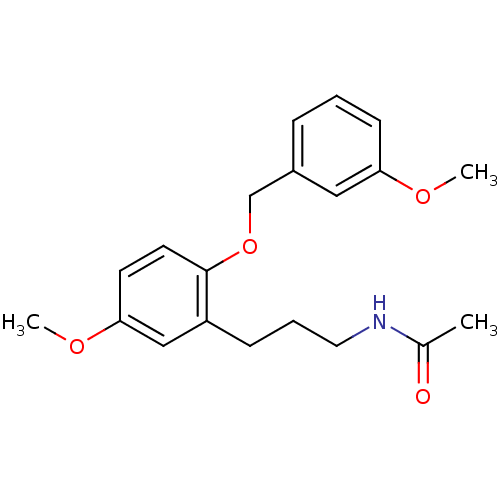
(CHEMBL1092646 | N-(3-(5-methoxy-2-(3-methoxybenzyl...)Show InChI InChI=1S/C20H25NO4/c1-15(22)21-11-5-7-17-13-19(24-3)9-10-20(17)25-14-16-6-4-8-18(12-16)23-2/h4,6,8-10,12-13H,5,7,11,14H2,1-3H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2582-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.084
BindingDB Entry DOI: 10.7270/Q2S75GG4 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50315178
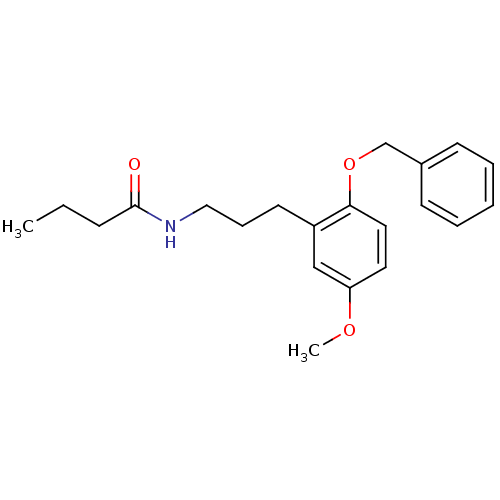
(CHEMBL1088825 | N-(3-(2-(benzyloxy)-5-methoxypheny...)Show InChI InChI=1S/C21H27NO3/c1-3-8-21(23)22-14-7-11-18-15-19(24-2)12-13-20(18)25-16-17-9-5-4-6-10-17/h4-6,9-10,12-13,15H,3,7-8,11,14,16H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2582-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.084
BindingDB Entry DOI: 10.7270/Q2S75GG4 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50315183
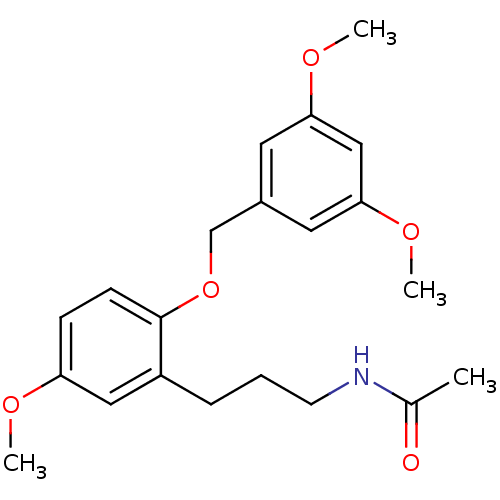
(CHEMBL1091998 | N-(3-(2-(3,5-dimethoxybenzyloxy)-5...)Show InChI InChI=1S/C21H27NO5/c1-15(23)22-9-5-6-17-12-18(24-2)7-8-21(17)27-14-16-10-19(25-3)13-20(11-16)26-4/h7-8,10-13H,5-6,9,14H2,1-4H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0326 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2582-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.084
BindingDB Entry DOI: 10.7270/Q2S75GG4 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50315176
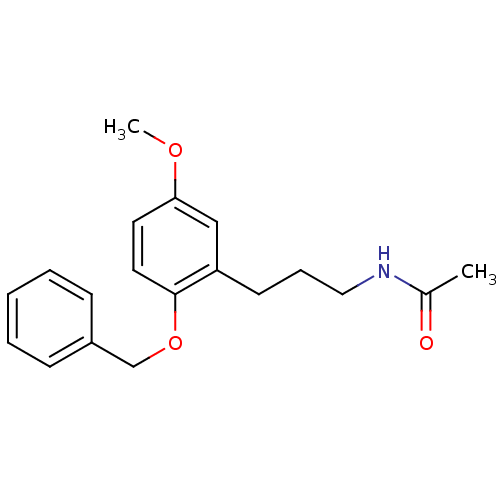
(CHEMBL1091160 | N-(3-(2-(benzyloxy)-5-methoxypheny...)Show InChI InChI=1S/C19H23NO3/c1-15(21)20-12-6-9-17-13-18(22-2)10-11-19(17)23-14-16-7-4-3-5-8-16/h3-5,7-8,10-11,13H,6,9,12,14H2,1-2H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0471 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2582-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.084
BindingDB Entry DOI: 10.7270/Q2S75GG4 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
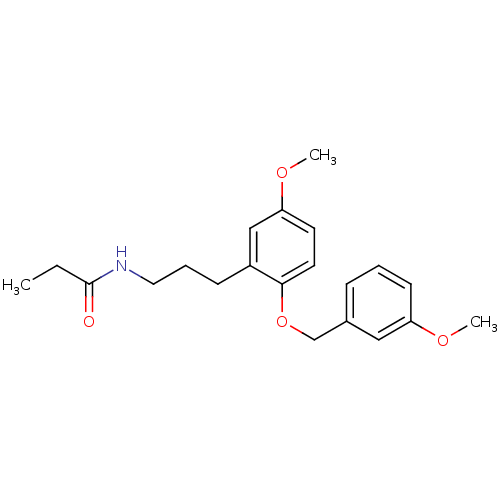
(Homo sapiens (Human)) | BDBM50426656
(CHEMBL2326200)Show InChI InChI=1S/C22H25NO3/c1-4-22(24)23-14-6-8-19-16-21(26-3)13-12-18(19)11-10-17-7-5-9-20(15-17)25-2/h5,7,9,12-13,15-16H,4,6,8,14H2,1-3H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human MT2 receptor expressed in CHO cells after 60 mins by microbeta scintillation method |
