 Found 212 hits with Last Name = 'verhagen' and Initial = 'j'
Found 212 hits with Last Name = 'verhagen' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026685

(CHEMBL3335373)Show SMILES CC(C)c1cccc(Nc2nc3cc(Oc4ccnc(NC(C)=O)c4)ccc3n2C)c1 Show InChI InChI=1S/C24H25N5O2/c1-15(2)17-6-5-7-18(12-17)27-24-28-21-13-19(8-9-22(21)29(24)4)31-20-10-11-25-23(14-20)26-16(3)30/h5-15H,1-4H3,(H,27,28)(H,25,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026695
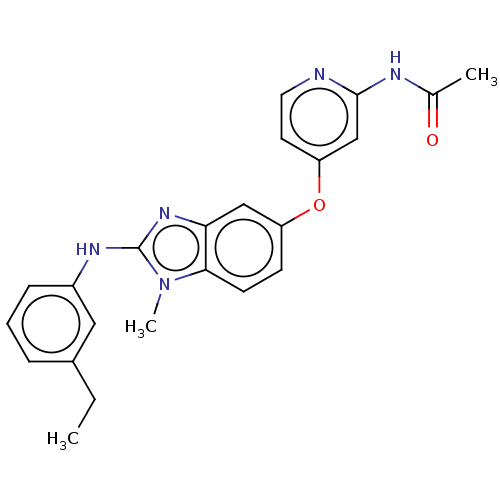
(CHEMBL3335372)Show SMILES CCc1cccc(Nc2nc3cc(Oc4ccnc(NC(C)=O)c4)ccc3n2C)c1 Show InChI InChI=1S/C23H23N5O2/c1-4-16-6-5-7-17(12-16)26-23-27-20-13-18(8-9-21(20)28(23)3)30-19-10-11-24-22(14-19)25-15(2)29/h5-14H,4H2,1-3H3,(H,26,27)(H,24,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026678
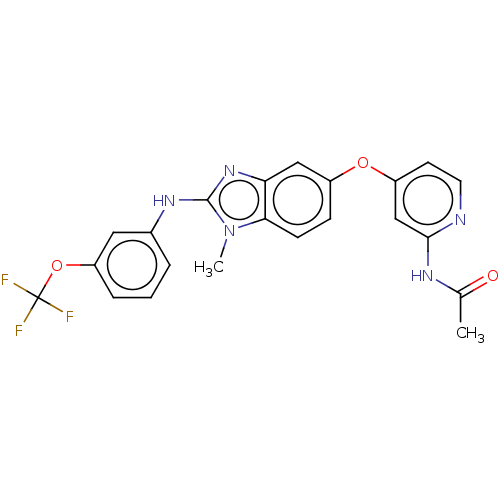
(CHEMBL3335374)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(OC(F)(F)F)c4)nc3c2)ccn1 Show InChI InChI=1S/C22H18F3N5O3/c1-13(31)27-20-12-16(8-9-26-20)32-15-6-7-19-18(11-15)29-21(30(19)2)28-14-4-3-5-17(10-14)33-22(23,24)25/h3-12H,1-2H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM31088
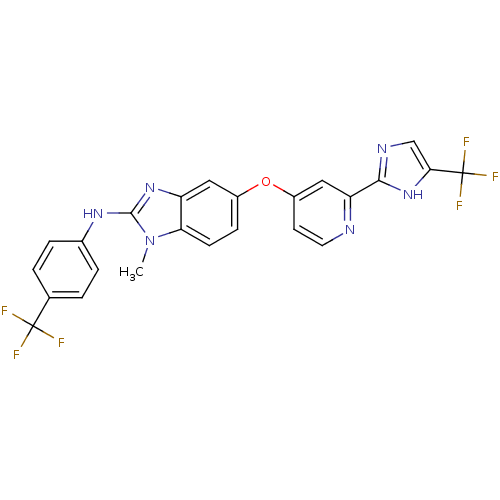
(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity towards Coagulation factor X was determined using chromogenic substrate, MeO-COD-CHG-Gly-Arg-pNA |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50026679
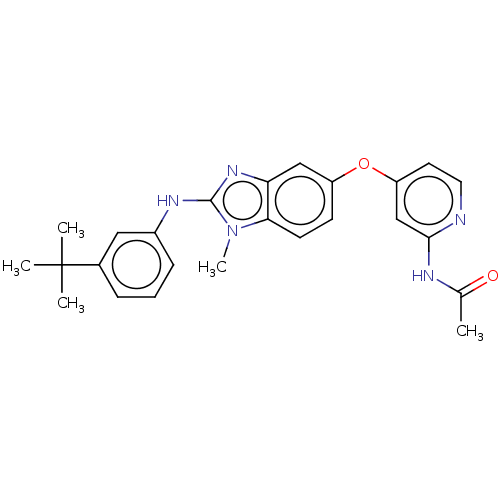
(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Histamine H1 receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344541

