 Found 57 hits with Last Name = 'wang' and Initial = 'jq'
Found 57 hits with Last Name = 'wang' and Initial = 'jq' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50151435
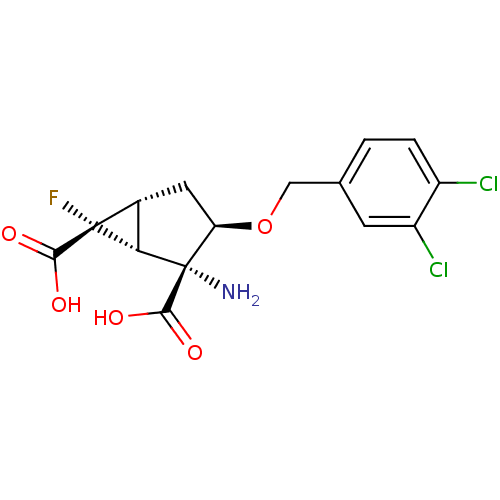
((1R,2R,3R,5R,6R)-2-Amino-3-(3,4-dichloro-benzyloxy...)Show SMILES N[C@@]1([C@H]2[C@@H](C[C@H]1OCc1ccc(Cl)c(Cl)c1)[C@]2(F)C(O)=O)C(O)=O Show InChI InChI=1S/C15H14Cl2FNO5/c16-8-2-1-6(3-9(8)17)5-24-10-4-7-11(14(7,18)12(20)21)15(10,19)13(22)23/h1-3,7,10-11H,4-5,19H2,(H,20,21)(H,22,23)/t7-,10-,11+,14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Binding affinity to mGLUR2 |
Bioorg Med Chem Lett 22: 1958-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.039
BindingDB Entry DOI: 10.7270/Q2T72JJ1 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50151435

((1R,2R,3R,5R,6R)-2-Amino-3-(3,4-dichloro-benzyloxy...)Show SMILES N[C@@]1([C@H]2[C@@H](C[C@H]1OCc1ccc(Cl)c(Cl)c1)[C@]2(F)C(O)=O)C(O)=O Show InChI InChI=1S/C15H14Cl2FNO5/c16-8-2-1-6(3-9(8)17)5-24-10-4-7-11(14(7,18)12(20)21)15(10,19)13(22)23/h1-3,7,10-11H,4-5,19H2,(H,20,21)(H,22,23)/t7-,10-,11+,14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Binding affinity to mGLUR3 |
Bioorg Med Chem Lett 22: 1958-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.039
BindingDB Entry DOI: 10.7270/Q2T72JJ1 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50084289

((S)-2-(4'-methoxy-biphenyl-4-sulfonylamino)-3-meth...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C18H21NO5S/c1-12(2)17(18(20)21)19-25(22,23)16-10-6-14(7-11-16)13-4-8-15(24-3)9-5-13/h4-12,17,19H,1-3H3,(H,20,21)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-2 |
Bioorg Med Chem Lett 13: 2217-22 (2003)
BindingDB Entry DOI: 10.7270/Q28G8K3B |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50084289

((S)-2-(4'-methoxy-biphenyl-4-sulfonylamino)-3-meth...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C18H21NO5S/c1-12(2)17(18(20)21)19-25(22,23)16-10-6-14(7-11-16)13-4-8-15(24-3)9-5-13/h4-12,17,19H,1-3H3,(H,20,21)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloproteinase-13 |
Bioorg Med Chem Lett 13: 2217-22 (2003)
BindingDB Entry DOI: 10.7270/Q28G8K3B |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50084289

((S)-2-(4'-methoxy-biphenyl-4-sulfonylamino)-3-meth...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C18H21NO5S/c1-12(2)17(18(20)21)19-25(22,23)16-10-6-14(7-11-16)13-4-8-15(24-3)9-5-13/h4-12,17,19H,1-3H3,(H,20,21)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-3 |
Bioorg Med Chem Lett 13: 2217-22 (2003)
BindingDB Entry DOI: 10.7270/Q28G8K3B |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50062522
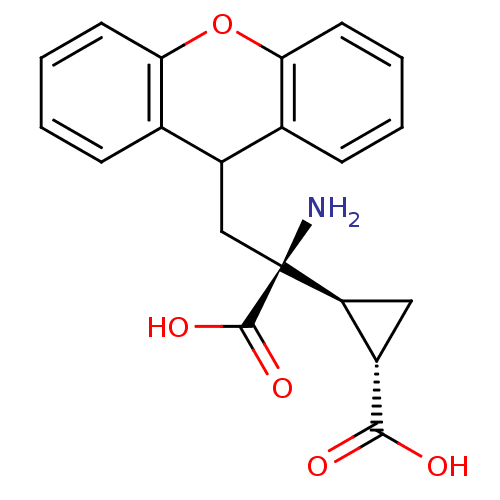
((1S,2S)-2-((S)-1-amino-1-carboxy-2-(9H-xanthen-9-y...)Show SMILES N[C@@](CC1c2ccccc2Oc2ccccc12)([C@H]1C[C@@H]1C(O)=O)C(O)=O Show InChI InChI=1S/C20H19NO5/c21-20(19(24)25,15-9-13(15)18(22)23)10-14-11-5-1-3-7-16(11)26-17-8-4-2-6-12(14)17/h1-8,13-15H,9-10,21H2,(H,22,23)(H,24,25)/t13-,15-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGLUR3 expressed in RGT cells assessed as inhibition of (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid-induced inhibi... |
Bioorg Med Chem Lett 22: 1958-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.039
BindingDB Entry DOI: 10.7270/Q2T72JJ1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50151435