Bioorg Med Chem 21: 547-52 (2012)
Article DOI: 10.1016/j.bmc.2012.10.060
BindingDB Entry DOI: 10.7270/Q29W0GTX |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
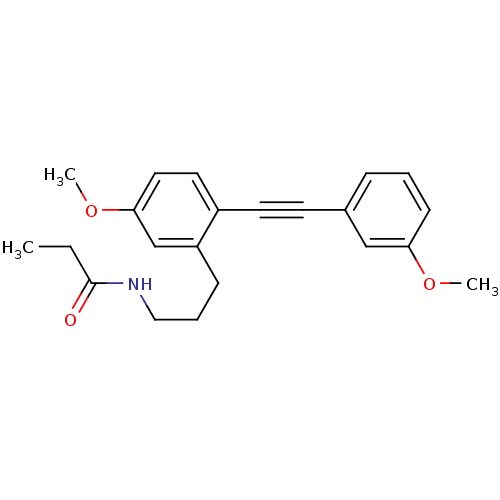
(Homo sapiens (Human)) | BDBM50426657
(CHEMBL2326199)Show InChI InChI=1S/C21H27NO4/c1-4-21(23)22-12-6-8-17-14-19(25-3)10-11-20(17)26-15-16-7-5-9-18(13-16)24-2/h5,7,9-11,13-14H,4,6,8,12,15H2,1-3H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.291 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human MT2 receptor expressed in CHO cells after 60 mins by microbeta scintillation method |
Bioorg Med Chem 21: 547-52 (2012)
Article DOI: 10.1016/j.bmc.2012.10.060
BindingDB Entry DOI: 10.7270/Q29W0GTX |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.296 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human melatonin MT1 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2582-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.084
BindingDB Entry DOI: 10.7270/Q2S75GG4 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1A
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.296 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human MT1 receptor expressed in CHO cells after 60 mins by microbeta scintillation method |
Bioorg Med Chem 21: 547-52 (2012)
Article DOI: 10.1016/j.bmc.2012.10.060
BindingDB Entry DOI: 10.7270/Q29W0GTX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM82505
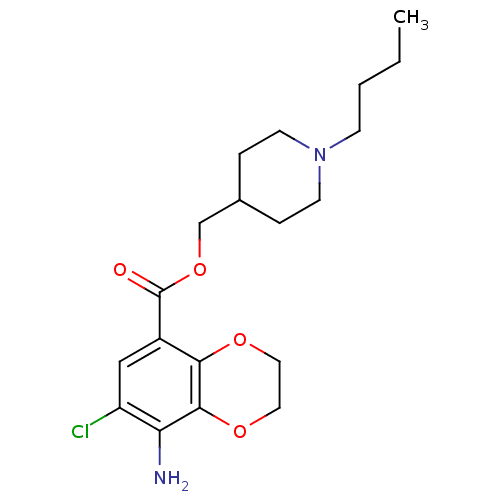
(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244490
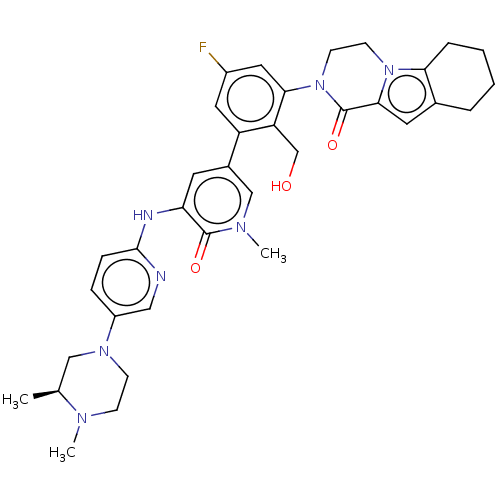
(CHEMBL4102992)Show SMILES C[C@H]1CN(CCN1C)c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C35H40FN7O3/c1-22-19-41(11-10-39(22)2)26-8-9-33(37-18-26)38-29-14-24(20-40(3)34(29)45)27-16-25(36)17-31(28(27)21-44)43-13-12-42-30-7-5-4-6-23(30)15-32(42)35(43)46/h8-9,14-18,20,22,44H,4-7,10-13,19,21H2,1-3H3,(H,37,38)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50426653
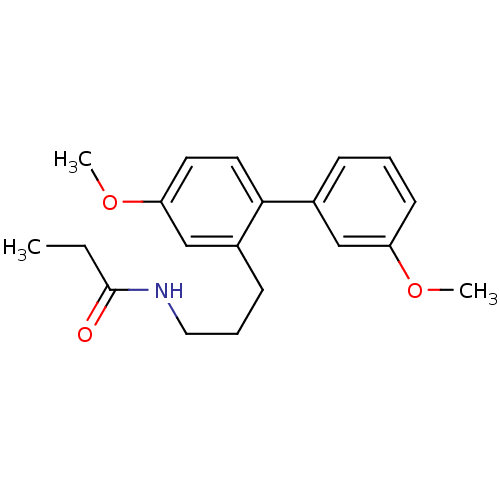
(CHEMBL2326203)Show InChI InChI=1S/C20H25NO3/c1-4-20(22)21-12-6-8-16-14-18(24-3)10-11-19(16)15-7-5-9-17(13-15)23-2/h5,7,9-11,13-14H,4,6,8,12H2,1-3H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.339 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human MT2 receptor expressed in CHO cells after 60 mins by microbeta scintillation method |
