

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from human histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from rat histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]R-alpha-methylistamine from mouse histamine H3 receptor by cell-based assay | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296179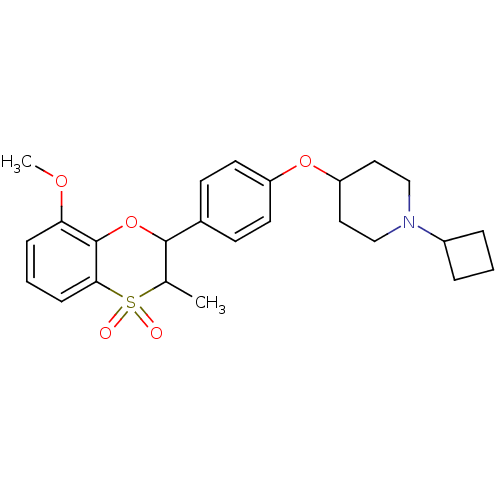 ((+/-)-1-Cyclobutyl-4-[4-(8-methoxy-3-methyl-4,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296178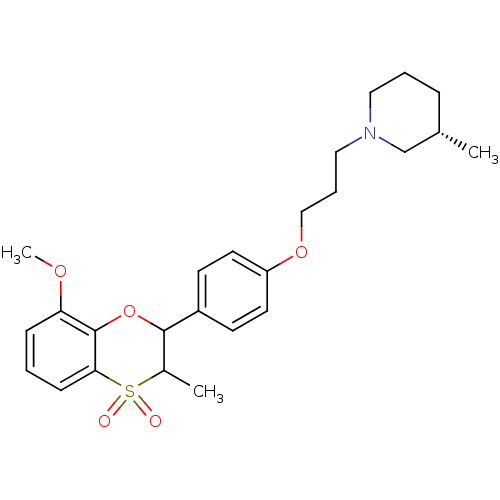 ((+/-)-(S)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296176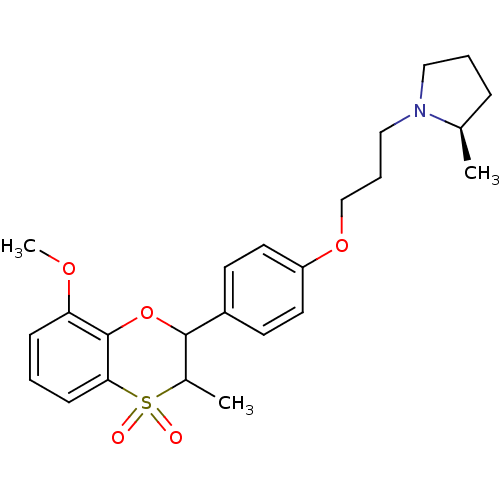 ((+/-)-(R)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296174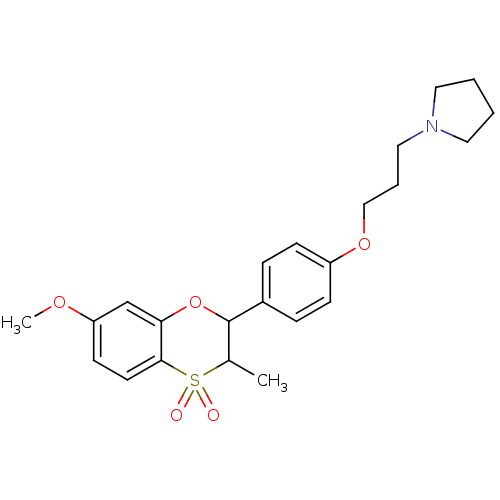 ((+/-)-1-{3-[4-(7-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296175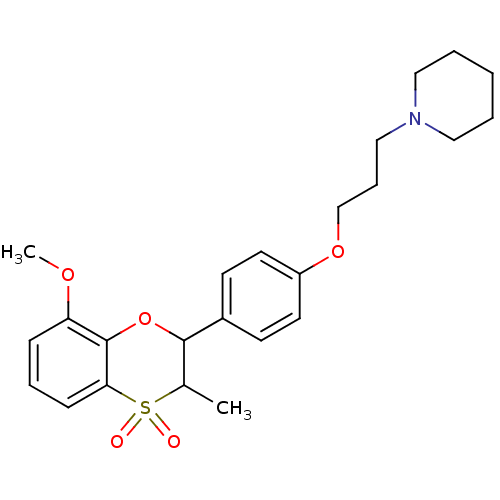 ((+/-)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296173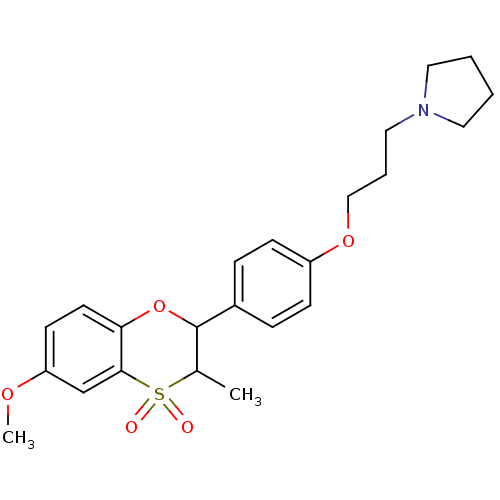 ((+/-)-1-{3-[4-(6-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296171 (1-{3-[4-((2S,3S)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296172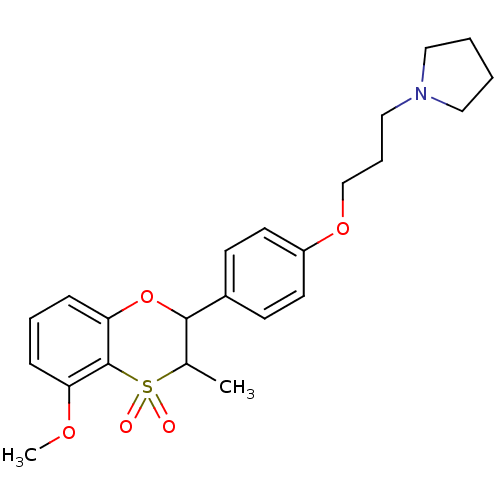 ((+/-)-1-{3-[4-(5-Methoxy-3-methyl-4,4-dioxo-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296188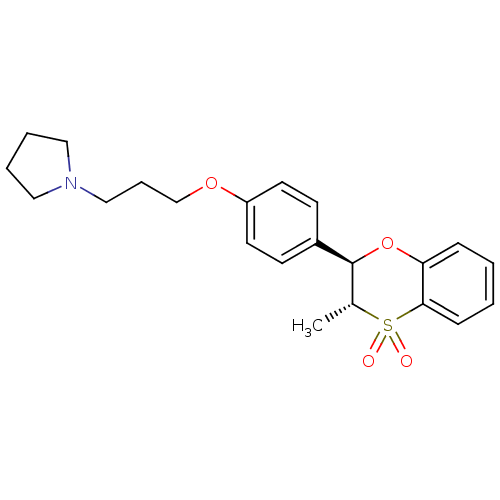 (CHEMBL558248 | trans-1-{3-[4-(3-Methyl-4,4-dioxo-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296181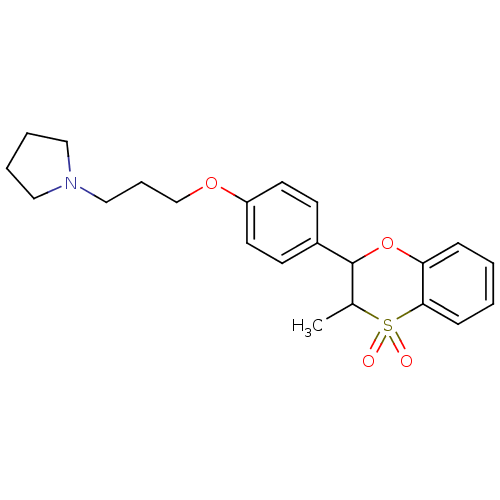 ((+/-)-1-{3-[4-(3-Methyl-4,4-dioxo-3,4-dihydro-2H-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296180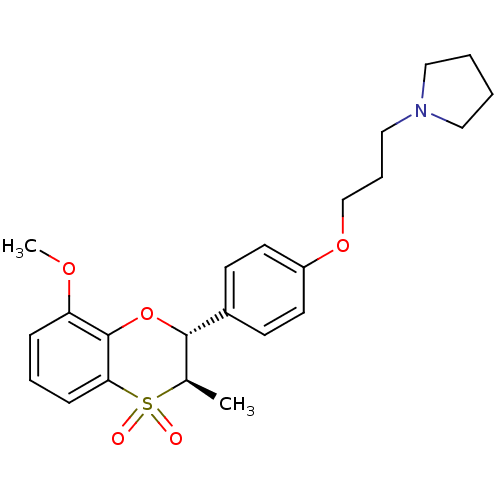 (1-{3-[4-((2R,3R)-8-Methoxy-3-methyl-4,4-dioxo-3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.607 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296177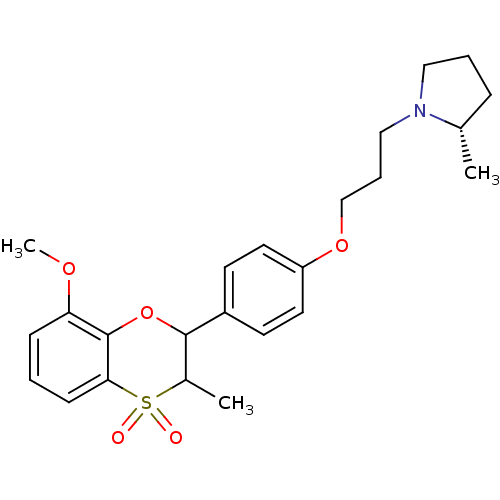 ((+/-)-(S)-1-{3-[4-(8-Methoxy-3-methyl-4,4-dioxo-3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296189 (1-{3-[4-(3-Methyl-4,4-dioxo-4H-4lambda*6*-benzo[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304315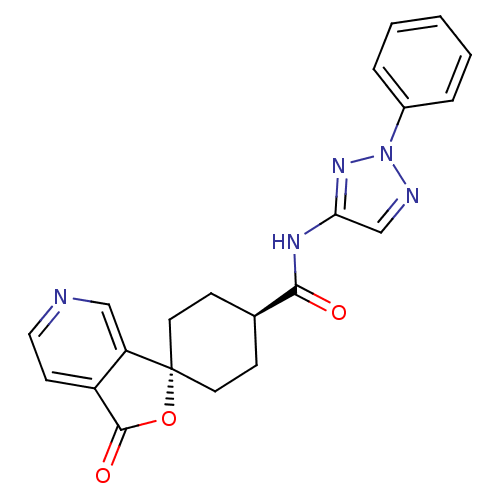 (CHEMBL593465 | trans-3-Oxo-N-(2-phenyl-2H-1,2,3-tr...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304301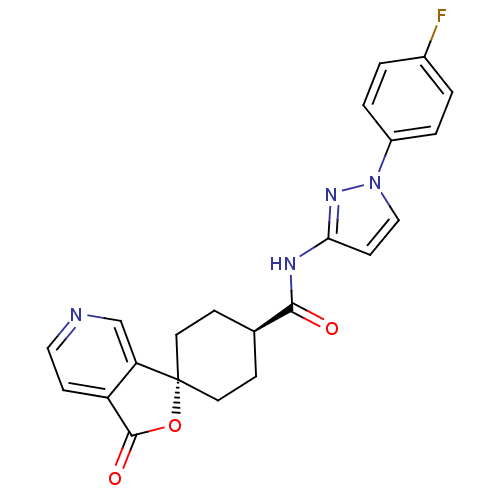 (CHEMBL595790 | trans-N-[1-(3-Fluorophenyl)-1H-pyra...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304313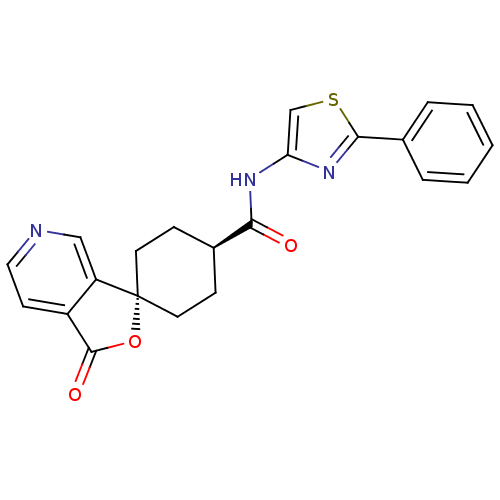 (CHEMBL607037 | trans-3-Oxo-N-(2-phenyl-1,3-thiazol...