(4-(2-(4-ethylphenylamino)benzo[d]oxazol-5-yloxy)-N...)Show SMILES CCc1ccc(Nc2nc3cc(Oc4ccnc(c4)C(=O)NC)ccc3o2)cc1 Show InChI InChI=1S/C22H20N4O3/c1-3-14-4-6-15(7-5-14)25-22-26-18-12-16(8-9-20(18)29-22)28-17-10-11-24-19(13-17)21(27)23-2/h4-13H,3H2,1-2H3,(H,23,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344540
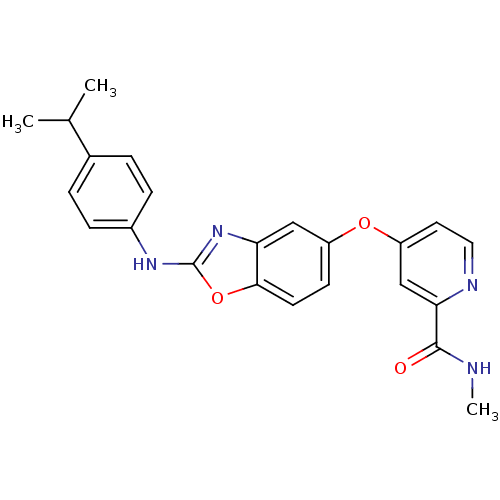
(4-(2-(4-isopropylphenylamino)benzo[d]oxazol-5-ylox...)Show SMILES CNC(=O)c1cc(Oc2ccc3oc(Nc4ccc(cc4)C(C)C)nc3c2)ccn1 Show InChI InChI=1S/C23H22N4O3/c1-14(2)15-4-6-16(7-5-15)26-23-27-19-12-17(8-9-21(19)30-23)29-18-10-11-25-20(13-18)22(28)24-3/h4-14H,1-3H3,(H,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344525
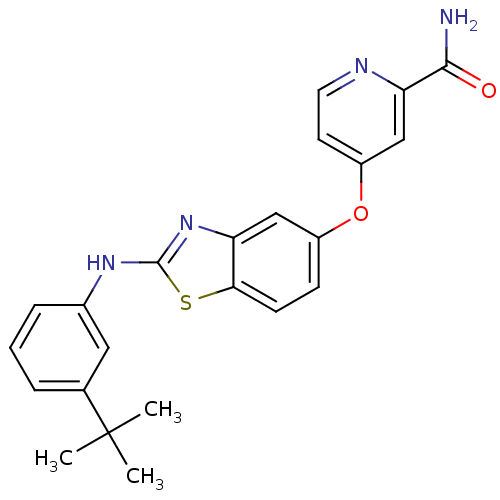
(4-(2-(3-tert-butylphenylamino)benzo[d]thiazol-5-yl...)Show SMILES CC(C)(C)c1cccc(Nc2nc3cc(Oc4ccnc(c4)C(N)=O)ccc3s2)c1 Show InChI InChI=1S/C23H22N4O2S/c1-23(2,3)14-5-4-6-15(11-14)26-22-27-18-12-16(7-8-20(18)30-22)29-17-9-10-25-19(13-17)21(24)28/h4-13H,1-3H3,(H2,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344548
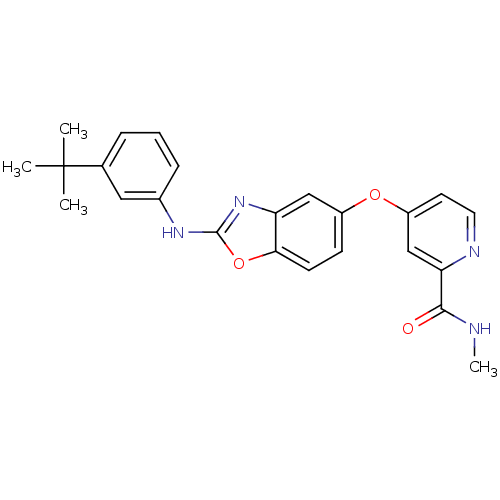
(4-(2-(3-tert-butyl phenylamino)benzo[d]oxazol-5-yl...)Show SMILES CNC(=O)c1cc(Oc2ccc3oc(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C24H24N4O3/c1-24(2,3)15-6-5-7-16(12-15)27-23-28-19-13-17(8-9-21(19)31-23)30-18-10-11-26-20(14-18)22(29)25-4/h5-14H,1-4H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344532
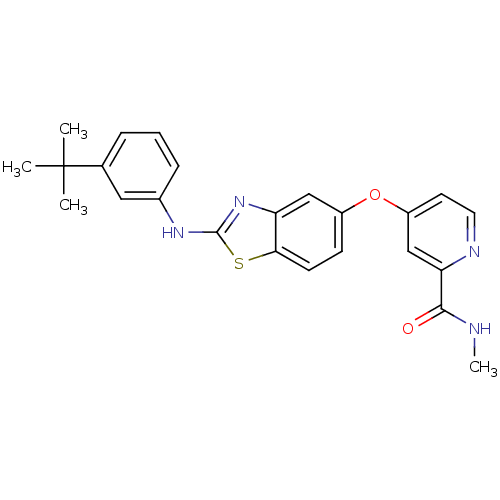
(4-(2-(3-tert-butylphenylamino)benzo[d]thiazol-5-yl...)Show SMILES CNC(=O)c1cc(Oc2ccc3sc(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C24H24N4O2S/c1-24(2,3)15-6-5-7-16(12-15)27-23-28-19-13-17(8-9-21(19)31-23)30-18-10-11-26-20(14-18)22(29)25-4/h5-14H,1-4H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50131822