Bioorg Med Chem 21: 547-52 (2012)
Article DOI: 10.1016/j.bmc.2012.10.060
BindingDB Entry DOI: 10.7270/Q29W0GTX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111951
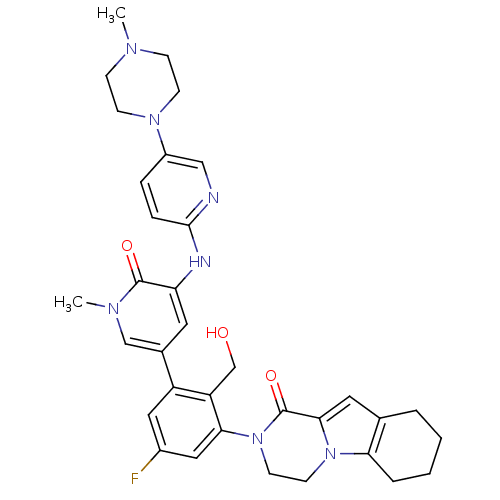
(US8618107, 197)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C34H38FN7O3/c1-38-9-11-40(12-10-38)25-7-8-32(36-19-25)37-28-15-23(20-39(2)33(28)44)26-17-24(35)18-30(27(26)21-43)42-14-13-41-29-6-4-3-5-22(29)16-31(41)34(42)45/h7-8,15-20,43H,3-6,9-14,21H2,1-2H3,(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244489

(CHEMBL4095379)Show SMILES C[C@@H]1CN(C)CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C35H40FN7O3/c1-22-19-39(2)10-11-41(22)26-8-9-33(37-18-26)38-29-14-24(20-40(3)34(29)45)27-16-25(36)17-31(28(27)21-44)43-13-12-42-30-7-5-4-6-23(30)15-32(42)35(43)46/h8-9,14-18,20,22,44H,4-7,10-13,19,21H2,1-3H3,(H,37,38)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM82505

(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.429 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2582-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.084
BindingDB Entry DOI: 10.7270/Q2S75GG4 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.429 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human MT2 receptor expressed in CHO cells after 60 mins by microbeta scintillation method |
Bioorg Med Chem 21: 547-52 (2012)
Article DOI: 10.1016/j.bmc.2012.10.060
BindingDB Entry DOI: 10.7270/Q29W0GTX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244491
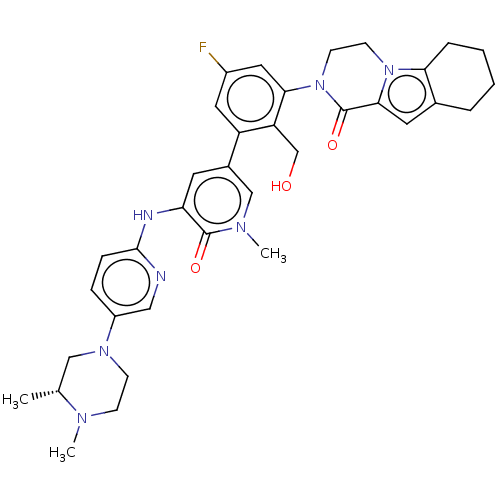
(CHEMBL4092794)Show SMILES C[C@@H]1CN(CCN1C)c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C35H40FN7O3/c1-22-19-41(11-10-39(22)2)26-8-9-33(37-18-26)38-29-14-24(20-40(3)34(29)45)27-16-25(36)17-31(28(27)21-44)43-13-12-42-30-7-5-4-6-23(30)15-32(42)35(43)46/h8-9,14-18,20,22,44H,4-7,10-13,19,21H2,1-3H3,(H,37,38)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244488
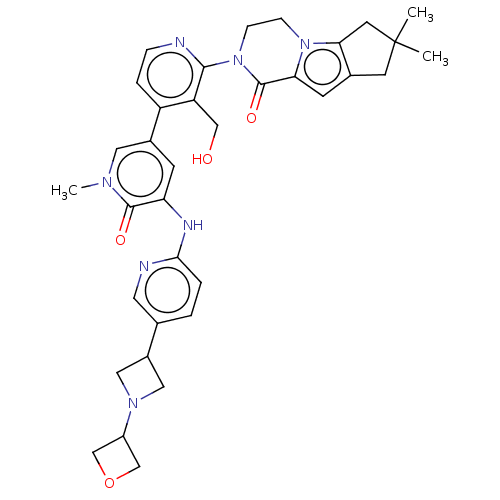
