 Found 919 hits with Last Name = 'mallon' and Initial = 'r'
Found 919 hits with Last Name = 'mallon' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM28123

(3-cyanoquinoline, 8 | 4-({3-chloro-4-[(1-methyl-1H...)Show SMILES COc1cc2c(Nc3ccc(Sc4nccn4C)c(Cl)c3)c(cnc2cc1OCCCN1CCOCC1)C#N Show InChI InChI=1S/C28H29ClN6O3S/c1-34-8-6-31-28(34)39-26-5-4-20(14-22(26)29)33-27-19(17-30)18-32-23-16-25(24(36-2)15-21(23)27)38-11-3-7-35-9-12-37-13-10-35/h4-6,8,14-16,18H,3,7,9-13H2,1-2H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 phosphorylation in LoVo cells |
Bioorg Med Chem Lett 13: 3031-4 (2003)
BindingDB Entry DOI: 10.7270/Q2P84B8T |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320098
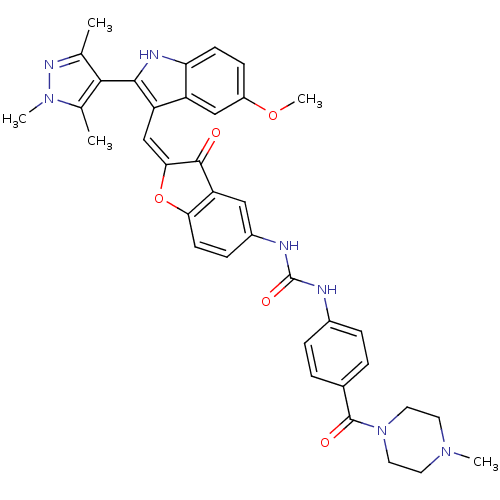
(1-(2-((5-methoxy-2-(1,3,5-trimethyl-1H-pyrazol-4-y...)Show SMILES COc1ccc2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N5CCN(C)CC5)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(-2.98,-34.6,;-2.98,-36.14,;-1.64,-36.92,;-1.65,-38.46,;-.31,-39.23,;1.02,-38.45,;2.49,-38.93,;3.4,-37.68,;2.49,-36.43,;3.09,-35.01,;2.32,-33.67,;2.95,-32.27,;1.8,-31.25,;1.8,-29.71,;.47,-28.94,;-.86,-29.72,;-2.2,-28.95,;-2.21,-27.41,;-.87,-26.64,;-3.55,-26.65,;-3.56,-25.12,;-2.23,-24.34,;-2.24,-22.81,;-3.57,-22.05,;-4.9,-22.84,;-4.88,-24.36,;-3.58,-20.52,;-2.26,-19.74,;-4.92,-19.76,;-4.92,-18.23,;-6.25,-17.48,;-7.57,-18.25,;-8.9,-17.49,;-7.56,-19.78,;-6.23,-20.55,;-.85,-31.25,;.47,-32.01,;.79,-33.52,;-.24,-34.67,;1.02,-36.91,;-.31,-36.14,;4.94,-37.68,;5.84,-38.93,;5.36,-40.39,;7.3,-38.45,;7.3,-36.91,;8.55,-36,;5.84,-36.43,;5.36,-34.96,)| Show InChI InChI=1S/C37H37N7O5/c1-21-33(22(2)43(4)41-21)34-28(27-19-26(48-5)11-12-30(27)40-34)20-32-35(45)29-18-25(10-13-31(29)49-32)39-37(47)38-24-8-6-23(7-9-24)36(46)44-16-14-42(3)15-17-44/h6-13,18-20,40H,14-17H2,1-5H3,(H2,38,39,47)/b32-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50349619
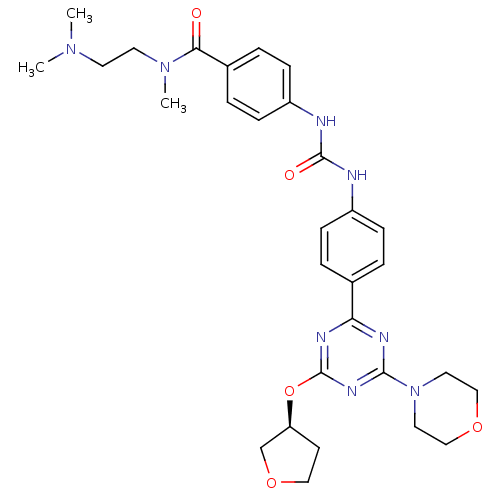