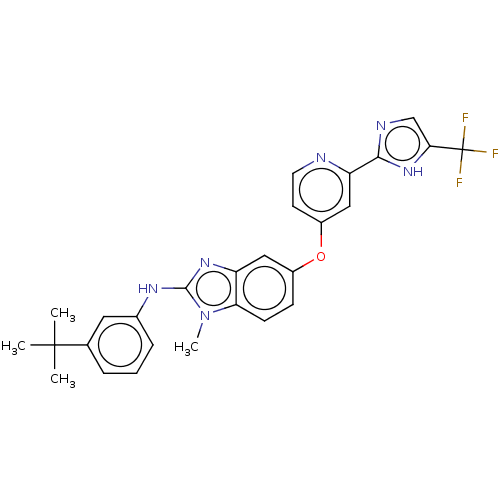
(CHEMBL3632719)Show SMILES Cn1c(Nc2cccc(c2)C(C)(C)C)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C27H25F3N6O/c1-26(2,3)16-6-5-7-17(12-16)33-25-34-20-13-18(8-9-22(20)36(25)4)37-19-10-11-31-21(14-19)24-32-15-23(35-24)27(28,29)30/h5-15H,1-4H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of Histamine H1 receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026692
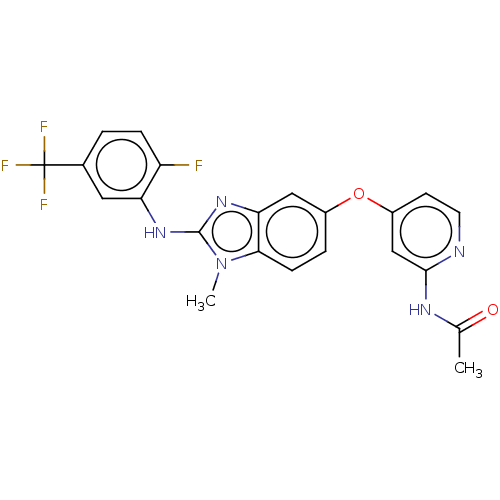
(CHEMBL3335381)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cc(ccc4F)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C22H17F4N5O2/c1-12(32)28-20-11-15(7-8-27-20)33-14-4-6-19-18(10-14)30-21(31(19)2)29-17-9-13(22(24,25)26)3-5-16(17)23/h3-11H,1-2H3,(H,29,30)(H,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026677
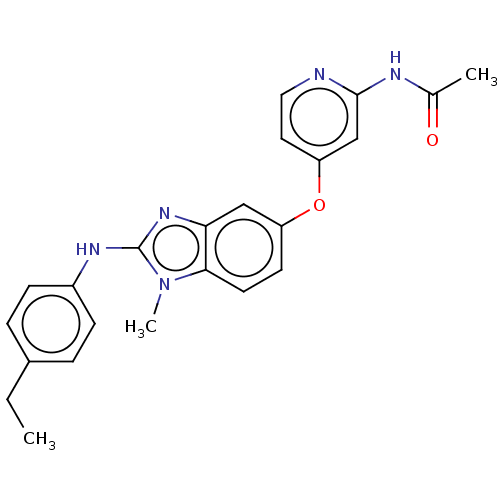
(CHEMBL3335377)Show SMILES CCc1ccc(Nc2nc3cc(Oc4ccnc(NC(C)=O)c4)ccc3n2C)cc1 Show InChI InChI=1S/C23H23N5O2/c1-4-16-5-7-17(8-6-16)26-23-27-20-13-18(9-10-21(20)28(23)3)30-19-11-12-24-22(14-19)25-15(2)29/h5-14H,4H2,1-3H3,(H,26,27)(H,24,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026683
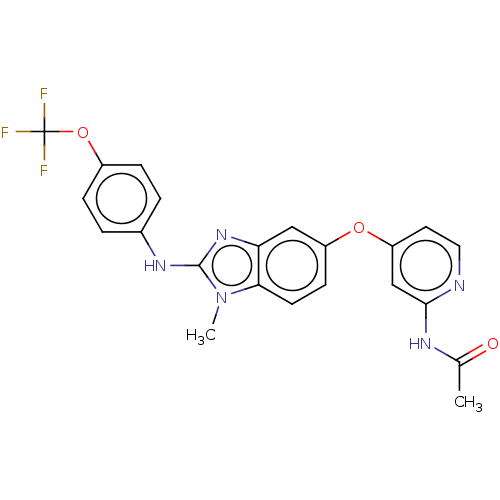
(CHEMBL3335379)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4ccc(OC(F)(F)F)cc4)nc3c2)ccn1 Show InChI InChI=1S/C22H18F3N5O3/c1-13(31)27-20-12-17(9-10-26-20)32-16-7-8-19-18(11-16)29-21(30(19)2)28-14-3-5-15(6-4-14)33-22(23,24)25/h3-12H,1-2H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344539
(4-(2-(4-butylphenylamino)benzo[d]oxazol-5-yloxy)-N...)Show SMILES CCCCc1ccc(Nc2nc3cc(Oc4ccnc(c4)C(=O)NC)ccc3o2)cc1 Show InChI InChI=1S/C24H24N4O3/c1-3-4-5-16-6-8-17(9-7-16)27-24-28-20-14-18(10-11-22(20)31-24)30-19-12-13-26-21(15-19)23(29)25-2/h6-15H,3-5H2,1-2H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344538