((1R,2R,3R,5R,6R)-2-Amino-3-(3,4-dichloro-benzyloxy...)Show SMILES N[C@@]1([C@H]2[C@@H](C[C@H]1OCc1ccc(Cl)c(Cl)c1)[C@]2(F)C(O)=O)C(O)=O Show InChI InChI=1S/C15H14Cl2FNO5/c16-8-2-1-6(3-9(8)17)5-24-10-4-7-11(14(7,18)12(20)21)15(10,19)13(22)23/h1-3,7,10-11H,4-5,19H2,(H,20,21)(H,22,23)/t7-,10-,11+,14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Antagonist activity at mGLUR2 expressed in CHO cells assessed as inhibition of glutamate-induced inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 22: 1958-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.039
BindingDB Entry DOI: 10.7270/Q2T72JJ1 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM50062522

((1S,2S)-2-((S)-1-amino-1-carboxy-2-(9H-xanthen-9-y...)Show SMILES N[C@@](CC1c2ccccc2Oc2ccccc12)([C@H]1C[C@@H]1C(O)=O)C(O)=O Show InChI InChI=1S/C20H19NO5/c21-20(19(24)25,15-9-13(15)18(22)23)10-14-11-5-1-3-7-16(11)26-17-8-4-2-6-12(14)17/h1-8,13-15H,9-10,21H2,(H,22,23)(H,24,25)/t13-,15-,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Antagonist activity at human mGLUR2 expressed in RGT cells assessed as inhibition of (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid-induced inhibi... |
Bioorg Med Chem Lett 22: 1958-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.039
BindingDB Entry DOI: 10.7270/Q2T72JJ1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Metabotropic glutamate receptor 3
(Homo sapiens (Human)) | BDBM50151435

((1R,2R,3R,5R,6R)-2-Amino-3-(3,4-dichloro-benzyloxy...)Show SMILES N[C@@]1([C@H]2[C@@H](C[C@H]1OCc1ccc(Cl)c(Cl)c1)[C@]2(F)C(O)=O)C(O)=O Show InChI InChI=1S/C15H14Cl2FNO5/c16-8-2-1-6(3-9(8)17)5-24-10-4-7-11(14(7,18)12(20)21)15(10,19)13(22)23/h1-3,7,10-11H,4-5,19H2,(H,20,21)(H,22,23)/t7-,10-,11+,14-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Antagonist activity at mGLUR3 expressed in CHO cells assessed as inhibition of glutamate-induced inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 22: 1958-62 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.039
BindingDB Entry DOI: 10.7270/Q2T72JJ1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP3A4 in presence of NADPH generating system by fluorescence assay |
J Med Chem 61: 5988-6001 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00335
BindingDB Entry DOI: 10.7270/Q2S1851N |
More data for this
Ligand-Target Pair | |
Thioredoxin reductase 1, cytoplasmic
(Homo sapiens (Human)) | BDBM50468676

(CHEMBL4281382)Show SMILES COc1cc(OC)c2c(c1)oc(-c1cc(OC)c(OC)c(OC)c1)c(OCCCOc1ccccc1\C=C\C(=O)\C=C\c1ccccn1)c2=O Show InChI InChI=1S/C39H37NO10/c1-43-29-23-31(44-2)35-32(24-29)50-37(26-21-33(45-3)38(47-5)34(22-26)46-4)39(36(35)42)49-20-10-19-48-30-13-7-6-11-25(30)14-16-28(41)17-15-27-12-8-9-18-40-27/h6-9,11-18,21-24H,10,19-20H2,1-5H3/b16-14+,17-15+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 783 | n/a | n/a | n/a | n/a | n/a | n/a |
Anhui Medical University
Curated by ChEMBL
| Assay Description
Inhibition of TrxR in human SGC7901 cells |
Eur J Med Chem 156: 493-509 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.013
BindingDB Entry DOI: 10.7270/Q2RV0RDJ |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50084289

((S)-2-(4'-methoxy-biphenyl-4-sulfonylamino)-3-meth...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C18H21NO5S/c1-12(2)17(18(20)21)19-25(22,23)16-10-6-14(7-11-16)13-4-8-15(24-3)9-5-13/h4-12,17,19H,1-3H3,(H,20,21)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-1 |
Bioorg Med Chem Lett 13: 2217-22 (2003)
BindingDB Entry DOI: 10.7270/Q28G8K3B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50346601

(NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50084289

((S)-2-(4'-methoxy-biphenyl-4-sulfonylamino)-3-meth...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C18H21NO5S/c1-12(2)17(18(20)21)19-25(22,23)16-10-6-14(7-11-16)13-4-8-15(24-3)9-5-13/h4-12,17,19H,1-3H3,(H,20,21)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-9 |
Bioorg Med Chem Lett 13: 2217-22 (2003)
BindingDB Entry DOI: 10.7270/Q28G8K3B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124241
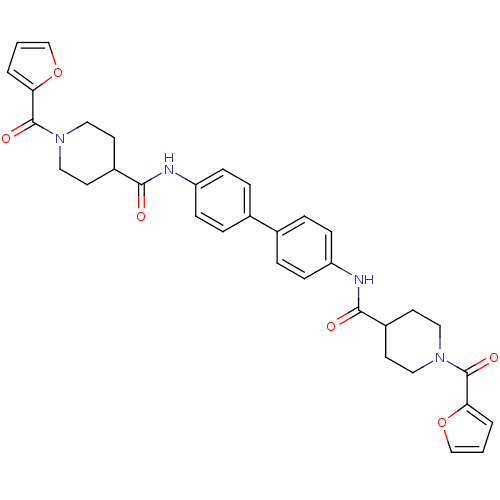
(N,N'-([1,1'-biphenyl]-4,4'-diyl)bis(1-...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)C2CCN(CC2)C(=O)c2ccco2)cc1)C1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C34H34N4O6/c39-31(25-13-17-37(18-14-25)33(41)29-3-1-21-43-29)35-27-9-5-23(6-10-27)24-7-11-28(12-8-24)36-32(40)26-15-19-38(20-16-26)34(42)30-4-2-22-44-30/h1-12,21-22,25-26H,13-20H2,(H,35,39)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124240
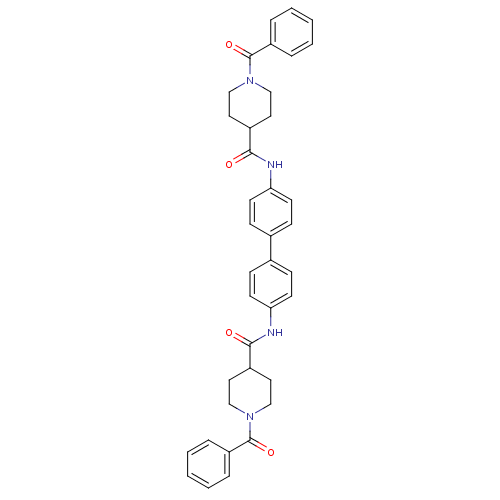
(N,N'-(biphenyl-4,4'-diyl)bis(1-benzoylpipe...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)C2CCN(CC2)C(=O)c2ccccc2)cc1)C1CCN(CC1)C(=O)c1ccccc1 Show InChI InChI=1S/C38H38N4O4/c43-35(29-19-23-41(24-20-29)37(45)31-7-3-1-4-8-31)39-33-15-11-27(12-16-33)28-13-17-34(18-14-28)40-36(44)30-21-25-42(26-22-30)38(46)32-9-5-2-6-10-32/h1-18,29-30H,19-26H2,(H,39,43)(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM50346601

(NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...)Show SMILES CC1(C)CC[C@@]2(CC[C@]3(C)C(=CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]2C1)C(O)=O |r,c:10| Show InChI InChI=1S/C30H48O3/c1-25(2)14-16-30(24(32)33)17-15-28(6)19(20(30)18-25)8-9-22-27(5)12-11-23(31)26(3,4)21(27)10-13-29(22,28)7/h8,20-23,31H,9-18H2,1-7H3,(H,32,33)/t20-,21-,22+,23-,27-,28+,29+,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124246
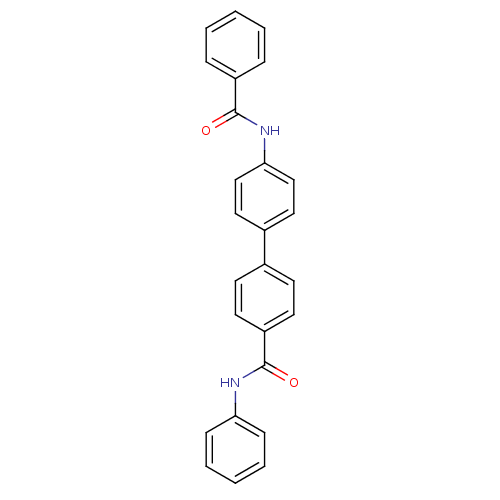