(CHEMBL4069790)Show SMILES Cn1cc(cc(Nc2ccc(cn2)C2CN(C2)C2COC2)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C35H39N7O4/c1-35(2)12-22-11-29-34(45)42(9-8-41(29)30(22)13-35)32-27(18-43)26(6-7-36-32)23-10-28(33(44)39(3)15-23)38-31-5-4-21(14-37-31)24-16-40(17-24)25-19-46-20-25/h4-7,10-11,14-15,24-25,43H,8-9,12-13,16-20H2,1-3H3,(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244494
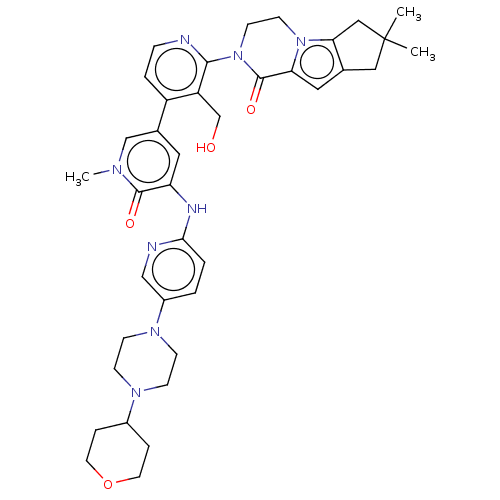
(CHEMBL4090117)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2CCOCC2)c1=O)-c1ccnc(N2CCn3c4CC(C)(C)Cc4cc3C2=O)c1CO Show InChI InChI=1S/C38H46N8O4/c1-38(2)20-25-19-32-37(49)46(15-14-45(32)33(25)21-38)35-30(24-47)29(6-9-39-35)26-18-31(36(48)42(3)23-26)41-34-5-4-28(22-40-34)44-12-10-43(11-13-44)27-7-16-50-17-8-27/h4-6,9,18-19,22-23,27,47H,7-8,10-17,20-21,24H2,1-3H3,(H,40,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615173

(CHEMBL5265782) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50426655
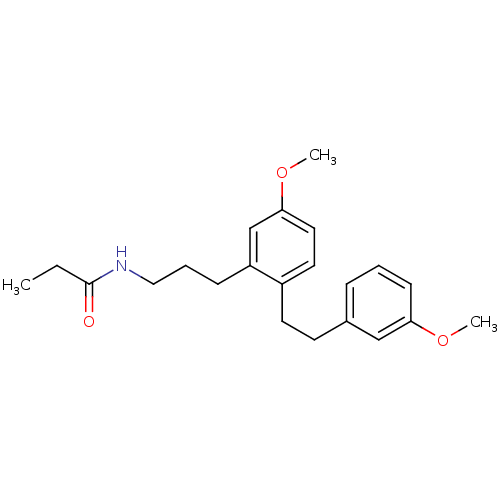
(CHEMBL2326201)Show InChI InChI=1S/C22H29NO3/c1-4-22(24)23-14-6-8-19-16-21(26-3)13-12-18(19)11-10-17-7-5-9-20(15-17)25-2/h5,7,9,12-13,15-16H,4,6,8,10-11,14H2,1-3H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human MT2 receptor expressed in CHO cells after 60 mins by microbeta scintillation method |
Bioorg Med Chem 21: 547-52 (2012)
Article DOI: 10.1016/j.bmc.2012.10.060
BindingDB Entry DOI: 10.7270/Q29W0GTX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244467
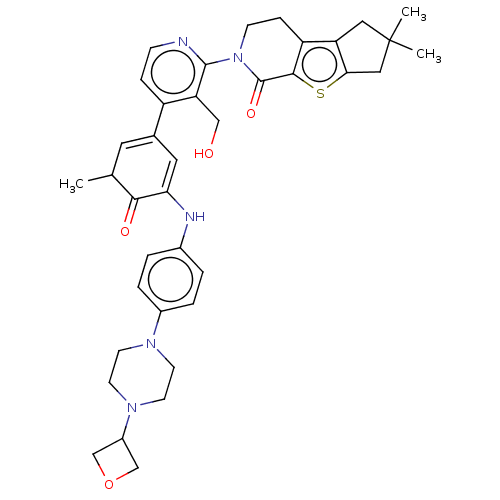
(CHEMBL4063638)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(N2CCc3c4CC(C)(C)Cc4sc3C2=O)c1CO |c:2,t:4| Show InChI InChI=1S/C38H43N5O4S/c1-23-16-24(17-32(34(23)45)40-25-4-6-26(7-5-25)41-12-14-42(15-13-41)27-21-47-22-27)28-8-10-39-36(31(28)20-44)43-11-9-29-30-18-38(2,3)19-33(30)48-35(29)37(43)46/h4-8,10,16-17,23,27,40,44H,9,11-15,18-22H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50054820
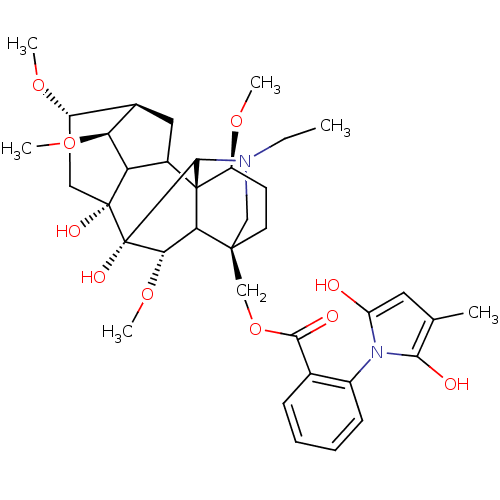
(Methyllycaconitine | [(1S,4S,5R,6S,8R,9R,13S,16S,1...)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)[C@@]34C5C[C@H]6[C@H](OC)C5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)C23)C14 |wU:4.4,42.46,36.39,44.48,39.43,wD:28.55,32.34,25.27,31.32,TLB:28:29:32:36.38.39,1:2:47:25.23.24,25:28:42.44:4.2.3,THB:42:48:47:25.23.24,29:28:42.44:4.2.3,2:48:36.35.29:47.44,(3.83,-6.7,;5.61,-4.92,;8.1,-4.92,;7.12,-7.5,;8.22,-6.42,;8.22,-7.94,;7.82,-9.45,;8.61,-10.78,;9.94,-10.01,;8.62,-12.33,;7.29,-13.1,;7.29,-14.64,;8.62,-15.41,;9.95,-14.64,;9.95,-13.1,;11.49,-13.09,;12.9,-13.7,;13.3,-15.19,;13.93,-12.55,;13.14,-11.22,;13.91,-9.88,;11.64,-11.55,;10.86,-10.2,;6.88,-5.65,;6.87,-4.11,;8.21,-3.34,;8.2,-1.8,;6.87,-1.03,;9.54,-4.11,;10.5,-3.18,;10.3,-1.71,;11.64,-1.06,;12.67,-2.15,;14.21,-2.15,;14.96,-3.46,;11.97,-3.45,;12.65,-4.9,;13.98,-5.67,;16.6,-3.1,;16.6,-1.56,;17.69,-.47,;19.02,-1.24,;11.93,-6.28,;12.7,-7.59,;10.46,-6.53,;10.06,-8.01,;11.39,-8.78,;9.55,-5.63,;9.53,-2.57,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22?,24+,25+,27?,28+,29?,30+,33?,34+,35-,36+,37+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244502

(CHEMBL4085043)Show SMILES C[C@H]1CN(C)CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2cc(F)cc(N3CCn4c5CCCCc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C35H40FN7O3/c1-22-19-39(2)10-11-41(22)26-8-9-33(37-18-26)38-29-14-24(20-40(3)34(29)45)27-16-25(36)17-31(28(27)21-44)43-13-12-42-30-7-5-4-6-23(30)15-32(42)35(43)46/h8-9,14-18,20,22,44H,4-7,10-13,19,21H2,1-3H3,(H,37,38)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111952
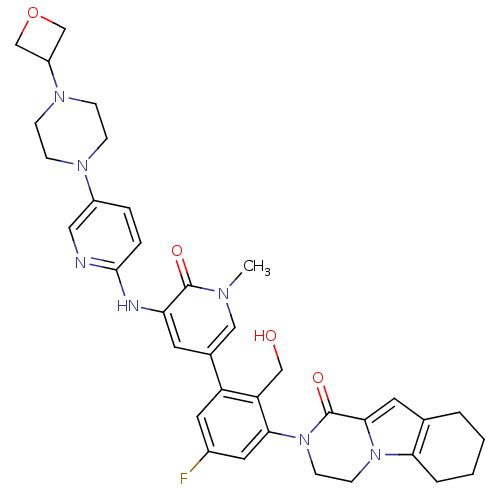
(US8618107, 210)Show SMILES Cn1cc(cc(Nc2ccc(cn2)N2CCN(CC2)C2COC2)c1=O)-c1cc(F)cc(N2CCn3c4CCCCc4cc3C2=O)c1CO Show InChI InChI=1S/C36H40FN7O4/c1-40-19-24(14-30(35(40)46)39-34-7-6-26(18-38-34)41-8-10-42(11-9-41)27-21-48-22-27)28-16-25(37)17-32(29(28)20-45)44-13-12-43-31-5-3-2-4-23(31)15-33(43)36(44)47/h6-7,14-19,27,45H,2-5,8-13,20-22H2,1H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50007872
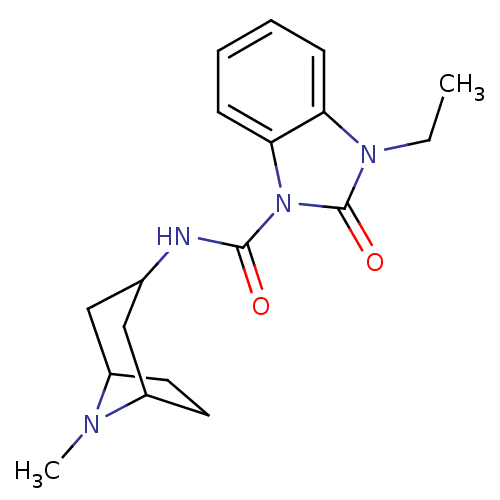
(3-Ethyl-2-oxo-2,3-dihydro-benzoimidazole-1-carboxy...)Show SMILES CCn1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:12:13:20:16.17| Show InChI InChI=1S/C18H24N4O2/c1-3-21-15-6-4-5-7-16(15)22(18(21)24)17(23)19-12-10-13-8-9-14(11-12)20(13)2/h4-7,12-14H,3,8-11H2,1-2H3,(H,19,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50049516
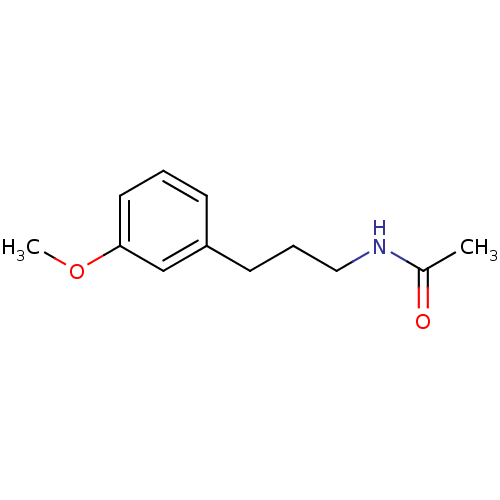
(CHEMBL50398 | N-(3-(3-methoxyphenyl)propyl)acetami...)Show InChI InChI=1S/C12H17NO2/c1-10(14)13-8-4-6-11-5-3-7-12(9-11)15-2/h3,5,7,9H,4,6,8H2,1-2H3,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.751 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2582-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.084