(CHEMBL1808977)Show SMILES CN(C)CCN(C)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(O[C@H]3CCOC3)nc(n2)N2CCOCC2)cc1 |r| Show InChI InChI=1S/C30H38N8O5/c1-36(2)13-14-37(3)27(39)22-6-10-24(11-7-22)32-29(40)31-23-8-4-21(5-9-23)26-33-28(38-15-18-41-19-16-38)35-30(34-26)43-25-12-17-42-20-25/h4-11,25H,12-20H2,1-3H3,(H2,31,32,40)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha expressed in SF9 insect cells after 2 hrs by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320101
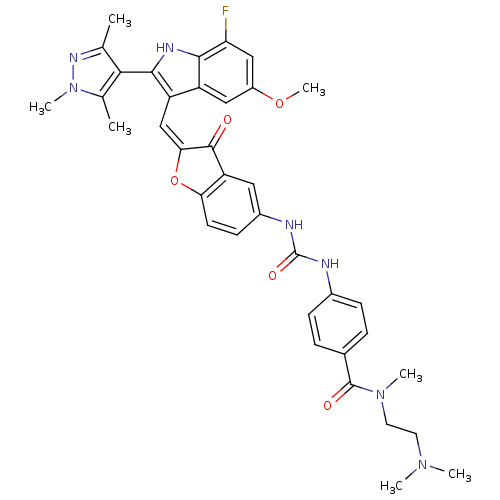
(CHEMBL1082621 | N-(2-(dimethylamino)ethyl)-4-(3-(2...)Show SMILES COc1cc(F)c2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N(C)CCN(C)C)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(16.64,-8.95,;16.64,-10.49,;17.98,-11.27,;17.98,-12.81,;19.32,-13.58,;19.32,-15.12,;20.65,-12.81,;22.12,-13.28,;23.02,-12.03,;22.12,-10.78,;22.71,-9.36,;21.95,-8.02,;22.57,-6.62,;21.42,-5.6,;21.42,-4.06,;20.1,-3.29,;18.76,-4.07,;17.42,-3.3,;17.42,-1.76,;18.75,-.99,;16.08,-1,;16.07,.53,;17.4,1.31,;17.39,2.84,;16.05,3.6,;14.73,2.81,;14.74,1.29,;16.04,5.13,;17.36,5.91,;14.71,5.89,;14.69,7.42,;13.38,5.11,;12.05,5.87,;10.73,5.09,;9.39,5.85,;10.74,3.56,;18.77,-5.6,;20.1,-6.36,;20.42,-7.87,;19.38,-9.02,;20.65,-11.26,;19.31,-10.49,;24.56,-12.03,;25.46,-13.28,;24.99,-14.74,;26.93,-12.8,;26.93,-11.26,;28.18,-10.35,;25.46,-10.78,;24.99,-9.32,)| Show InChI InChI=1S/C37H38FN7O5/c1-20-32(21(2)45(6)42-20)34-27(26-17-25(49-7)18-29(38)33(26)41-34)19-31-35(46)28-16-24(12-13-30(28)50-31)40-37(48)39-23-10-8-22(9-11-23)36(47)44(5)15-14-43(3)4/h8-13,16-19,41H,14-15H2,1-7H3,(H2,39,40,48)/b31-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320097
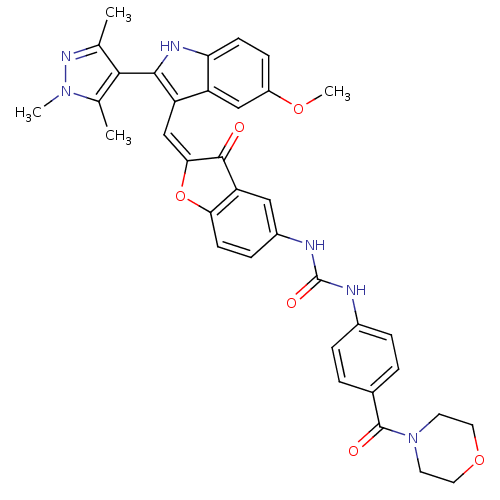