(4-(4-benzamidophenyl)-N-phenylbenzamide (25))Show SMILES O=C(Nc1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H20N2O2/c29-25(21-7-3-1-4-8-21)28-24-17-15-20(16-18-24)19-11-13-22(14-12-19)26(30)27-23-9-5-2-6-10-23/h1-18H,(H,27,30)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124239
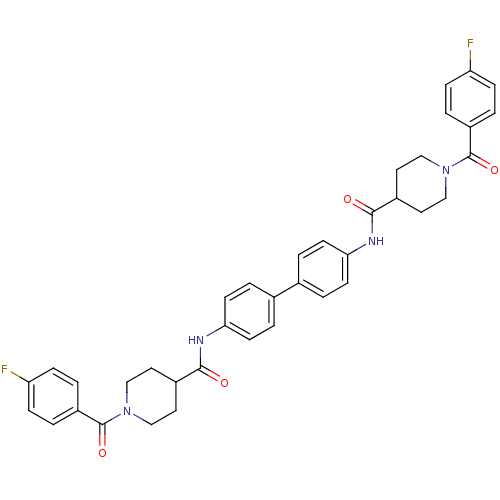
(N,N'-([1,1'-biphenyl]-4,4'-diyl)bis(1-...)Show SMILES Fc1ccc(cc1)C(=O)N1CCC(CC1)C(=O)Nc1ccc(cc1)-c1ccc(NC(=O)C2CCN(CC2)C(=O)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C38H36F2N4O4/c39-31-9-1-29(2-10-31)37(47)43-21-17-27(18-22-43)35(45)41-33-13-5-25(6-14-33)26-7-15-34(16-8-26)42-36(46)28-19-23-44(24-20-28)38(48)30-3-11-32(40)12-4-30/h1-16,27-28H,17-24H2,(H,41,45)(H,42,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124248
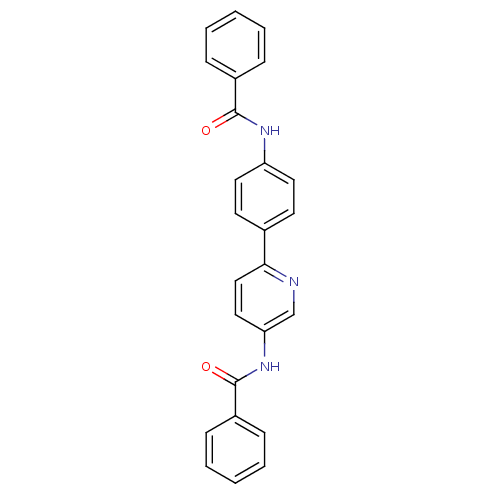
(N-(6-(4-benzamidophenyl)pyridin-3-yl)benzamide (28...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)c2ccccc2)cn1)c1ccccc1 Show InChI InChI=1S/C25H19N3O2/c29-24(19-7-3-1-4-8-19)27-21-13-11-18(12-14-21)23-16-15-22(17-26-23)28-25(30)20-9-5-2-6-10-20/h1-17H,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124249
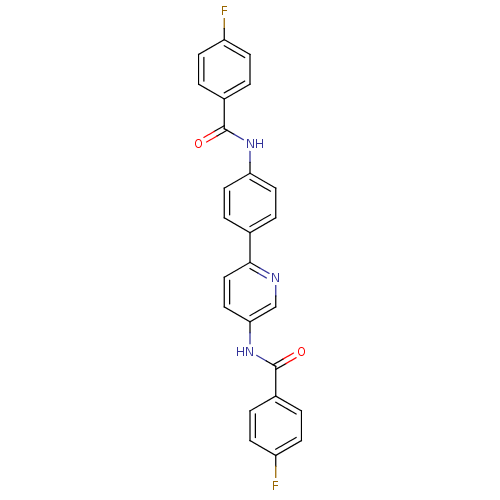
(4-fluoro-N-(6-(4-(4-fluorobenzamido)phenyl)pyridin...)Show SMILES Fc1ccc(cc1)C(=O)Nc1ccc(cc1)-c1ccc(NC(=O)c2ccc(F)cc2)cn1 Show InChI InChI=1S/C25H17F2N3O2/c26-19-7-1-17(2-8-19)24(31)29-21-11-5-16(6-12-21)23-14-13-22(15-28-23)30-25(32)18-3-9-20(27)10-4-18/h1-15H,(H,29,31)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124250
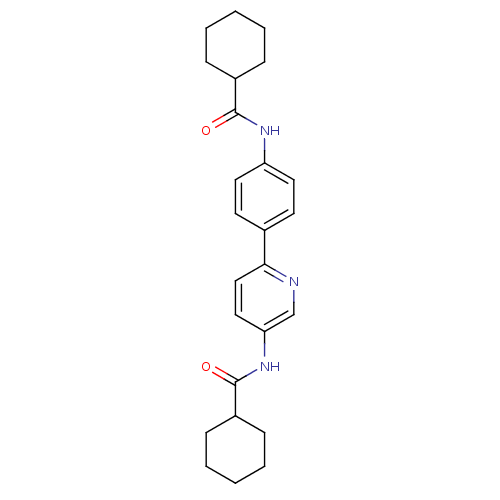
(N-(6-(4-(cyclohexanecarboxamido)phenyl)pyridin-3-y...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)C2CCCCC2)cn1)C1CCCCC1 Show InChI InChI=1S/C25H31N3O2/c29-24(19-7-3-1-4-8-19)27-21-13-11-18(12-14-21)23-16-15-22(17-26-23)28-25(30)20-9-5-2-6-10-20/h11-17,19-20H,1-10H2,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50084289

