

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insulin receptor (Homo sapiens (Human)) | BDBM50256480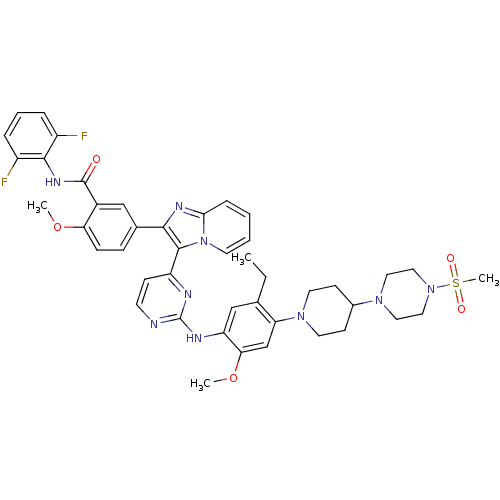 (CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to insulin receptor by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256480 (CHEMBL466397 | N-(2,6-difluorophenyl)-5-(3-(2-(5-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to IGF1R by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin receptor (Homo sapiens (Human)) | BDBM50256478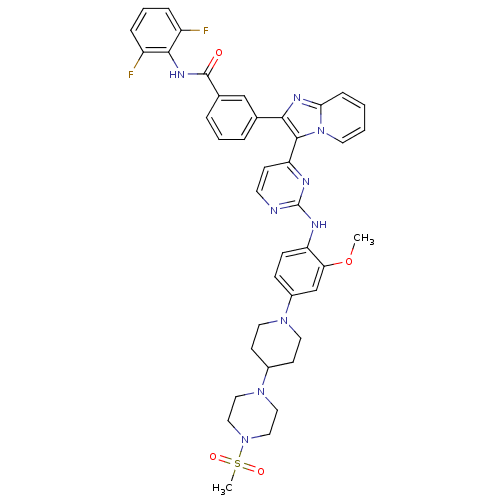 (CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to insulin receptor by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50257064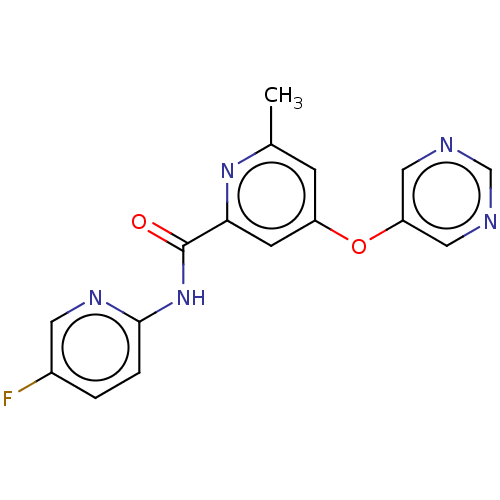 (CHEMBL2386850) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Radiology and Radiological Sciences, Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center , Nashville, Tennessee 37232, United States. Curated by ChEMBL | Assay Description Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat mGlu5 receptor expressed in HEK293A cell membranes after 1 hr by scintillatio... | J Med Chem 60: 5072-5085 (2017) Article DOI: 10.1021/acs.jmedchem.7b00410 BindingDB Entry DOI: 10.7270/Q2JH3PM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50084137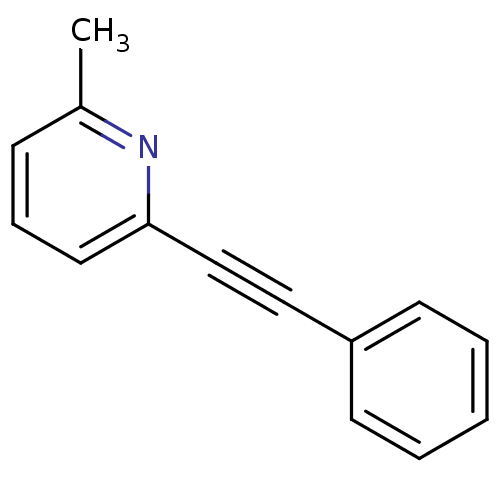 (2-Methyl-6-(phenylethynyl)pyridine | 2-Methyl-6-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]3methoxy-5-(pyridin-2-ylethynyl)pyridine from rat mGluR5 | Bioorg Med Chem Lett 19: 6623-6 (2009) Article DOI: 10.1016/j.bmcl.2009.10.024 BindingDB Entry DOI: 10.7270/Q2TX3FHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256478 (CHEMBL507714 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity to IGF1R by liquid scintillation counting | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50442525 (CHEMBL2440659 | US8796295, Table 2: Compound: 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine from mGlu5 receptor allosteric binding site (unknown origin) | Bioorg Med Chem Lett 23: 5779-85 (2013) Article DOI: 10.1016/j.bmcl.2013.09.001 BindingDB Entry DOI: 10.7270/Q2X92CSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50047700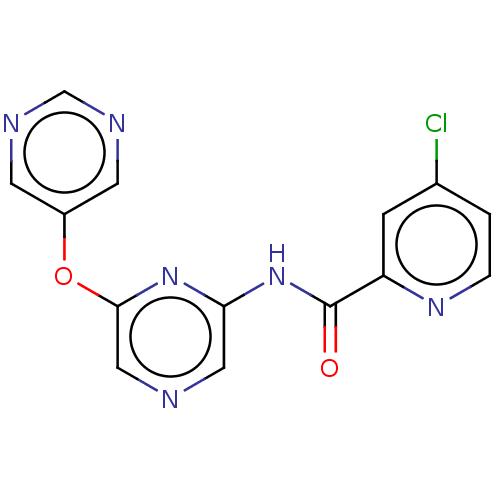 (CHEMBL3314850) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]methoxyPEPy from rat mGlu5 expressed in HEK293A cells after 1 hr by scintillation counting method | Bioorg Med Chem Lett 24: 3307-14 (2014) Article DOI: 10.1016/j.bmcl.2014.06.003 BindingDB Entry DOI: 10.7270/Q2ZW1NJP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50323286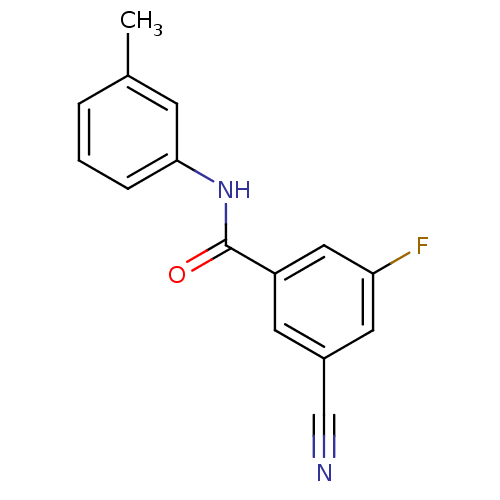 (3-cyano-5-fluoro-N-m-tolylbenzamide | CHEMBL120920...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPy from rat mGlu5 receptor expressed in HEK293A cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 4390-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.064 BindingDB Entry DOI: 10.7270/Q2V9888D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50323308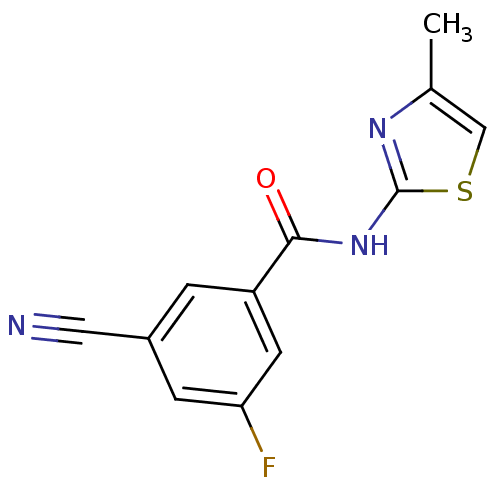 (3-cyano-5-fluoro-N-(4-methylthiazol-2-yl)benzamide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPy from rat mGlu5 receptor expressed in HEK293A cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 4390-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.064 BindingDB Entry DOI: 10.7270/Q2V9888D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50311887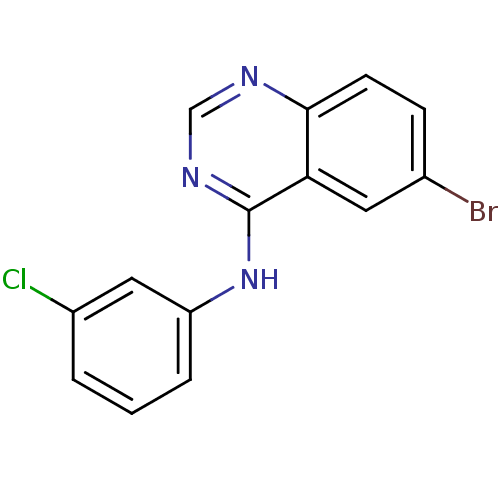 (6-bromo-N-(3-chlorophenyl)quinazolin-4-amine | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]3methoxy-5-(pyridin-2-ylethynyl)pyridine from rat mGluR5 | Bioorg Med Chem Lett 19: 6623-6 (2009) Article DOI: 10.1016/j.bmcl.2009.10.024 BindingDB Entry DOI: 10.7270/Q2TX3FHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50258619 (3-Cyano-5-fluoro-N-(6-methylpyridin-2-yl)-benzamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 157 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPy from rat mGlu5 receptor expressed in HEK293A cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 4390-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.064 BindingDB Entry DOI: 10.7270/Q2V9888D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50323289 (CHEMBL1209202 | N-(3-chlorophenyl)-3-cyano-5-fluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 206 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]methoxy-PEPy from rat mGlu5 receptor expressed in HEK293A cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 4390-4 (2010) Article DOI: 10.1016/j.bmcl.2010.06.064 BindingDB Entry DOI: 10.7270/Q2V9888D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50257064 (CHEMBL2386850) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Radiology and Radiological Sciences, Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center , Nashville, Tennessee 37232, United States. Curated by ChEMBL | Assay Description Negative allosteric modulation of rat mGlu5 receptor expressed in HEK293A cells assessed as inhibition of glutamate induced-calcium mobilization prei... | J Med Chem 60: 5072-5085 (2017) Article DOI: 10.1021/acs.jmedchem.7b00410 BindingDB