(CHEMBL1778397 | N-methyl-4-(2-(4-(trifluoromethoxy...)Show SMILES CNC(=O)c1cc(Oc2ccc3oc(Nc4ccc(OC(F)(F)F)cc4)nc3c2)ccn1 Show InChI InChI=1S/C21H15F3N4O4/c1-25-19(29)17-11-15(8-9-26-17)30-14-6-7-18-16(10-14)28-20(31-18)27-12-2-4-13(5-3-12)32-21(22,23)24/h2-11H,1H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439721
(CHEMBL2418953)Show SMILES CC(=O)Nc1cn2nc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C14H11F3N6O/c1-7(24)20-11-6-23-12(21-11)3-2-10(22-23)8-4-9(14(15,16)17)13(18)19-5-8/h2-6H,1H3,(H2,18,19)(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344530
(4-(2-(4-bromophenylamino)benzo[d]thiazol-5-yloxy)-...)Show SMILES CNC(=O)c1cc(Oc2ccc3sc(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C20H15BrN4O2S/c1-22-19(26)17-11-15(8-9-23-17)27-14-6-7-18-16(10-14)25-20(28-18)24-13-4-2-12(21)3-5-13/h2-11H,1H3,(H,22,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026680

(CHEMBL3335385)Show SMILES CC(C)N1CCC(CC1)C(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cc(ccc4F)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C29H30F4N6O2/c1-17(2)39-12-9-18(10-13-39)27(40)37-26-16-21(8-11-34-26)41-20-5-7-25-24(15-20)36-28(38(25)3)35-23-14-19(29(31,32)33)4-6-22(23)30/h4-8,11,14-18H,9-10,12-13H2,1-3H3,(H,35,36)(H,34,37,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1/2/3
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140266
(CHEMBL3753366)Show InChI InChI=1S/C16H18N6O2/c1-9(2)24-16-15(17)18-6-12(20-16)11-4-5-14-21-13(19-10(3)23)8-22(14)7-11/h4-9H,1-3H3,(H2,17,18)(H,19,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380371
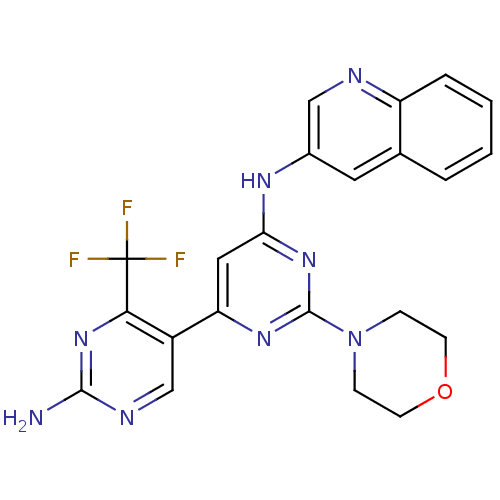
(CHEMBL2017970)Show SMILES Nc1ncc(-c2cc(Nc3cnc4ccccc4c3)nc(n2)N2CCOCC2)c(n1)C(F)(F)F Show InChI InChI=1S/C22H19F3N8O/c23-22(24,25)19-15(12-28-20(26)32-19)17-10-18(31-21(30-17)33-5-7-34-8-6-33)29-14-9-13-3-1-2-4-16(13)27-11-14/h1-4,9-12H,5-8H2,(H2,26,28,32)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380366
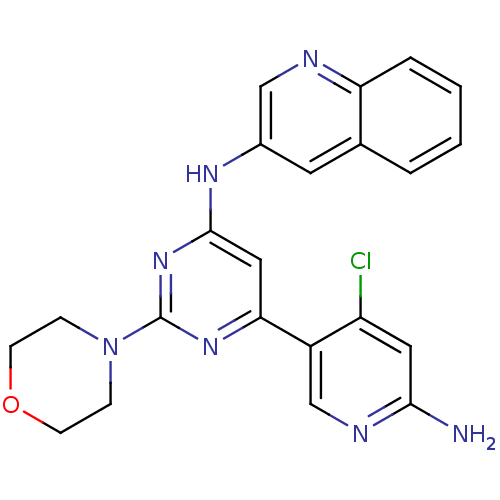
(CHEMBL2017965)Show SMILES Nc1cc(Cl)c(cn1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C22H20ClN7O/c23-17-10-20(24)26-13-16(17)19-11-21(29-22(28-19)30-5-7-31-8-6-30)27-15-9-14-3-1-2-4-18(14)25-12-15/h1-4,9-13H,5-8H2,(H2,24,26)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380369