((S)-2-(4'-methoxy-biphenyl-4-sulfonylamino)-3-meth...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C18H21NO5S/c1-12(2)17(18(20)21)19-25(22,23)16-10-6-14(7-11-16)13-4-8-15(24-3)9-5-13/h4-12,17,19H,1-3H3,(H,20,21)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-7 |
Bioorg Med Chem Lett 13: 2217-22 (2003)
BindingDB Entry DOI: 10.7270/Q28G8K3B |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM124240

(N,N'-(biphenyl-4,4'-diyl)bis(1-benzoylpipe...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)C2CCN(CC2)C(=O)c2ccccc2)cc1)C1CCN(CC1)C(=O)c1ccccc1 Show InChI InChI=1S/C38H38N4O4/c43-35(29-19-23-41(24-20-29)37(45)31-7-3-1-4-8-31)39-33-15-11-27(12-16-33)28-13-17-34(18-14-28)40-36(44)30-21-25-42(26-22-30)38(46)32-9-5-2-6-10-32/h1-18,29-30H,19-26H2,(H,39,43)(H,40,44) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM81939
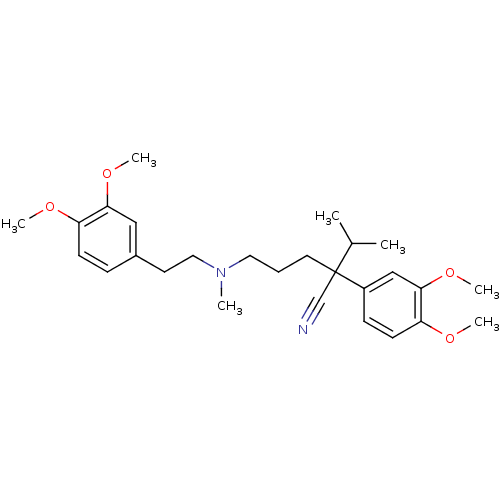
(CAS_52-53-9 | NSC_62969 | VERAPAMIL)Show SMILES COc1ccc(CCN(C)CCCC(C#N)(C(C)C)c2ccc(OC)c(OC)c2)cc1OC Show InChI InChI=1S/C27H38N2O4/c1-20(2)27(19-28,22-10-12-24(31-5)26(18-22)33-7)14-8-15-29(3)16-13-21-9-11-23(30-4)25(17-21)32-6/h9-12,17-18,20H,8,13-16H2,1-7H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 8.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP3A4 in presence of NADPH generating system by fluorescence assay |
J Med Chem 61: 5988-6001 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00335
BindingDB Entry DOI: 10.7270/Q2S1851N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124238
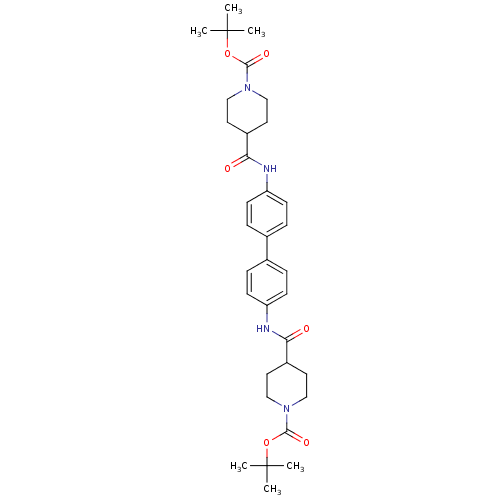
(di-tert-butyl4,4'-(([1,1'-biphenyl]-4,4�...)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)C(=O)Nc1ccc(cc1)-c1ccc(NC(=O)C2CCN(CC2)C(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C34H46N4O6/c1-33(2,3)43-31(41)37-19-15-25(16-20-37)29(39)35-27-11-7-23(8-12-27)24-9-13-28(14-10-24)36-30(40)26-17-21-38(22-18-26)32(42)44-34(4,5)6/h7-14,25-26H,15-22H2,1-6H3,(H,35,39)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124245
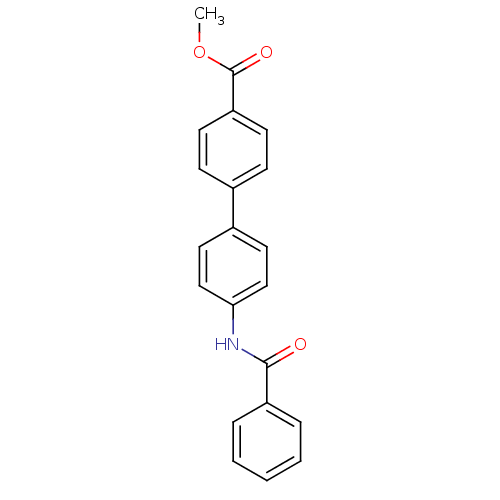
(Methyl 4-(4-benzamidophenyl)benzoate (23))Show InChI InChI=1S/C21H17NO3/c1-25-21(24)18-9-7-15(8-10-18)16-11-13-19(14-12-16)22-20(23)17-5-3-2-4-6-17/h2-14H,1H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124247
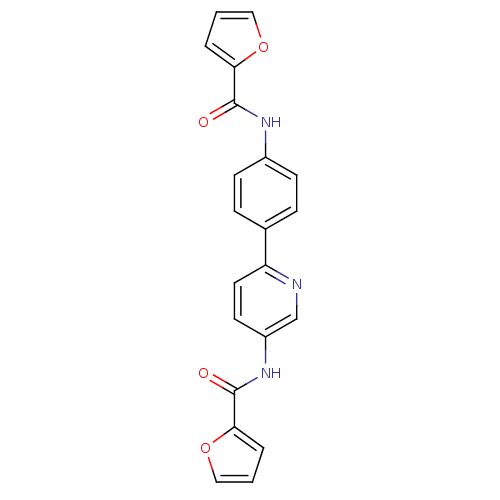
(N-(6-(4-(furan-2-carboxamido)phenyl)pyridin-3-yl)f...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)c2ccco2)cn1)c1ccco1 Show InChI InChI=1S/C21H15N3O4/c25-20(18-3-1-11-27-18)23-15-7-5-14(6-8-15)17-10-9-16(13-22-17)24-21(26)19-4-2-12-28-19/h1-13H,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124234
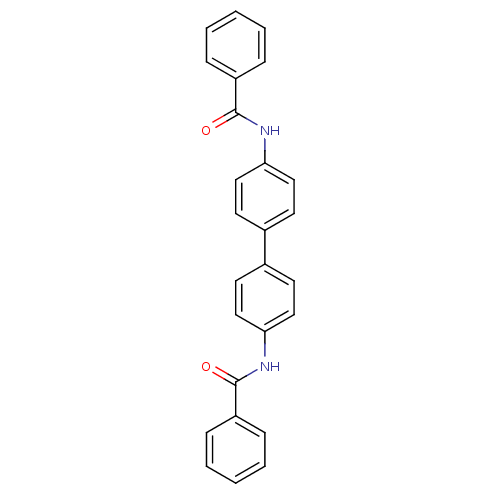
(N,N'-(biphenyl-4,4'-diyl)dibenzamide (3))Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)c2ccccc2)cc1)c1ccccc1 Show InChI InChI=1S/C26H20N2O2/c29-25(21-7-3-1-4-8-21)27-23-15-11-19(12-16-23)20-13-17-24(18-14-20)28-26(30)22-9-5-2-6-10-22/h1-18H,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124243
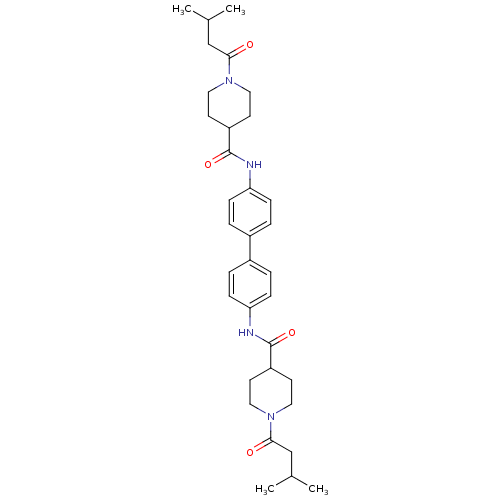
(N,N'-([1,1'-biphenyl]-4,4'-diyl)bis(1-...)Show SMILES CC(C)CC(=O)N1CCC(CC1)C(=O)Nc1ccc(cc1)-c1ccc(NC(=O)C2CCN(CC2)C(=O)CC(C)C)cc1 Show InChI InChI=1S/C34H46N4O4/c1-23(2)21-31(39)37-17-13-27(14-18-37)33(41)35-29-9-5-25(6-10-29)26-7-11-30(12-8-26)36-34(42)28-15-19-38(20-16-28)32(40)22-24(3)4/h5-12,23-24,27-28H,13-22H2,1-4H3,(H,35,41)(H,36,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM124241