Entry DOI: 10.7270/Q2JH3PM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50311876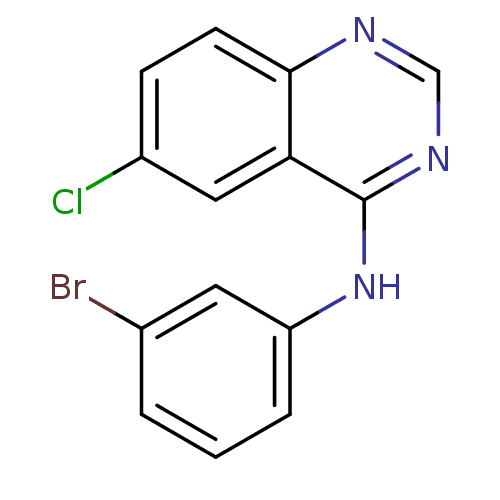 (CHEMBL1075626 | N-(3-bromophenyl)-6-chloroquinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 249 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]3methoxy-5-(pyridin-2-ylethynyl)pyridine from rat mGluR5 | Bioorg Med Chem Lett 19: 6623-6 (2009) Article DOI: 10.1016/j.bmcl.2009.10.024 BindingDB Entry DOI: 10.7270/Q2TX3FHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50257087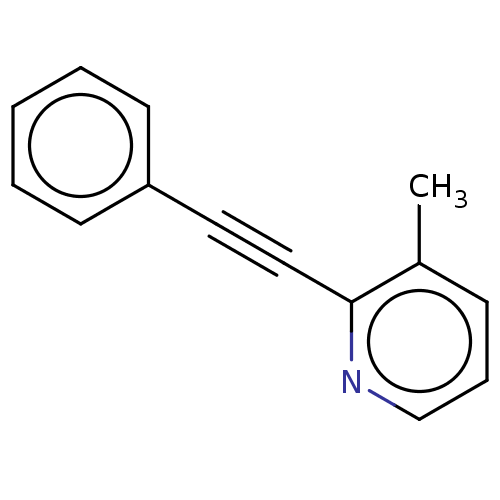 (CHEMBL4102569) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 388 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Radiology and Radiological Sciences, Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center , Nashville, Tennessee 37232, United States. Curated by ChEMBL | Assay Description Displacement of [3H]-3-methoxy-5-(pyridin-2-ylethynyl)pyridine from rat mGlu5 receptor expressed in HEK293A cell membranes after 1 hr by scintillatio... | J Med Chem 60: 5072-5085 (2017) Article DOI: 10.1021/acs.jmedchem.7b00410 BindingDB Entry DOI: 10.7270/Q2JH3PM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50311858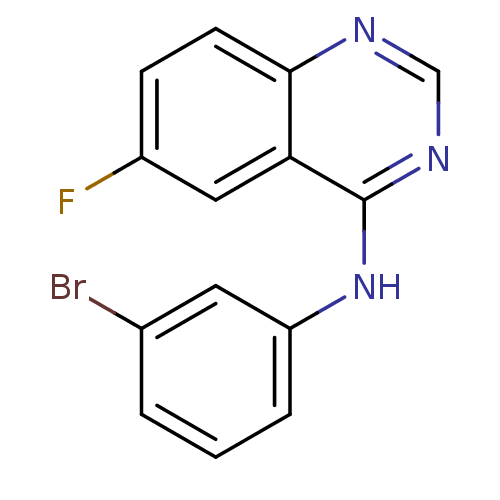 (CHEMBL1076333 | N-(3-bromophenyl)-6-fluoroquinazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 736 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Medical Center Curated by ChEMBL | Assay Description Displacement of [3H]3methoxy-5-(pyridin-2-ylethynyl)pyridine from rat mGluR5 | Bioorg Med Chem Lett 19: 6623-6 (2009) Article DOI: 10.1016/j.bmcl.2009.10.024 BindingDB Entry DOI: 10.7270/Q2TX3FHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22165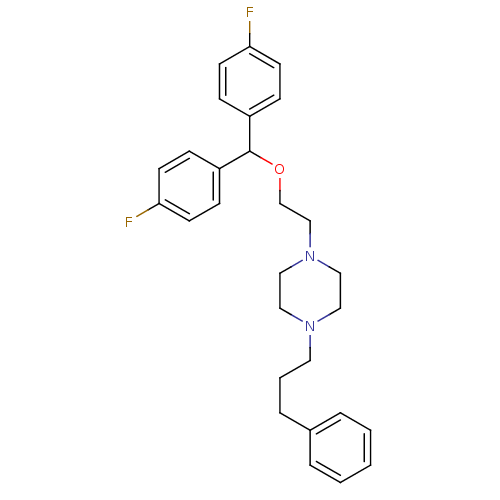 (1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt Center for Neuroscience Drug Discovery, Vanderbilt University, Nashville, TN 37232, USA; Department of Pharmacology, Vanderbilt University, Nashville, TN 37232, USA. Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from recombinant human DAT expressed in CHOK1 cells after 3 hrs by scintillation counting method | Bioorg Med Chem Lett 27: 4858-4866 (2017) Article DOI: 10.1016/j.bmcl.2017.09.042 BindingDB Entry DOI: 10.7270/Q2V1278Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28206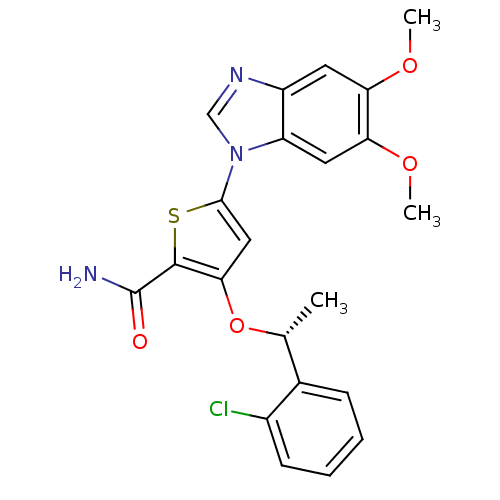 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(5,6-dimethoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28208 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-methoxy-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256469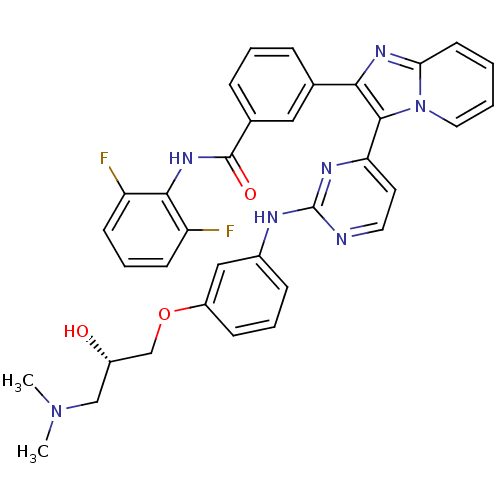 ((S)-N-(2,6-difluorophenyl)-3-(3-(2-(3-(3-(dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256467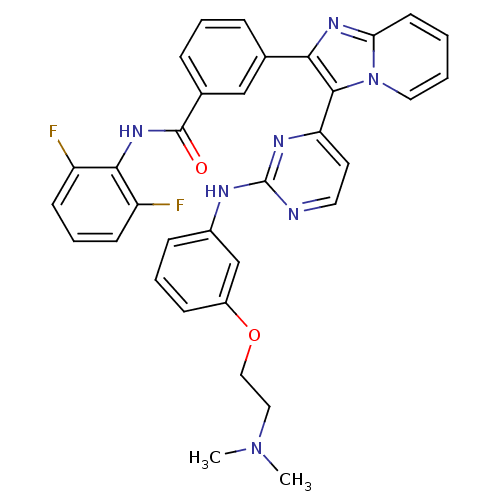 (CHEMBL448668 | N-(2,6-difluorophenyl)-3-(3-(2-(3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28210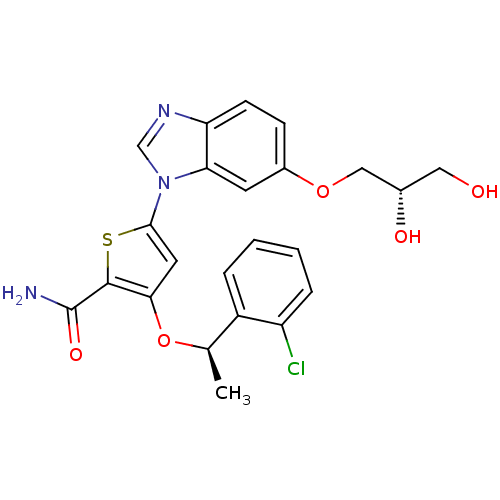 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28216 (5-{6-[(1-methylpiperidin-4-yl)oxy]-1H-1,3-benzodia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256410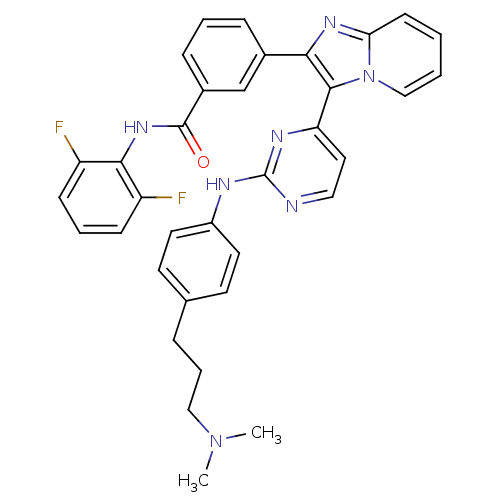 (CHEMBL447668 | N-(2,6-difluorophenyl)-3-(3-(2-(3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28175 (5-(6-methoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(trifl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM25120 (5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28177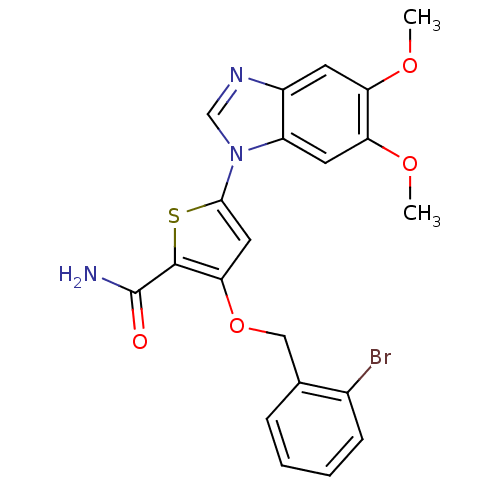 (3-[(2-bromophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28178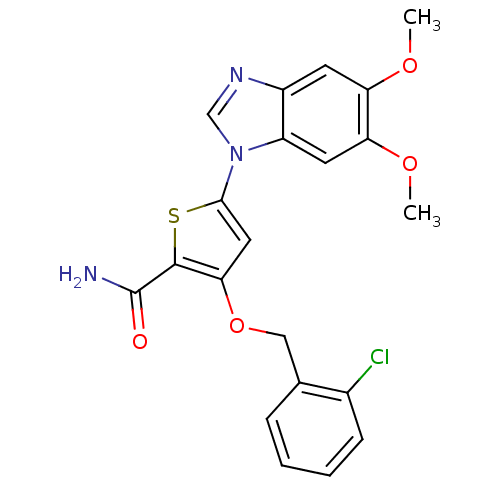 (3-[(2-chlorophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28194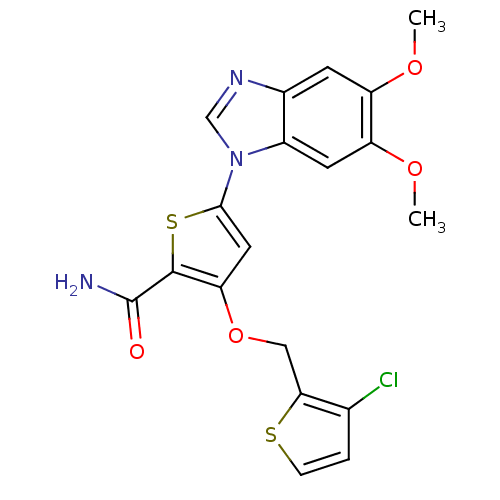 (3-[(3-chlorothiophen-2-yl)methoxy]-5-(5,6-dimethox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28200 (5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28178 (3-[(2-chlorophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256472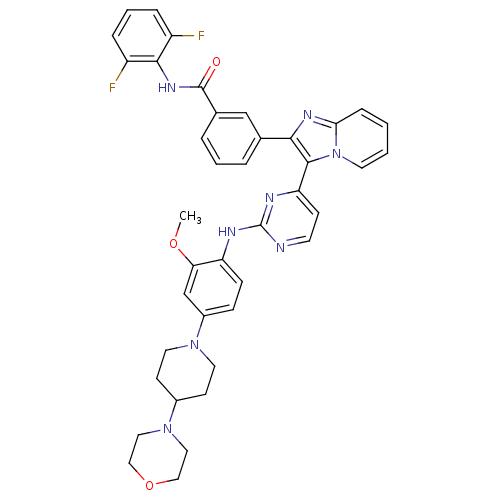 (CHEMBL502652 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256473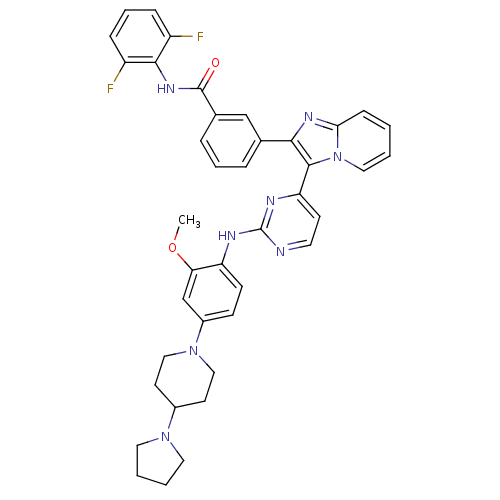 (CHEMBL502198 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256474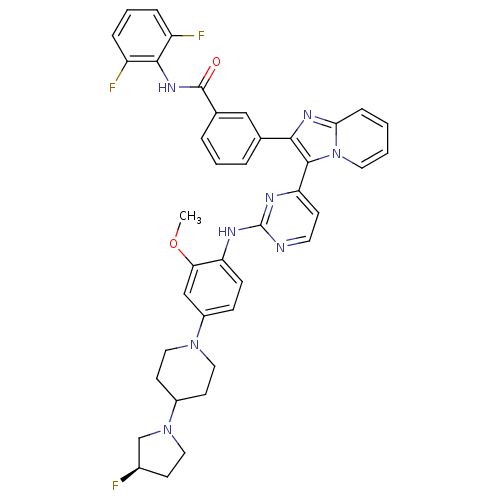 ((R)-N-(2,6-difluorophenyl)-3-(3-(2-(4-(4-(3-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256475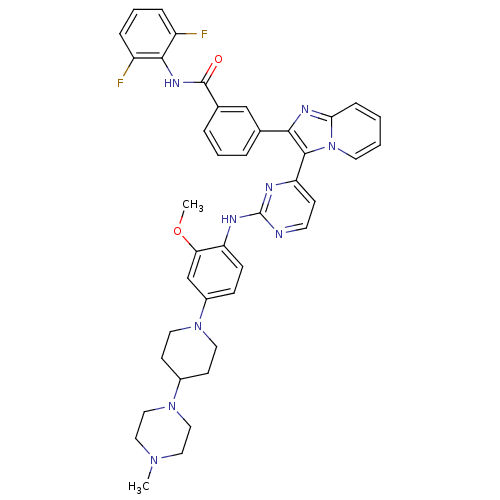 (CHEMBL449110 | N-(2,6-difluorophenyl)-3-(3-(2-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256470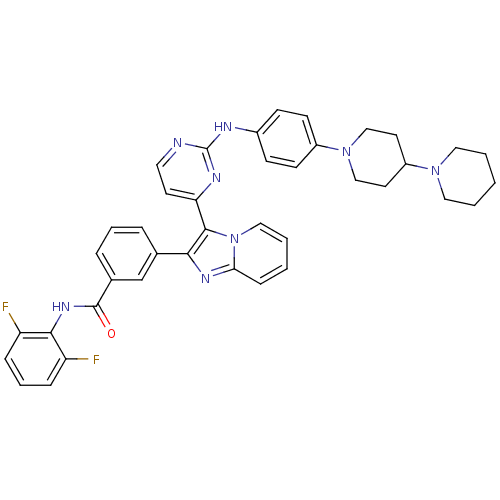 (3-(3-(2-(4-(1,4'-bipiperidin-1'-yl)phenylamino)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256471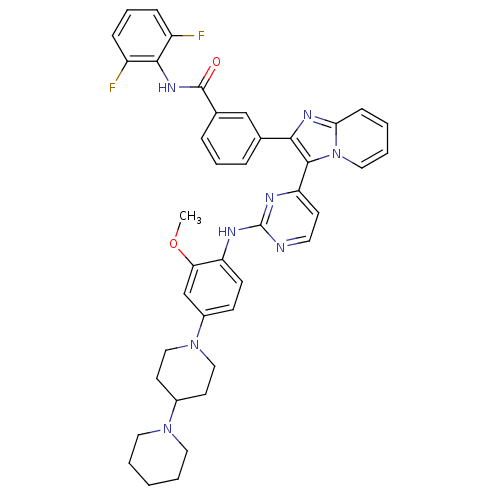 (3-(3-(2-(4-(1,4'-bipiperidin-1'-yl)-2-methoxypheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28217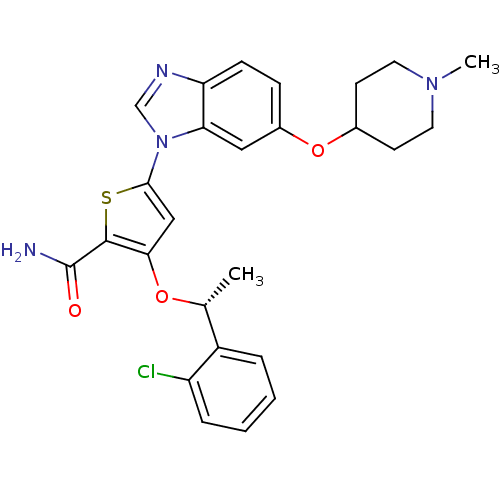 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(1-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28209 (5-(6-methoxy-1H-1,3-benzodiazol-1-yl)-3-[(1R)-1-[2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28212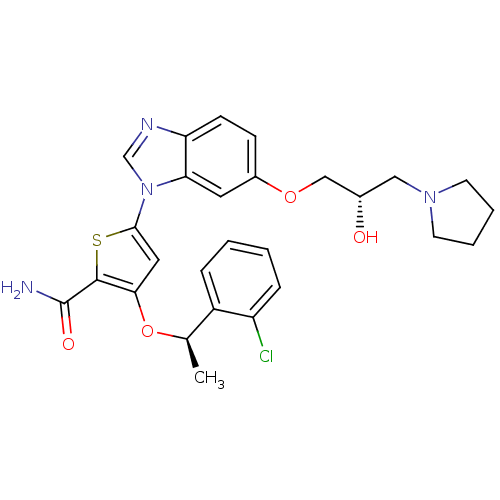 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28215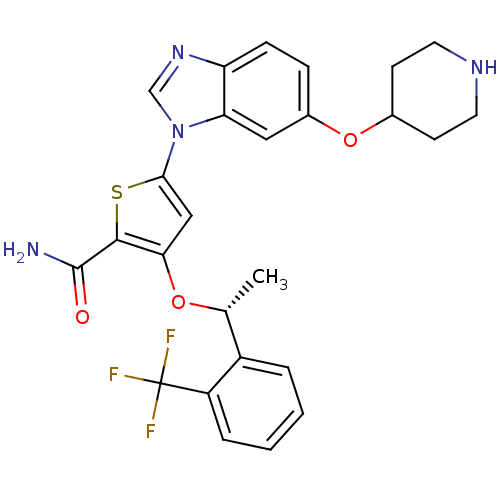 (5-[6-(piperidin-4-yloxy)-1H-1,3-benzodiazol-1-yl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28218 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4S)-1-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Insulin-like growth factor 1 receptor (Homo sapiens (Human)) | BDBM50256477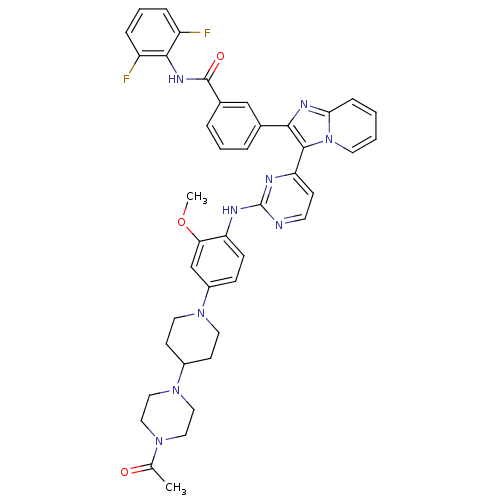 (3-(3-(2-(4-(4-(4-acetylpiperazin-1-yl)piperidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of GST-tagged IGF1R (957-1367) (unknown origin) expressed in baculovirus by time-resolved fluorescence assay | Bioorg Med Chem Lett 19: 1004-8 (2009) Article DOI: 10.1016/j.bmcl.2008.11.058 BindingDB Entry DOI: 10.7270/Q24T6J8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50257058 (CHEMBL2386849) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Radiology and Radiological Sciences, Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center , Nashville, Tennessee 37232, United States. Curated by ChEMBL | Assay Description Negative allosteric modulation of rat mGlu5 receptor expressed in HEK293A cells assessed as inhibition of glutamate induced-calcium mobilization prei... | J Med Chem 60: 5072-5085 (2017) Article DOI: 10.1021/acs.jmedchem.7b00410 BindingDB Entry DOI: 10.7270/Q2JH3PM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50257058 (CHEMBL2386849) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Radiology and Radiological Sciences, Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center , Nashville, Tennessee 37232, United States. Curated by ChEMBL | Assay Description Negative allosteric modulation of rat mGlu5 receptor expressed in HEK293A cells assessed as inhibition of