(1-(2-((5-methoxy-2-(1,3,5-trimethyl-1H-pyrazol-4-y...)Show SMILES COc1ccc2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N5CCOCC5)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(-2.53,-10.46,;-2.53,-12,;-1.2,-12.77,;-1.2,-14.32,;.14,-15.09,;1.47,-14.31,;2.94,-14.78,;3.85,-13.53,;2.94,-12.28,;3.54,-10.86,;2.77,-9.53,;3.4,-8.12,;2.25,-7.1,;2.25,-5.57,;.92,-4.79,;-.41,-5.57,;-1.75,-4.8,;-1.76,-3.26,;-.43,-2.49,;-3.1,-2.5,;-3.11,-.97,;-1.78,-.2,;-1.79,1.34,;-3.12,2.1,;-4.45,1.31,;-4.44,-.22,;-3.14,3.63,;-1.81,4.41,;-4.47,4.39,;-4.48,5.92,;-5.81,6.68,;-7.13,5.91,;-7.13,4.37,;-5.79,3.61,;-.41,-7.11,;.92,-7.86,;1.24,-9.37,;.21,-10.52,;1.47,-12.77,;.14,-12,;5.39,-13.53,;6.29,-14.78,;5.81,-16.25,;7.75,-14.31,;7.75,-12.77,;9,-11.86,;6.29,-12.29,;5.81,-10.82,)| Show InChI InChI=1S/C36H34N6O6/c1-20-32(21(2)41(3)40-20)33-27(26-18-25(46-4)10-11-29(26)39-33)19-31-34(43)28-17-24(9-12-30(28)48-31)38-36(45)37-23-7-5-22(6-8-23)35(44)42-13-15-47-16-14-42/h5-12,17-19,39H,13-16H2,1-4H3,(H2,37,38,45)/b31-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50320101

(CHEMBL1082621 | N-(2-(dimethylamino)ethyl)-4-(3-(2...)Show SMILES COc1cc(F)c2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N(C)CCN(C)C)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(16.64,-8.95,;16.64,-10.49,;17.98,-11.27,;17.98,-12.81,;19.32,-13.58,;19.32,-15.12,;20.65,-12.81,;22.12,-13.28,;23.02,-12.03,;22.12,-10.78,;22.71,-9.36,;21.95,-8.02,;22.57,-6.62,;21.42,-5.6,;21.42,-4.06,;20.1,-3.29,;18.76,-4.07,;17.42,-3.3,;17.42,-1.76,;18.75,-.99,;16.08,-1,;16.07,.53,;17.4,1.31,;17.39,2.84,;16.05,3.6,;14.73,2.81,;14.74,1.29,;16.04,5.13,;17.36,5.91,;14.71,5.89,;14.69,7.42,;13.38,5.11,;12.05,5.87,;10.73,5.09,;9.39,5.85,;10.74,3.56,;18.77,-5.6,;20.1,-6.36,;20.42,-7.87,;19.38,-9.02,;20.65,-11.26,;19.31,-10.49,;24.56,-12.03,;25.46,-13.28,;24.99,-14.74,;26.93,-12.8,;26.93,-11.26,;28.18,-10.35,;25.46,-10.78,;24.99,-9.32,)| Show InChI InChI=1S/C37H38FN7O5/c1-20-32(21(2)45(6)42-20)34-27(26-17-25(49-7)18-29(38)33(26)41-34)19-31-35(46)28-16-24(12-13-30(28)50-31)40-37(48)39-23-10-8-22(9-11-23)36(47)44(5)15-14-43(3)4/h8-13,16-19,41H,14-15H2,1-7H3,(H2,39,40,48)/b31-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50349632