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50265209 (1'-(5-chloro-1H-benzo[d]imidazol-2-yl)-3H-spiro[is...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human NPY Y5 receptor transfected in mouse LMtk cells assessed as inhibition of neuropeptide Y-induced increase in intercellul... | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304298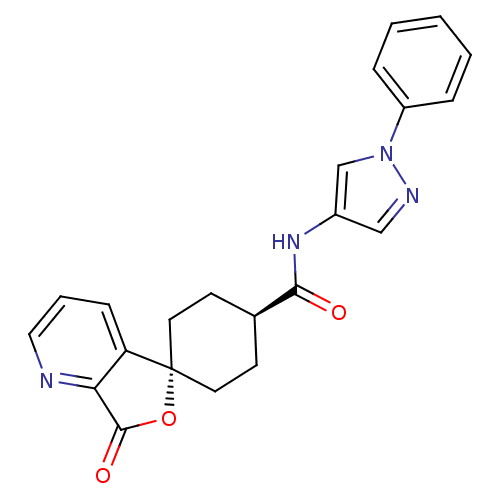 (CHEMBL595120 | trans-3-Oxo-N-(1-phenyl-1H-pyrazol-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304300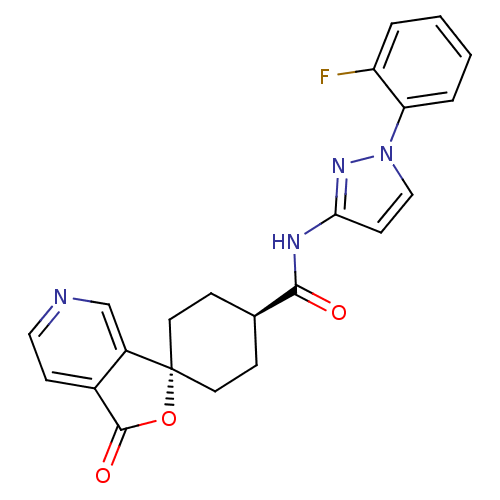 (CHEMBL595573 | trans-N-[1-(2-fluorophenyl)-3-pyraz...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50264726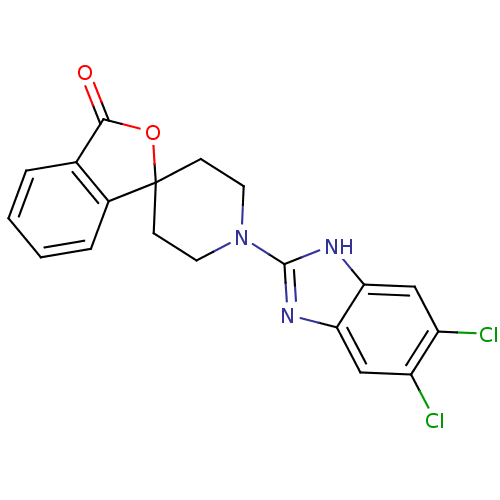 (1'-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)-3H-spir...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human NPY Y5 receptor transfected in mouse LMtk cells assessed as inhibition of neuropeptide Y-induced increase in intercellul... | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50265298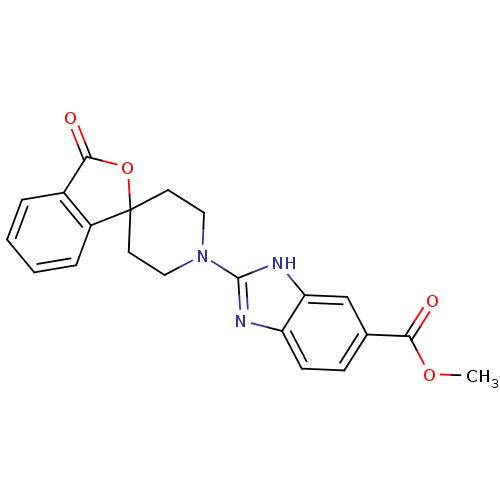 (CHEMBL496327 | methyl 2-(3-oxo-3H-spiro[isobenzofu...