(CHEMBL2017968)Show SMILES Nc1ncc(c(N)n1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C21H21N9O/c22-19-15(12-25-20(23)29-19)17-10-18(28-21(27-17)30-5-7-31-8-6-30)26-14-9-13-3-1-2-4-16(13)24-11-14/h1-4,9-12H,5-8H2,(H,26,27,28)(H4,22,23,25,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026694
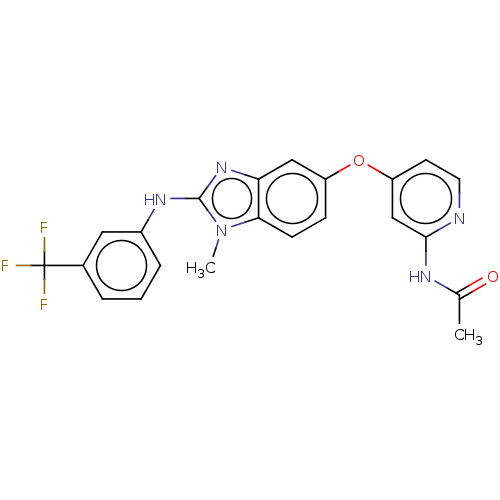
(CHEMBL3335375)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C22H18F3N5O2/c1-13(31)27-20-12-17(8-9-26-20)32-16-6-7-19-18(11-16)29-21(30(19)2)28-15-5-3-4-14(10-15)22(23,24)25/h3-12H,1-2H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Reduced farnesylation of H-ras transformed NIH3T3 cells |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140265
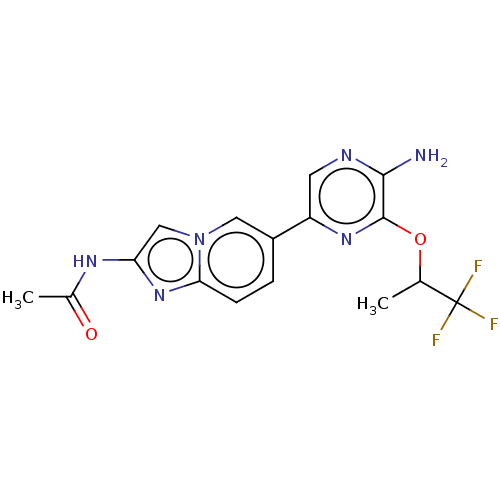
(CHEMBL3753450)Show SMILES CC(Oc1nc(cnc1N)-c1ccc2nc(NC(C)=O)cn2c1)C(F)(F)F Show InChI InChI=1S/C16H15F3N6O2/c1-8(16(17,18)19)27-15-14(20)21-5-11(23-15)10-3-4-13-24-12(22-9(2)26)7-25(13)6-10/h3-8H,1-2H3,(H2,20,21)(H,22,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344543
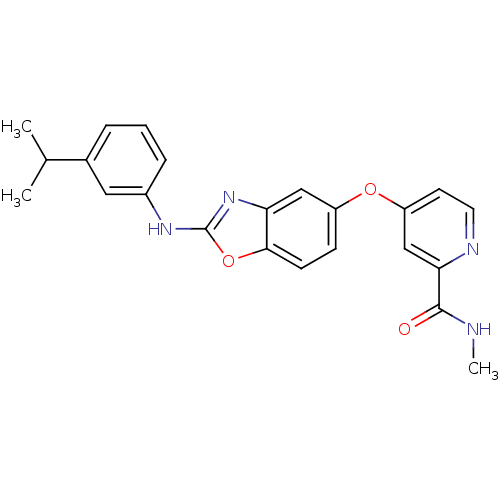
(4-(2-(3-isopropylphenylamino)benzo[d]oxazol-5-ylox...)Show SMILES CNC(=O)c1cc(Oc2ccc3oc(Nc4cccc(c4)C(C)C)nc3c2)ccn1 Show InChI InChI=1S/C23H22N4O3/c1-14(2)15-5-4-6-16(11-15)26-23-27-19-12-17(7-8-21(19)30-23)29-18-9-10-25-20(13-18)22(28)24-3/h4-14H,1-3H3,(H,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344542