(N,N'-([1,1'-biphenyl]-4,4'-diyl)bis(1-...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)C2CCN(CC2)C(=O)c2ccco2)cc1)C1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C34H34N4O6/c39-31(25-13-17-37(18-14-25)33(41)29-3-1-21-43-29)35-27-9-5-23(6-10-27)24-7-11-28(12-8-24)36-32(40)26-15-19-38(20-16-26)34(42)30-4-2-22-44-30/h1-12,21-22,25-26H,13-20H2,(H,35,39)(H,36,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124236
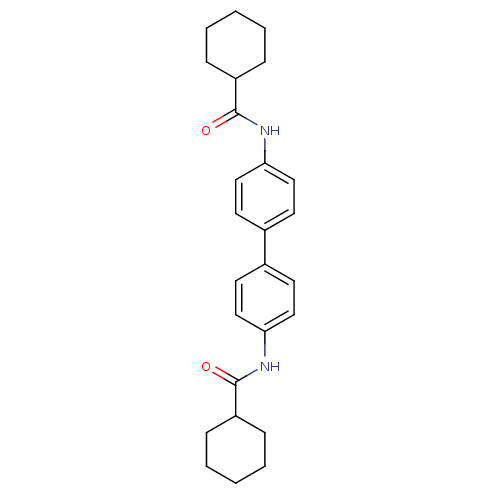
(N,N'-([1,1'-biphenyl]-4,4'-diyl)dicycl...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)C2CCCCC2)cc1)C1CCCCC1 Show InChI InChI=1S/C26H32N2O2/c29-25(21-7-3-1-4-8-21)27-23-15-11-19(12-16-23)20-13-17-24(18-14-20)28-26(30)22-9-5-2-6-10-22/h11-18,21-22H,1-10H2,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124242
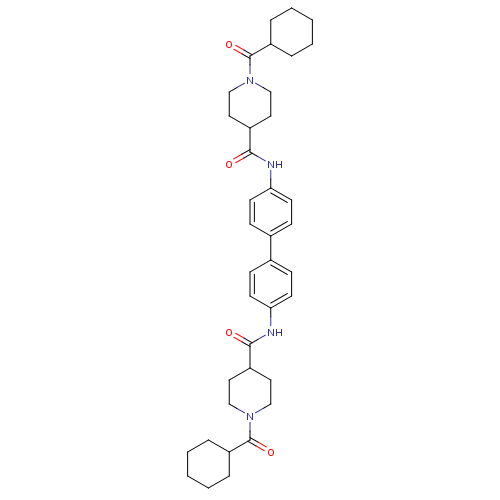
(N,N'-([1,1'-biphenyl]-4,4'-diyl)bis(1-...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)C2CCN(CC2)C(=O)C2CCCCC2)cc1)C1CCN(CC1)C(=O)C1CCCCC1 Show InChI InChI=1S/C38H50N4O4/c43-35(29-19-23-41(24-20-29)37(45)31-7-3-1-4-8-31)39-33-15-11-27(12-16-33)28-13-17-34(18-14-28)40-36(44)30-21-25-42(26-22-30)38(46)32-9-5-2-6-10-32/h11-18,29-32H,1-10,19-26H2,(H,39,43)(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM50391109

(CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F
(Homo sapiens (Human)) | BDBM50391109

(CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124237
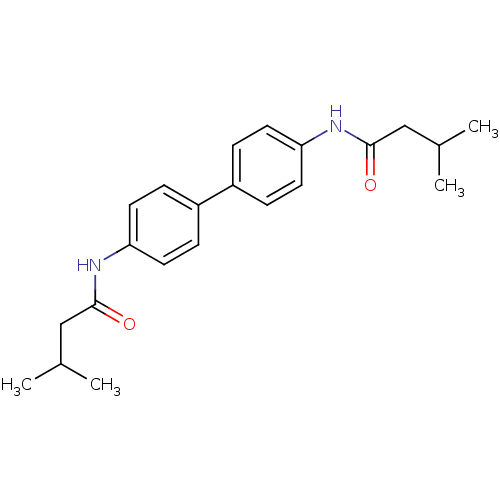
(N,N'-([1,1'-biphenyl]-4,4'-diyl)bis(3-...)Show SMILES CC(C)CC(=O)Nc1ccc(cc1)-c1ccc(NC(=O)CC(C)C)cc1 Show InChI InChI=1S/C22H28N2O2/c1-15(2)13-21(25)23-19-9-5-17(6-10-19)18-7-11-20(12-8-18)24-22(26)14-16(3)4/h5-12,15-16H,13-14H2,1-4H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50288831
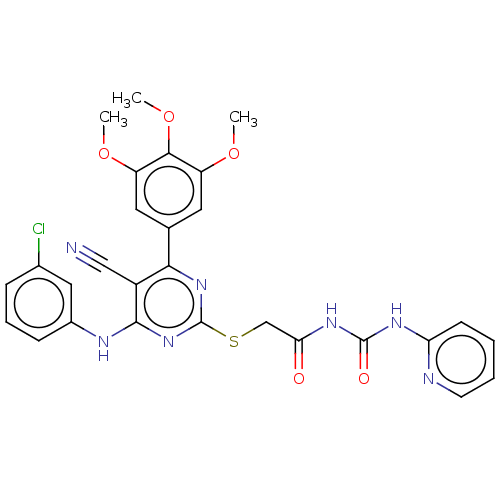
(CHEMBL4161738)Show SMILES COc1cc(cc(OC)c1OC)-c1nc(SCC(=O)NC(=O)Nc2ccccn2)nc(Nc2cccc(Cl)c2)c1C#N Show InChI InChI=1S/C28H24ClN7O5S/c1-39-20-11-16(12-21(40-2)25(20)41-3)24-19(14-30)26(32-18-8-6-7-17(29)13-18)36-28(35-24)42-15-23(37)34-27(38)33-22-9-4-5-10-31-22/h4-13H,15H2,1-3H3,(H,32,35,36)(H2,31,33,34,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhengzhou University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CYP3A4 in presence of NADPH generating system by fluorescence assay |
J Med Chem 61: 5988-6001 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00335
BindingDB Entry DOI: 10.7270/Q2S1851N |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM124249

(4-fluoro-N-(6-(4-(4-fluorobenzamido)phenyl)pyridin...)Show SMILES Fc1ccc(cc1)C(=O)Nc1ccc(cc1)-c1ccc(NC(=O)c2ccc(F)cc2)cn1 Show InChI InChI=1S/C25H17F2N3O2/c26-19-7-1-17(2-8-19)24(31)29-21-11-5-16(6-12-21)23-14-13-22(15-28-23)30-25(32)18-3-9-20(27)10-4-18/h1-15H,(H,29,31)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM124238

(di-tert-butyl4,4'-(([1,1'-biphenyl]-4,4�...)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)C(=O)Nc1ccc(cc1)-c1ccc(NC(=O)C2CCN(CC2)C(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C34H46N4O6/c1-33(2,3)43-31(41)37-19-15-25(16-20-37)29(39)35-27-11-7-23(8-12-27)24-9-13-28(14-10-24)36-30(40)26-17-21-38(22-18-26)32(42)44-34(4,5)6/h7-14,25-26H,15-22H2,1-6H3,(H,35,39)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124244
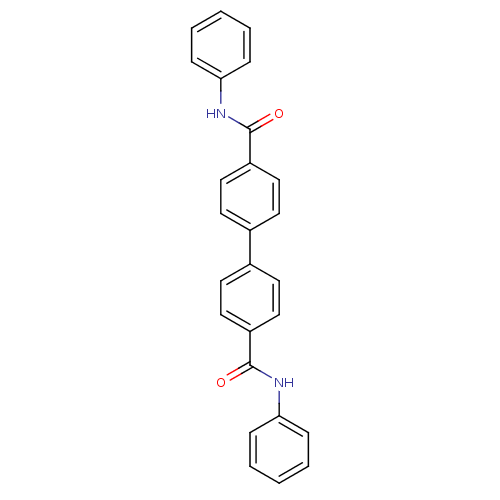
(N-phenyl-4-[4-(phenylcarbamoyl)phenyl]benzamide (2...)Show SMILES O=C(Nc1ccccc1)c1ccc(cc1)-c1ccc(cc1)C(=O)Nc1ccccc1 Show InChI InChI=1S/C26H20N2O2/c29-25(27-23-7-3-1-4-8-23)21-15-11-19(12-16-21)20-13-17-22(18-14-20)26(30)28-24-9-5-2-6-10-24/h1-18H,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124235
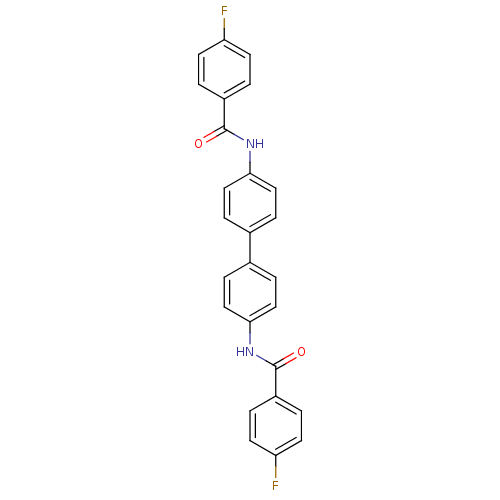
(N,N'-([1,1'-biphenyl]-4,4'-diyl)bis(4-...)Show SMILES Fc1ccc(cc1)C(=O)Nc1ccc(cc1)-c1ccc(NC(=O)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C26H18F2N2O2/c27-21-9-1-19(2-10-21)25(31)29-23-13-5-17(6-14-23)18-7-15-24(16-8-18)30-26(32)20-3-11-22(28)12-4-20/h1-16H,(H,29,31)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124233
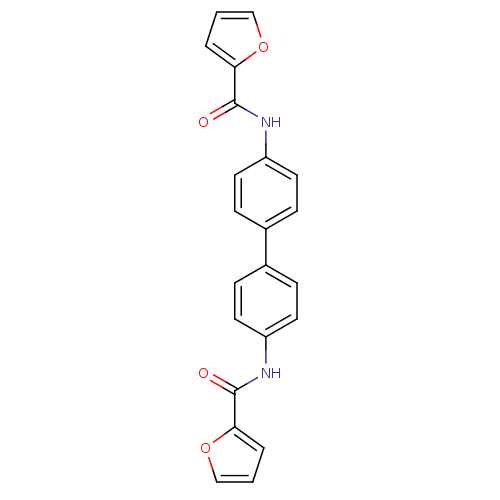
(N‐{4‐[4‐(furan‐2‐ami...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)c2ccco2)cc1)c1ccco1 Show InChI InChI=1S/C22H16N2O4/c25-21(19-3-1-13-27-19)23-17-9-5-15(6-10-17)16-7-11-18(12-8-16)24-22(26)20-4-2-14-28-20/h1-14H,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM124249