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human NPY Y5 receptor transfected in mouse LMtk cells assessed as inhibition of neuropeptide Y-induced increase in intercellul... | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304303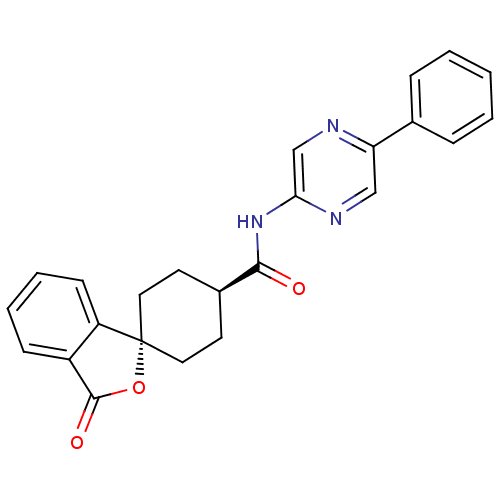 (CHEMBL593934 | trans-3-Oxo-N-(5-phenylpyrazin-2-yl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50264726 (1'-(5,6-dichloro-1H-benzo[d]imidazol-2-yl)-3H-spir...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPY Y5 receptor transfected in mouse LMtk cells | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50264364 (CHEMBL491229 | trans-4-[5-(4-Fluoro-phenyl)-1H-ben...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant NPYY5 receptor expressed in mouse LMtk cells | Bioorg Med Chem Lett 18: 4997-5001 (2008) Article DOI: 10.1016/j.bmcl.2008.08.021 BindingDB Entry DOI: 10.7270/Q25H7G2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50304297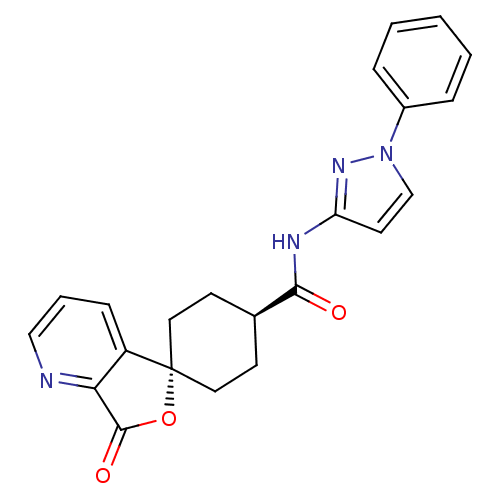 (CHEMBL594163 | trans-3-Oxo-N-(1-phenyl-1H-pyrazol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of rat Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50296185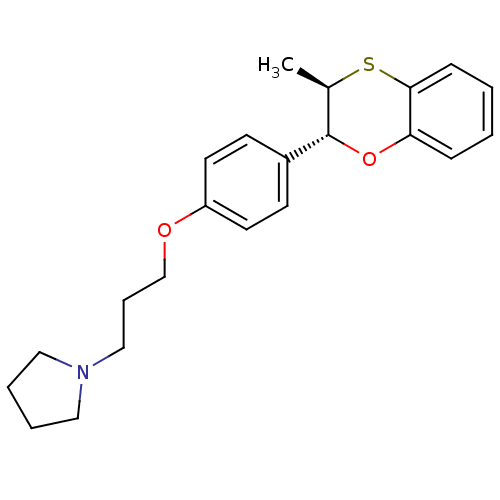 (CHEMBL550393 | trans-1-(3-(4-(3-methyl-2,3-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of R-alpha-methylistamine-induced [35S]GTPgammaS binding by cel... | Bioorg Med Chem Lett 19: 4232-6 (2009) Article