(4-(2-(4-bromophenylamino)benzo[d]oxazol-5-yloxy)-N...)Show SMILES CNC(=O)c1cc(Oc2ccc3oc(Nc4ccc(Br)cc4)nc3c2)ccn1 Show InChI InChI=1S/C20H15BrN4O3/c1-22-19(26)17-11-15(8-9-23-17)27-14-6-7-18-16(10-14)25-20(28-18)24-13-4-2-12(21)3-5-13/h2-11H,1H3,(H,22,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140269
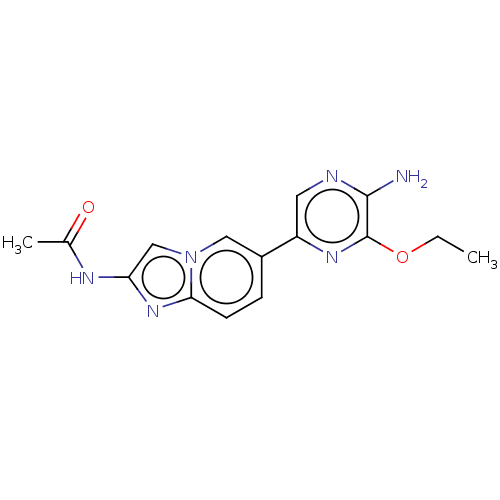
(CHEMBL3752653)Show InChI InChI=1S/C15H16N6O2/c1-3-23-15-14(16)17-6-11(19-15)10-4-5-13-20-12(18-9(2)22)8-21(13)7-10/h4-8H,3H2,1-2H3,(H2,16,17)(H,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140270
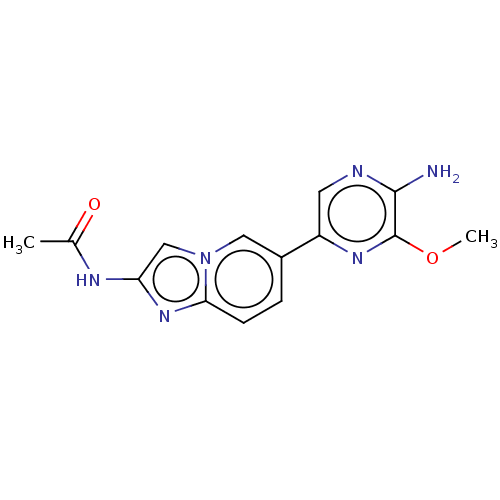
(CHEMBL3752503)Show InChI InChI=1S/C14H14N6O2/c1-8(21)17-11-7-20-6-9(3-4-12(20)19-11)10-5-16-13(15)14(18-10)22-2/h3-7H,1-2H3,(H2,15,16)(H,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140267
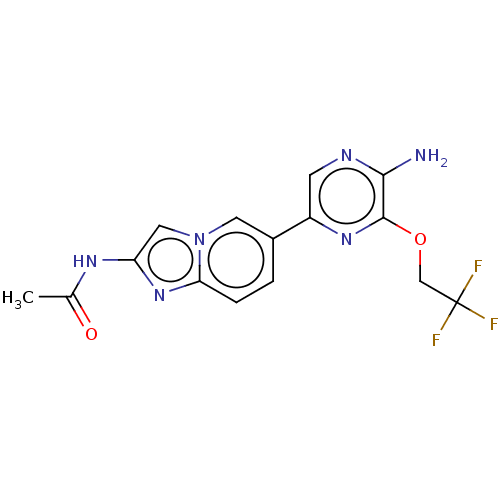
(CHEMBL3752019)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(OCC(F)(F)F)n1 Show InChI InChI=1S/C15H13F3N6O2/c1-8(25)21-11-6-24-5-9(2-3-12(24)23-11)10-4-20-13(19)14(22-10)26-7-15(16,17)18/h2-6H,7H2,1H3,(H2,19,20)(H,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344527

(4-(2-(4-isopropylphenylamino)benzo[d]thiazol-5-ylo...)Show SMILES CNC(=O)c1cc(Oc2ccc3sc(Nc4ccc(cc4)C(C)C)nc3c2)ccn1 Show InChI InChI=1S/C23H22N4O2S/c1-14(2)15-4-6-16(7-5-15)26-23-27-19-12-17(8-9-21(19)30-23)29-18-10-11-25-20(13-18)22(28)24-3/h4-14H,1-3H3,(H,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50026679

(CHEMBL3335371)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-16(31)27-23-15-20(11-12-26-23)32-19-9-10-22-21(14-19)29-24(30(22)5)28-18-8-6-7-17(13-18)25(2,3)4/h6-15H,1-5H3,(H,28,29)(H,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards Kappa opioid receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026676

(CHEMBL3335380)Show SMILES CC(=O)Nc1cc(Oc2ccc3n(C)c(Nc4ccc(cc4)C(F)(F)F)nc3c2)ccn1 Show InChI InChI=1S/C22H18F3N5O2/c1-13(31)27-20-12-17(9-10-26-20)32-16-7-8-19-18(11-16)29-21(30(19)2)28-15-5-3-14(4-6-15)22(23,24)25/h3-12H,1-2H3,(H,28,29)(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026693
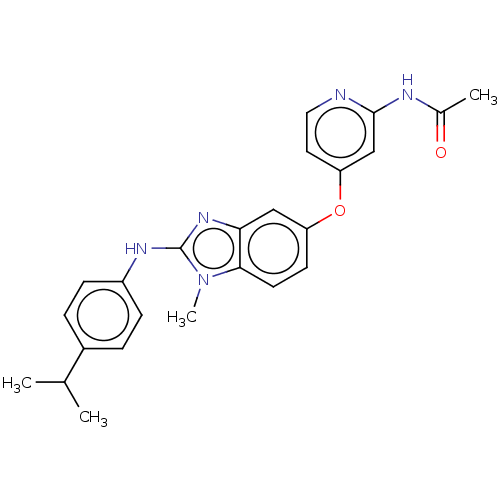
(CHEMBL3335378)Show SMILES CC(C)c1ccc(Nc2nc3cc(Oc4ccnc(NC(C)=O)c4)ccc3n2C)cc1 Show InChI InChI=1S/C24H25N5O2/c1-15(2)17-5-7-18(8-6-17)27-24-28-21-13-19(9-10-22(21)29(24)4)31-20-11-12-25-23(14-20)26-16(3)30/h5-15H,1-4H3,(H,27,28)(H,25,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344534