BindingDB Entry DOI: 10.7270/Q2S75GG4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50056404
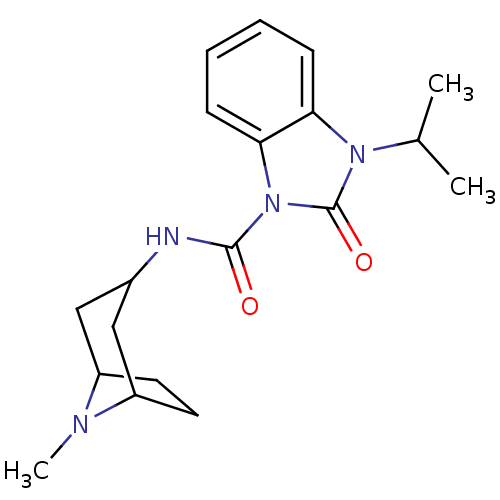
(3-Isopropyl-2-oxo-2,3-dihydro-benzoimidazole-1-car...)Show SMILES CC(C)n1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:13:14:21:17.18| Show InChI InChI=1S/C19H26N4O2/c1-12(2)22-16-6-4-5-7-17(16)23(19(22)25)18(24)20-13-10-14-8-9-15(11-13)21(14)3/h4-7,12-15H,8-11H2,1-3H3,(H,20,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244493
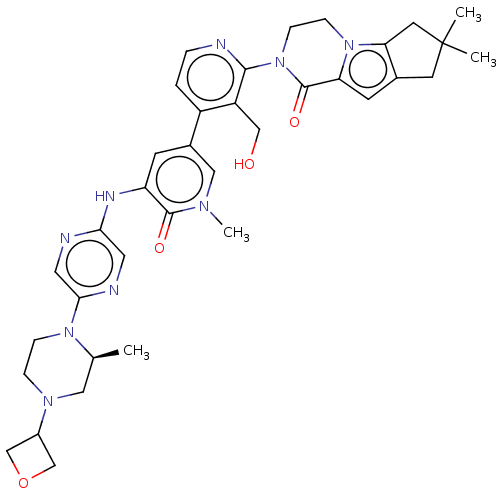
(CHEMBL4070991)Show SMILES C[C@H]1CN(CCN1c1cnc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)cn1)C1COC1 |r| Show InChI InChI=1S/C36H43N9O4/c1-22-17-42(25-20-49-21-25)7-8-43(22)32-16-38-31(15-39-32)40-28-11-24(18-41(4)34(28)47)26-5-6-37-33(27(26)19-46)45-10-9-44-29(35(45)48)12-23-13-36(2,3)14-30(23)44/h5-6,11-12,15-16,18,22,25,46H,7-10,13-14,17,19-21H2,1-4H3,(H,38,40)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244440
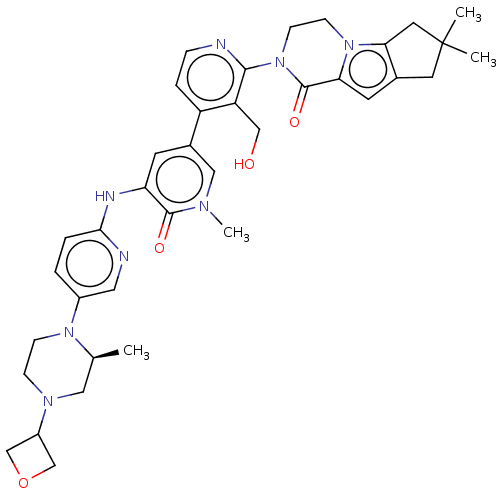
(CHEMBL4065122)Show SMILES C[C@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C37H44N8O4/c1-23-18-42(27-21-49-22-27)9-10-43(23)26-5-6-33(39-17-26)40-30-13-25(19-41(4)35(30)47)28-7-8-38-34(29(28)20-46)45-12-11-44-31(36(45)48)14-24-15-37(2,3)16-32(24)44/h5-8,13-14,17,19,23,27,46H,9-12,15-16,18,20-22H2,1-4H3,(H,39,40)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244492
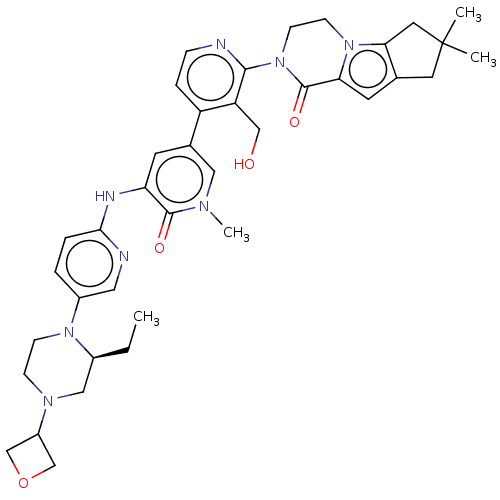
(CHEMBL4087543)Show SMILES CC[C@H]1CN(CCN1c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1)C1COC1 |r| Show InChI InChI=1S/C38H46N8O4/c1-5-26-20-43(28-22-50-23-28)10-11-44(26)27-6-7-34(40-18-27)41-31-14-25(19-42(4)36(31)48)29-8-9-39-35(30(29)21-47)46-13-12-45-32(37(46)49)15-24-16-38(2,3)17-33(24)45/h6-9,14-15,18-19,26,28,47H,5,10-13,16-17,20-23H2,1-4H3,(H,40,41)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50056404

(3-Isopropyl-2-oxo-2,3-dihydro-benzoimidazole-1-car...)Show SMILES CC(C)n1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:13:14:21:17.18| Show InChI InChI=1S/C19H26N4O2/c1-12(2)22-16-6-4-5-7-17(16)23(19(22)25)18(24)20-13-10-14-8-9-15(11-13)21(14)3/h4-7,12-15H,8-11H2,1-3H3,(H,20,24) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244500