DOI: 10.1016/j.bmcl.2009.05.101 BindingDB Entry DOI: 10.7270/Q20G3K69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50301656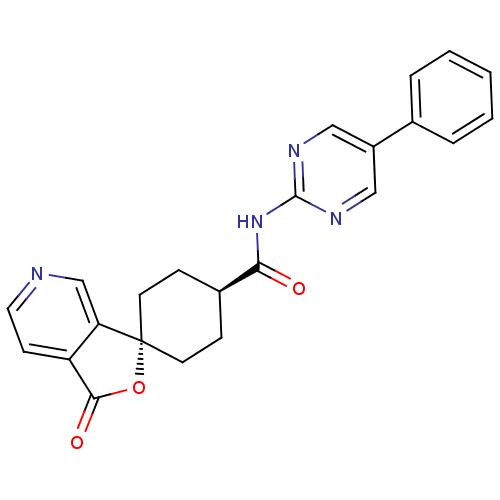 (CHEMBL567527 | trans-1'-oxo-N-(5-phenylpyrimidin-2...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50265297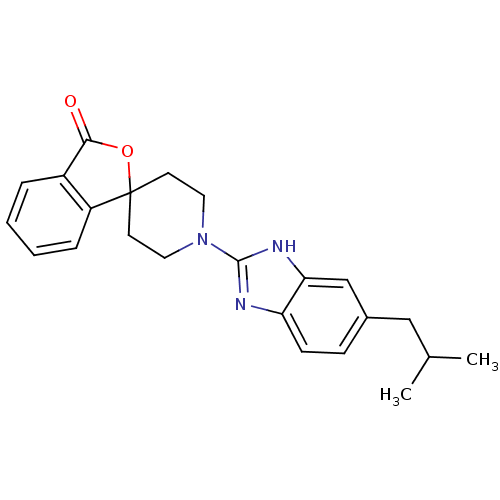 (1'-(5-isobutyl-1H-benzo[d]imidazol-2-yl)-3H-spiro[...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPY Y5 receptor transfected in mouse LMtk cells | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50265267 (1'-(5-phenyl-1H-benzo[d]imidazol-2-yl)-3H-spiro[is...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human NPY Y5 receptor transfected in mouse LMtk cells assessed as inhibition of neuropeptide Y-induced increase in intercellul... | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50265299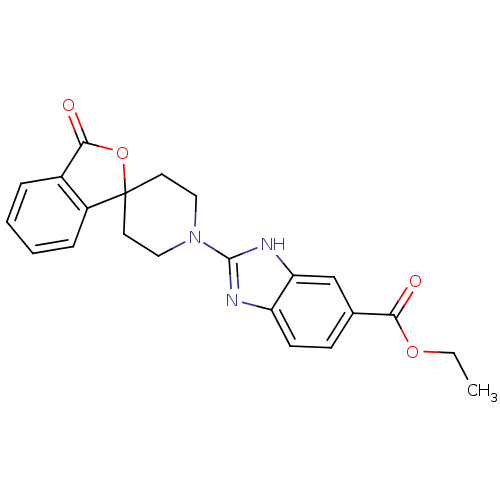 (CHEMBL524644 | ethyl 2-(3-oxo-3H-spiro[isobenzofur...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPY Y5 receptor transfected in mouse LMtk cells | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304316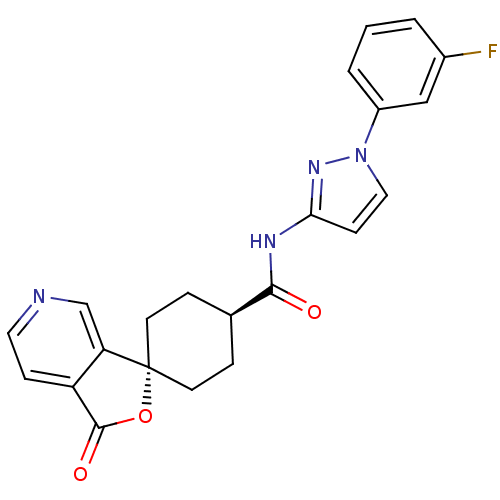 (CHEMBL594164 | trans-N-[1-(4-Fluorophenyl)-1H-pyra...