(4-fluoro-N-(6-(4-(4-fluorobenzamido)phenyl)pyridin...)Show SMILES Fc1ccc(cc1)C(=O)Nc1ccc(cc1)-c1ccc(NC(=O)c2ccc(F)cc2)cn1 Show InChI InChI=1S/C25H17F2N3O2/c26-19-7-1-17(2-8-19)24(31)29-21-11-5-16(6-12-21)23-14-13-22(15-28-23)30-25(32)18-3-9-20(27)10-4-18/h1-15H,(H,29,31)(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM124238

(di-tert-butyl4,4'-(([1,1'-biphenyl]-4,4�...)Show SMILES CC(C)(C)OC(=O)N1CCC(CC1)C(=O)Nc1ccc(cc1)-c1ccc(NC(=O)C2CCN(CC2)C(=O)OC(C)(C)C)cc1 Show InChI InChI=1S/C34H46N4O6/c1-33(2,3)43-31(41)37-19-15-25(16-20-37)29(39)35-27-11-7-23(8-12-27)24-9-13-28(14-10-24)36-30(40)26-17-21-38(22-18-26)32(42)44-34(4,5)6/h7-14,25-26H,15-22H2,1-6H3,(H,35,39)(H,36,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM124250

(N-(6-(4-(cyclohexanecarboxamido)phenyl)pyridin-3-y...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)C2CCCCC2)cn1)C1CCCCC1 Show InChI InChI=1S/C25H31N3O2/c29-24(19-7-3-1-4-8-19)27-21-13-11-18(12-14-21)23-16-15-22(17-26-23)28-25(30)20-9-5-2-6-10-20/h11-17,19-20H,1-10H2,(H,27,29)(H,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM124248

(N-(6-(4-benzamidophenyl)pyridin-3-yl)benzamide (28...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)c2ccccc2)cn1)c1ccccc1 Show InChI InChI=1S/C25H19N3O2/c29-24(19-7-3-1-4-8-19)27-21-13-11-18(12-14-21)23-16-15-22(17-26-23)28-25(30)20-9-5-2-6-10-20/h1-17H,(H,27,29)(H,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM124251
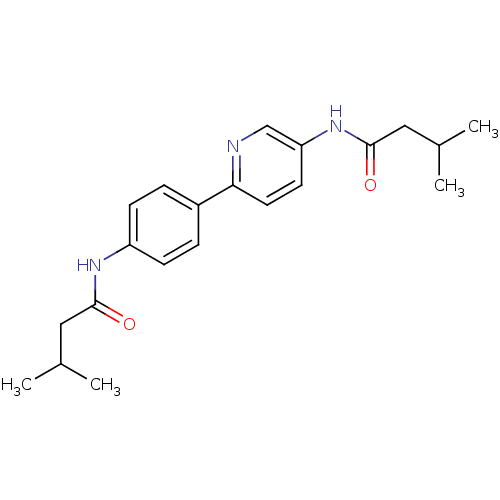
(3-methyl-N-(6-(4-(3-methylbutanamido)phenyl)pyridi...)Show SMILES CC(C)CC(=O)Nc1ccc(cc1)-c1ccc(NC(=O)CC(C)C)cn1 Show InChI InChI=1S/C21H27N3O2/c1-14(2)11-20(25)23-17-7-5-16(6-8-17)19-10-9-18(13-22-19)24-21(26)12-15(3)4/h5-10,13-15H,11-12H2,1-4H3,(H,23,25)(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50391109

(CHEMBL179166 | Sodium orthovanadate (SOV) | Vanada...) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 6
(Homo sapiens (Human)) | BDBM124233

(N‐{4‐[4‐(furan‐2‐ami...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)c2ccco2)cc1)c1ccco1 Show InChI InChI=1S/C22H16N2O4/c25-21(19-3-1-13-27-19)23-17-9-5-15(6-10-17)16-7-11-18(12-8-16)24-22(26)20-4-2-14-28-20/h1-14H,(H,23,25)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM124233

(N‐{4‐[4‐(furan‐2‐ami...)Show SMILES O=C(Nc1ccc(cc1)-c1ccc(NC(=O)c2ccco2)cc1)c1ccco1 Show InChI InChI=1S/C22H16N2O4/c25-21(19-3-1-13-27-19)23-17-9-5-15(6-10-17)16-7-11-18(12-8-16)24-22(26)20-4-2-14-28-20/h1-14H,(H,23,25)(H,24,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jiangnan University
| Assay Description
IC50 values were determined by pNPP assay. |
Bioorg Med Chem Lett 24: 1889-94 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.015
BindingDB Entry DOI: 10.7270/Q2PC3122 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data