(CHEMBL4093188)Show SMILES CC1C=C(C=C(Nc2ccc(cc2)N2CCN(CC2)C2COC2)C1=O)c1ccnc(N2Cc3cc(sc3C2=O)C(C)(C)C)c1CO |c:2,t:4| Show InChI InChI=1S/C36H41N5O4S/c1-22-15-23(28-9-10-37-34(29(28)19-42)41-18-24-17-31(36(2,3)4)46-33(24)35(41)44)16-30(32(22)43)38-25-5-7-26(8-6-25)39-11-13-40(14-12-39)27-20-45-21-27/h5-10,15-17,22,27,38,42H,11-14,18-21H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244486

(CHEMBL4097832)Show SMILES COCCCN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C37H46N8O4/c1-37(2)20-25-19-31-36(48)45(16-15-44(31)32(25)21-37)34-29(24-46)28(8-9-38-34)26-18-30(35(47)41(3)23-26)40-33-7-6-27(22-39-33)43-13-11-42(12-14-43)10-5-17-49-4/h6-9,18-19,22-23,46H,5,10-17,20-21,24H2,1-4H3,(H,39,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50107863
((-)-cytisine | (1R,9R)-7,11-diazatricyclo[7.3.1.0~...)Show InChI InChI=1S/C11H14N2O/c14-11-3-1-2-10-9-4-8(5-12-6-9)7-13(10)11/h1-3,8-9,12H,4-7H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244501
(CHEMBL4062634)Show SMILES COCCN1CCN(CC1)c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1 Show InChI InChI=1S/C36H44N8O4/c1-36(2)19-24-18-30-35(47)44(14-13-43(30)31(24)20-36)33-28(23-45)27(7-8-37-33)25-17-29(34(46)40(3)22-25)39-32-6-5-26(21-38-32)42-11-9-41(10-12-42)15-16-48-4/h5-8,17-18,21-22,45H,9-16,19-20,23H2,1-4H3,(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85135
(RS 57639)Show SMILES COc1cc(N)c(Cl)cc1C(=O)OCC1CCN(CCc2ccc3OCCOc3c2)CC1 Show InChI InChI=1S/C24H29ClN2O5/c1-29-22-14-20(26)19(25)13-18(22)24(28)32-15-17-5-8-27(9-6-17)7-4-16-2-3-21-23(12-16)31-11-10-30-21/h2-3,12-14,17H,4-11,15,26H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM82505

(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM85135

(RS 57639)Show SMILES COc1cc(N)c(Cl)cc1C(=O)OCC1CCN(CCc2ccc3OCCOc3c2)CC1 Show InChI InChI=1S/C24H29ClN2O5/c1-29-22-14-20(26)19(25)13-18(22)24(28)32-15-17-5-8-27(9-6-17)7-4-16-2-3-21-23(12-16)31-11-10-30-21/h2-3,12-14,17H,4-11,15,26H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111939