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50301651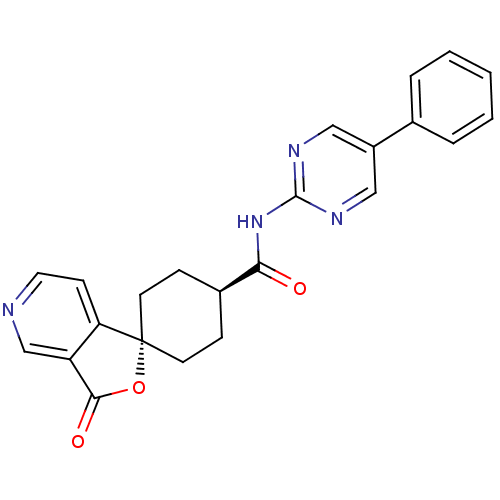 (CHEMBL568370 | trans-3'-oxo-N-(5-phenylpyrimidin-2...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50304300 (CHEMBL595573 | trans-N-[1-(2-fluorophenyl)-3-pyraz...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of rat Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50264493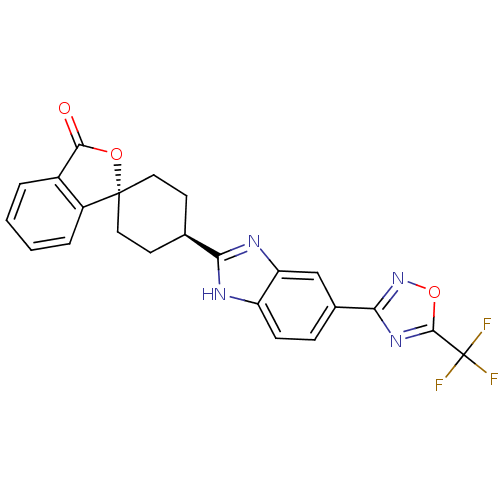 (CHEMBL489608 | trans-4-[5-(5-Trifluoromethyl-[1,2,...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant NPYY5 receptor expressed in mouse LMtk cells | Bioorg Med Chem Lett 18: 4997-5001 (2008) Article DOI: 10.1016/j.bmcl.2008.08.021 BindingDB Entry DOI: 10.7270/Q25H7G2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50304301 (CHEMBL595790 | trans-N-[1-(3-Fluorophenyl)-1H-pyra...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of rat Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304312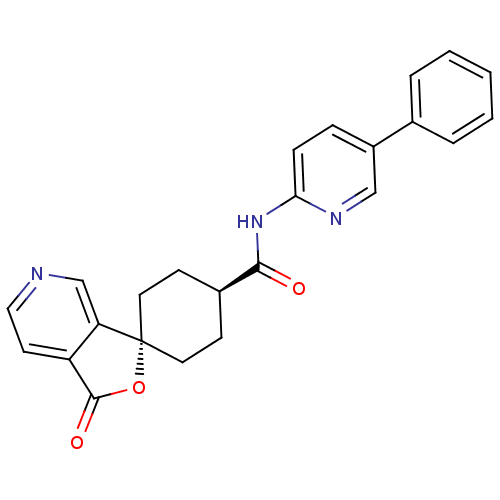 (CHEMBL604480 | trans-3-Oxo-N-(5-phenylpyridin-2-yl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304310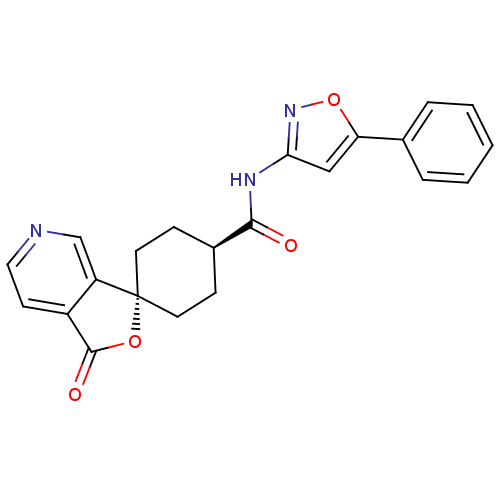 (CHEMBL596264 | trans-3-Oxo-N-(5-phenylisoxazol-3-y...