(4-(2-(3-isopropylphenylamino)benzo[d]oxazol-5-ylox...)Show SMILES CC(C)c1cccc(Nc2nc3cc(Oc4ccnc(c4)C(N)=O)ccc3o2)c1 Show InChI InChI=1S/C22H20N4O3/c1-13(2)14-4-3-5-15(10-14)25-22-26-18-11-16(6-7-20(18)29-22)28-17-8-9-24-19(12-17)21(23)27/h3-13H,1-2H3,(H2,23,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344531

(4-(2-(4-chlorophenylamino)benzo[d]thiazol-5-yloxy)...)Show SMILES CNC(=O)c1cc(Oc2ccc3sc(Nc4ccc(Cl)cc4)nc3c2)ccn1 Show InChI InChI=1S/C20H15ClN4O2S/c1-22-19(26)17-11-15(8-9-23-17)27-14-6-7-18-16(10-14)25-20(28-18)24-13-4-2-12(21)3-5-13/h2-11H,1H3,(H,22,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50344526

(CHEMBL1778409 | N-methyl-4-(2-(4-(trifluoromethoxy...)Show SMILES CNC(=O)c1cc(Oc2ccc3sc(Nc4ccc(OC(F)(F)F)cc4)nc3c2)ccn1 Show InChI InChI=1S/C21H15F3N4O3S/c1-25-19(29)17-11-15(8-9-26-17)30-14-6-7-18-16(10-14)28-20(32-18)27-12-2-4-13(5-3-12)31-21(22,23)24/h2-11H,1H3,(H,25,29)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of c-Raf assessed as phosphorylation of MEK1/2 by ELISA |
Bioorg Med Chem Lett 21: 3286-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.023
BindingDB Entry DOI: 10.7270/Q2R78FH0 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50439711
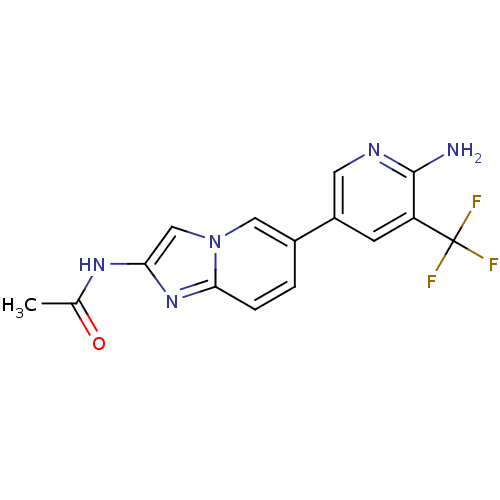
(CHEMBL2418954)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cnc(N)c(c1)C(F)(F)F Show InChI InChI=1S/C15H12F3N5O/c1-8(24)21-12-7-23-6-9(2-3-13(23)22-12)10-4-11(15(16,17)18)14(19)20-5-10/h2-7H,1H3,(H2,19,20)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50380367
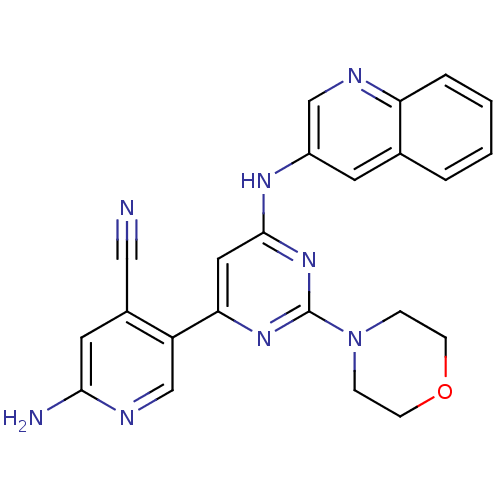
(CHEMBL2017966)Show SMILES Nc1cc(C#N)c(cn1)-c1cc(Nc2cnc3ccccc3c2)nc(n1)N1CCOCC1 Show InChI InChI=1S/C23H20N8O/c24-12-16-10-21(25)27-14-18(16)20-11-22(30-23(29-20)31-5-7-32-8-6-31)28-17-9-15-3-1-2-4-19(15)26-13-17/h1-4,9-11,13-14H,5-8H2,(H2,25,27)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using 1-alpha-phosphotidylinositol as substrate by ATP depletion assay |
ACS Med Chem Lett 2: 774-779 (2011)
Article DOI: 10.1021/ml200156t
BindingDB Entry DOI: 10.7270/Q2N29XXW |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1/2/3
(Homo sapiens (Human)) | BDBM26028
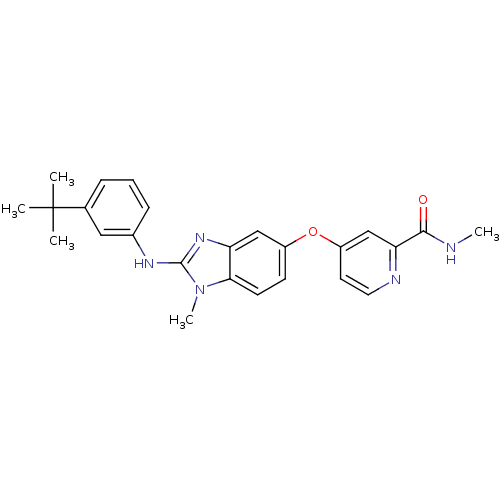
(4-({2-[(3-tert-butylphenyl)(methyl)amino]-1H-1,3-b...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-25(2,3)16-7-6-8-17(13-16)28-24-29-20-14-18(9-10-22(20)30(24)5)32-19-11-12-27-21(15-19)23(31)26-4/h6-15H,1-5H3,(H,26,31)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibitory activity towards acetylcholine esterase (AChE) |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM26028