(US8618107, 105)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2CCc3c4CC(C)(C)Cc4sc3C2=O)c1CO Show InChI InChI=1S/C29H29N5O3S/c1-29(2)12-20-19-8-10-34(28(37)26(19)38-24(20)13-29)23-6-4-5-18(21(23)15-35)17-11-22(27(36)33(3)14-17)32-25-7-9-30-16-31-25/h4-7,9,11,14,16,35H,8,10,12-13,15H2,1-3H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged BTK expressed in baculovirus expression system by Z-LYTE assay |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50244497

(CHEMBL4074792)Show SMILES C[C@@H]1CN([C@@H](C)CN1C1COC1)c1ccc(Nc2cc(cn(C)c2=O)-c2ccnc(N3CCn4c5CC(C)(C)Cc5cc4C3=O)c2CO)nc1 |r| Show InChI InChI=1S/C38H46N8O4/c1-23-18-46(28-21-50-22-28)24(2)17-45(23)27-6-7-34(40-16-27)41-31-12-26(19-42(5)36(31)48)29-8-9-39-35(30(29)20-47)44-11-10-43-32(37(44)49)13-25-14-38(3,4)15-33(25)43/h6-9,12-13,16,19,23-24,28,47H,10-11,14-15,17-18,20-22H2,1-5H3,(H,40,41)/t23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant C-terminal polyhistidine tagged BTK (1 to 659 residues)-mediated synthetic peptide substrate phosphorylation expresse... |
J Med Chem 61: 2227-2245 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01712
BindingDB Entry DOI: 10.7270/Q2H134F7 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM586099
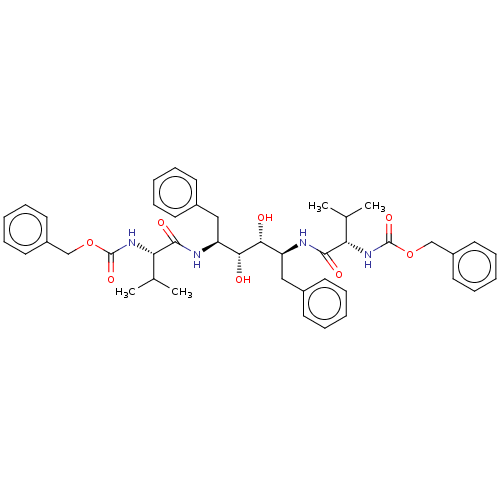
(BDBM50064200 | TL-3)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.5 | -52.4 | n/a | n/a | n/a | n/a | n/a | 5.6 | 37 |
The Scripps Research Institute
| Assay Description
Inhibition of HIV-protease activity for selected acids at P3-P3' positions. |
Chem Biol 9: 891-6 (2002)
Article DOI: 10.1016/S1074-5521(02)00184-9
BindingDB Entry DOI: 10.7270/Q2GM85QP |
More data for this
Ligand-Target Pair | |
Tissue alpha-L-fucosidase
(Homo sapiens (Human)) | BDBM50072685
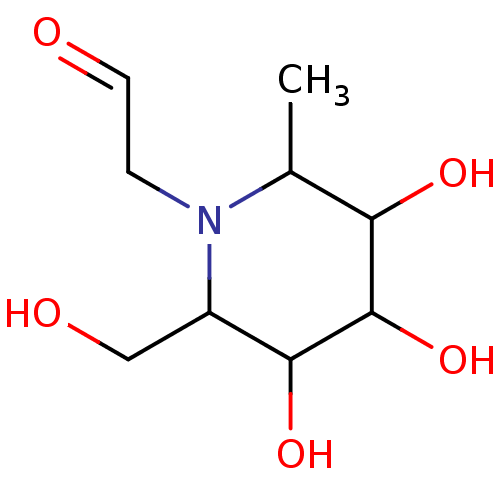
((3,4,5-Trihydroxy-2-hydroxymethyl-6-methyl-piperid...)Show InChI InChI=1S/C9H17NO5/c1-5-7(13)9(15)8(14)6(4-12)10(5)2-3-11/h3,5-9,12-15H,2,4H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against alpha-1,2-Fucosidase obtained from Arthrobacter oxidans F1 |
Bioorg Med Chem Lett 8: 3353-8 (1999)
BindingDB Entry DOI: 10.7270/Q2MK6C1B |
More data for this
Ligand-Target Pair | |
Microtubule-associated protein tau
(Homo sapiens (Human)) | BDBM50615175
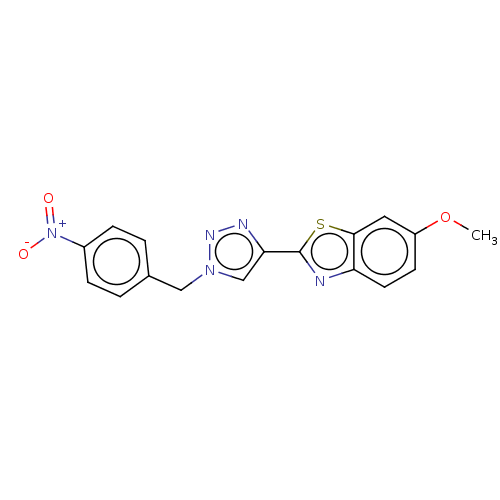
(CHEMBL5285051)Show SMILES COc1ccc2nc(sc2c1)-c1cn(Cc2ccc(cc2)[N+]([O-])=O)nn1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | >1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data