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50301644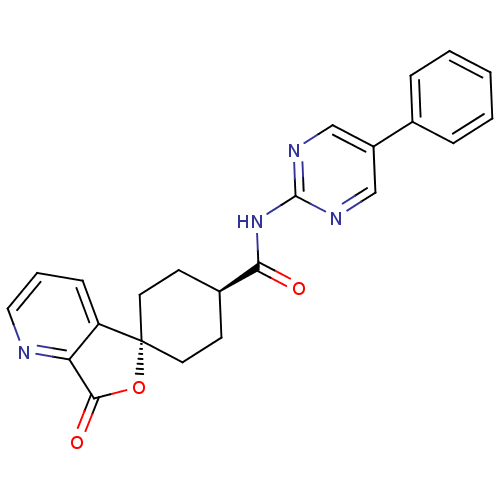 (CHEMBL568144 | trans-3-Oxo-N-(5-phenylpyrimidin-2-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50264395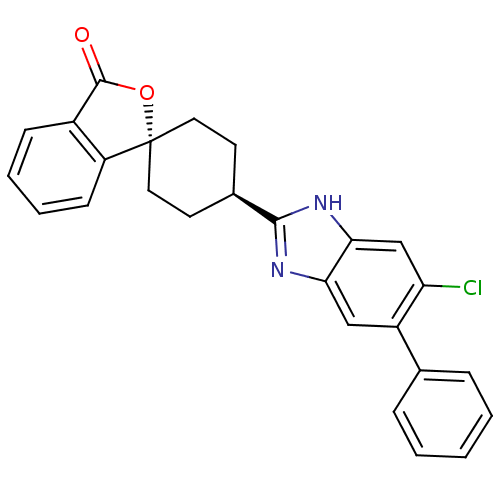 (CHEMBL490824 | trans-4-(5-Chloro-6-phenyl-1H-benzo...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant NPYY5 receptor expressed in mouse LMtk cells | Bioorg Med Chem Lett 18: 4997-5001 (2008) Article DOI: 10.1016/j.bmcl.2008.08.021 BindingDB Entry DOI: 10.7270/Q25H7G2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304309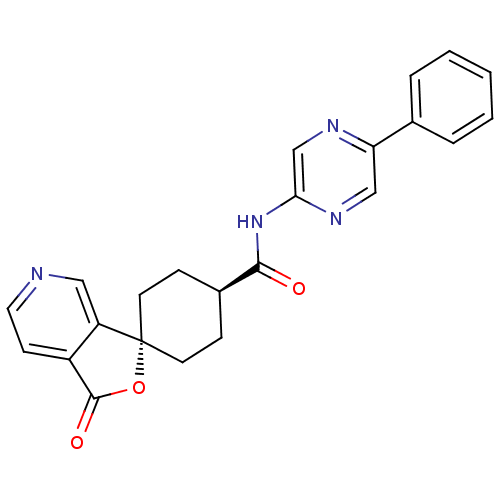 (CHEMBL607144 | trans-3-Oxo-N-(5-phenylpyrazin-2-yl...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304314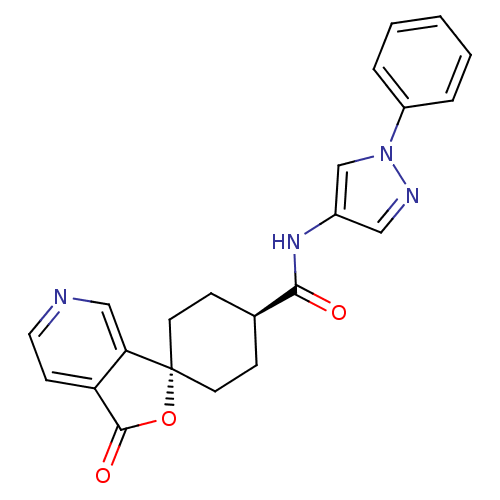 (CHEMBL596025 | trans-3-Oxo-N-(1-phenyl-1H-pyrazol-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50304299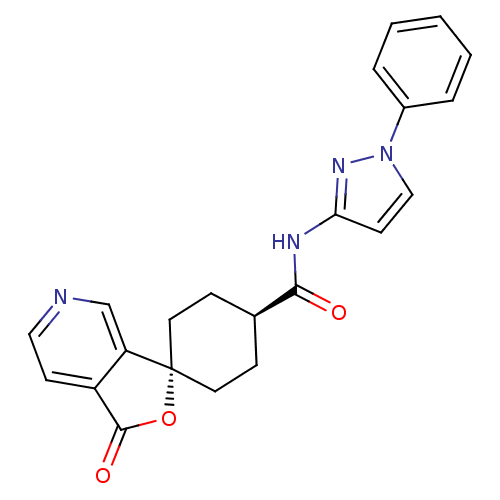 (CHEMBL595560 | trans-3-Oxo-N-(1-phenyl-1H-pyrazol-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibition of rat Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50264441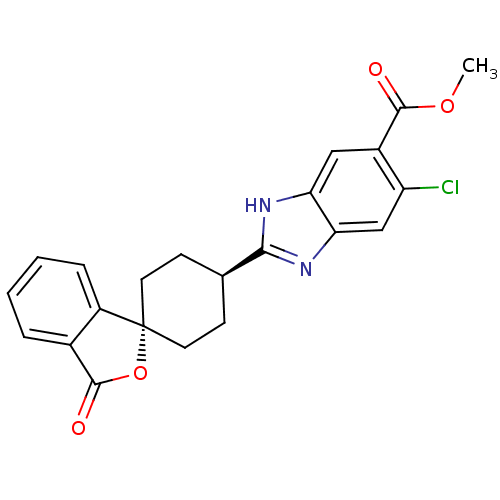 (CHEMBL491239 | trans-6-Chloro-2-(3'-oxo-3'H-spiro[...