(4-({2-[(3-tert-butylphenyl)(methyl)amino]-1H-1,3-b...)Show SMILES CNC(=O)c1cc(Oc2ccc3n(C)c(Nc4cccc(c4)C(C)(C)C)nc3c2)ccn1 Show InChI InChI=1S/C25H27N5O2/c1-25(2,3)16-7-6-8-17(13-16)28-24-29-20-14-18(9-10-22(20)30(24)5)32-19-11-12-27-21(15-19)23(31)26-4/h6-15H,1-5H3,(H,26,31)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into biotin-linked K-ras decapeptide (CVIM) by bovine farnesyltransferase |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50140273
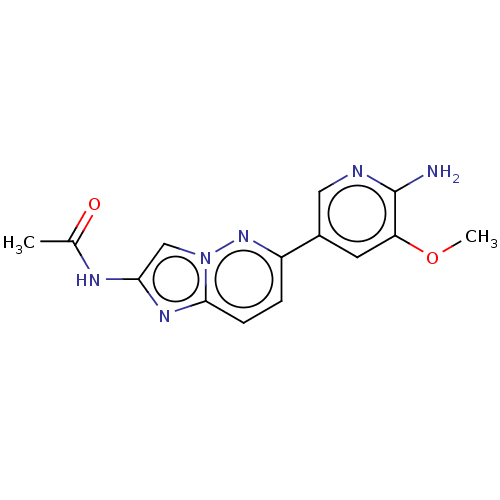
(CHEMBL3752760)Show InChI InChI=1S/C14H14N6O2/c1-8(21)17-12-7-20-13(18-12)4-3-10(19-20)9-5-11(22-2)14(15)16-6-9/h3-7H,1-2H3,(H2,15,16)(H,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using 1 alpha-phosphatidylinositol as substrate assessed as ATP depletion after 5 mins by KinaseGlo assay |
Bioorg Med Chem Lett 26: 742-6 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.003
BindingDB Entry DOI: 10.7270/Q2XP76SB |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM31088

(1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-y...)Show SMILES Cn1c(Nc2ccc(cc2)C(F)(F)F)nc2cc(Oc3ccnc(c3)-c3ncc([nH]3)C(F)(F)F)ccc12 Show InChI InChI=1S/C24H16F6N6O/c1-36-19-7-6-15(10-17(19)34-22(36)33-14-4-2-13(3-5-14)23(25,26)27)37-16-8-9-31-18(11-16)21-32-12-20(35-21)24(28,29)30/h2-12H,1H3,(H,32,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Binding affinity towards Kappa opioid receptor |
ACS Med Chem Lett 6: 961-5 (2015)
Article DOI: 10.1021/ml500526p
BindingDB Entry DOI: 10.7270/Q28W3G4S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50026674

(CHEMBL3335386)Show SMILES CC(C)N1CCN(CC(=O)Nc2cc(Oc3ccc4n(C)c(Nc5cc(ccc5F)C(F)(F)F)nc4c3)ccn2)CC1 Show InChI InChI=1S/C29H31F4N7O2/c1-18(2)40-12-10-39(11-13-40)17-27(41)37-26-16-21(8-9-34-26)42-20-5-7-25-24(15-20)36-28(38(25)3)35-23-14-19(29(31,32)33)4-6-22(23)30/h4-9,14-16,18H,10-13,17H2,1-3H3,(H,35,36)(H,34,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of BRAF V600E mutant (unknown origin) |
ACS Med Chem Lett 5: 989-92 (2014)
Article DOI: 10.1021/ml5002272
BindingDB Entry DOI: 10.7270/Q2RN39FP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data