glutamate induced-calcium mobilization prei... | J Med Chem 60: 5072-5085 (2017) Article DOI: 10.1021/acs.jmedchem.7b00410 BindingDB Entry DOI: 10.7270/Q2JH3PM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50257041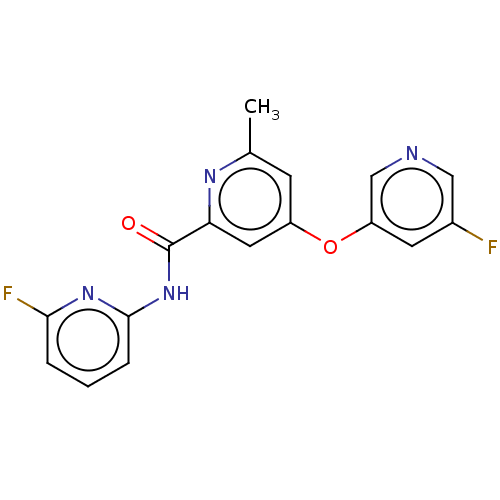 (CHEMBL4077672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Radiology and Radiological Sciences, Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center , Nashville, Tennessee 37232, United States. Curated by ChEMBL | Assay Description Negative allosteric modulation of rat mGlu5 receptor expressed in HEK293A cells assessed as inhibition of glutamate induced-calcium mobilization prei... | J Med Chem 60: 5072-5085 (2017) Article DOI: 10.1021/acs.jmedchem.7b00410 BindingDB Entry DOI: 10.7270/Q2JH3PM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50257041 (CHEMBL4077672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Radiology and Radiological Sciences, Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center , Nashville, Tennessee 37232, United States. Curated by ChEMBL | Assay Description Negative allosteric modulation of rat mGlu5 receptor expressed in HEK293A cells assessed as inhibition of glutamate induced-calcium mobilization prei... | J Med Chem 60: 5072-5085 (2017) Article DOI: 10.1021/acs.jmedchem.7b00410 BindingDB Entry DOI: 10.7270/Q2JH3PM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28211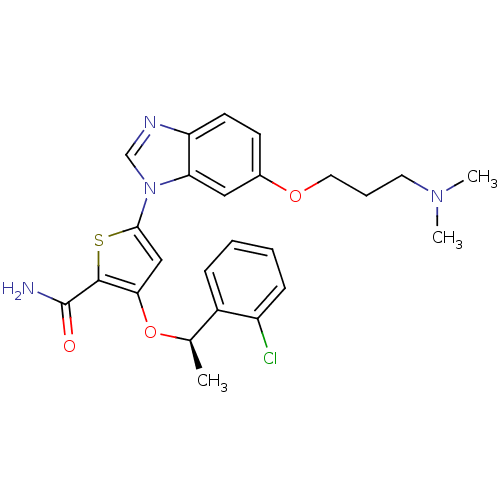 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[3-(dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28219 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4R)-1-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2770 total ) | Next | Last >> |