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant NPYY5 receptor expressed in mouse LMtk cells | Bioorg Med Chem Lett 18: 4997-5001 (2008) Article DOI: 10.1016/j.bmcl.2008.08.021 BindingDB Entry DOI: 10.7270/Q25H7G2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50264853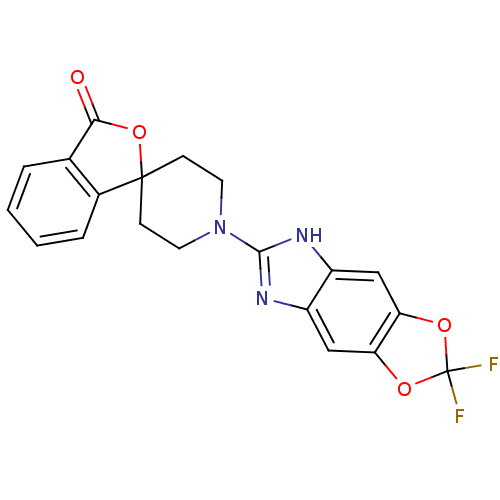 (1'-(2,2-difluoro-5H-[1,3]dioxolo[4,5-f]benzimidazo...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Binding affinity to human NPY Y5 receptor transfected in mouse LMtk cells | Bioorg Med Chem Lett 18: 5010-4 (2008) Article DOI: 10.1016/j.bmcl.2008.08.018 BindingDB Entry DOI: 10.7270/Q26D5STS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50264328 (CHEMBL489587 | trans-4-(5-Phenyl-1H-benzoimidazol-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant NPYY5 receptor expressed in mouse LMtk cells | Bioorg Med Chem Lett 18: 4997-5001 (2008) Article DOI: 10.1016/j.bmcl.2008.08.021 BindingDB Entry DOI: 10.7270/Q25H7G2P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50304299 (CHEMBL595560 | trans-3-Oxo-N-(1-phenyl-1H-pyrazol-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]PYY from human recombinant Y5 receptor | Bioorg Med Chem 17: 6971-82 (2009) Article DOI: 10.1016/j.bmc.2009.08.019 BindingDB Entry DOI: 10.7270/Q2P26Z62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 349 total ) | Next | Last >> |