(CHEMBL1808990)Show SMILES O=C(Nc1ccc(cc1)C(=O)NCCN1CCCC1)Nc1ccc(cc1)-c1nc(O[C@H]2CCOC2)nc(n1)N1CCOCC1 |r| Show InChI InChI=1S/C31H38N8O5/c40-28(32-12-15-38-13-1-2-14-38)23-5-9-25(10-6-23)34-30(41)33-24-7-3-22(4-8-24)27-35-29(39-16-19-42-20-17-39)37-31(36-27)44-26-11-18-43-21-26/h3-10,26H,1-2,11-21H2,(H,32,40)(H2,33,34,41)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320099
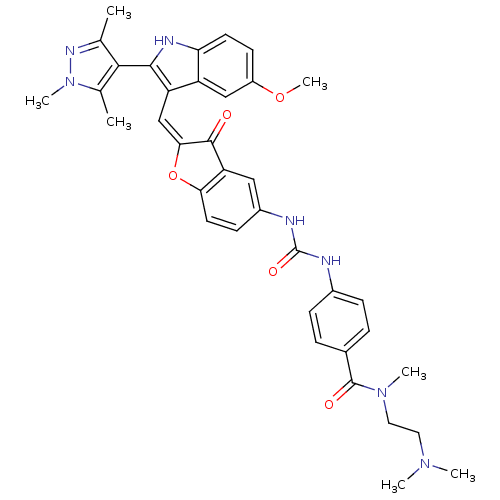
(CHEMBL1082619 | N-(2-(dimethylamino)ethyl)-4-(3-(2...)Show SMILES COc1ccc2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N(C)CCN(C)C)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(17.28,-35.65,;17.28,-37.19,;18.62,-37.96,;18.61,-39.51,;19.95,-40.28,;21.28,-39.5,;22.75,-39.97,;23.66,-38.72,;22.75,-37.47,;23.35,-36.05,;22.58,-34.72,;23.21,-33.32,;22.06,-32.29,;22.06,-30.76,;20.73,-29.99,;19.4,-30.77,;18.06,-30,;18.05,-28.46,;19.39,-27.68,;16.71,-27.69,;16.7,-26.16,;18.03,-25.39,;18.02,-23.86,;16.69,-23.1,;15.36,-23.88,;15.38,-25.41,;16.67,-21.56,;18,-20.79,;15.34,-20.81,;15.33,-19.27,;14.02,-21.59,;12.68,-20.83,;11.36,-21.61,;10.03,-20.85,;11.38,-23.14,;19.4,-32.3,;20.73,-33.06,;21.05,-34.56,;20.02,-35.71,;21.28,-37.96,;19.95,-37.19,;25.2,-38.72,;26.1,-39.97,;25.62,-41.44,;27.56,-39.5,;27.56,-37.96,;28.81,-37.05,;26.1,-37.48,;25.62,-36.01,)| Show InChI InChI=1S/C37H39N7O5/c1-21-33(22(2)44(6)41-21)34-28(27-19-26(48-7)13-14-30(27)40-34)20-32-35(45)29-18-25(12-15-31(29)49-32)39-37(47)38-24-10-8-23(9-11-24)36(46)43(5)17-16-42(3)4/h8-15,18-20,40H,16-17H2,1-7H3,(H2,38,39,47)/b32-20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50349628
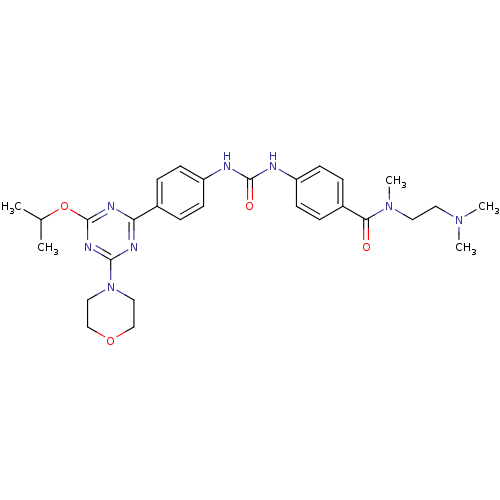
(CHEMBL1808986)Show SMILES CC(C)Oc1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N(C)CCN(C)C)cc1)N1CCOCC1 Show InChI InChI=1S/C29H38N8O4/c1-20(2)41-29-33-25(32-27(34-29)37-16-18-40-19-17-37)21-6-10-23(11-7-21)30-28(39)31-24-12-8-22(9-13-24)26(38)36(5)15-14-35(3)4/h6-13,20H,14-19H2,1-5H3,(H2,30,31,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308782
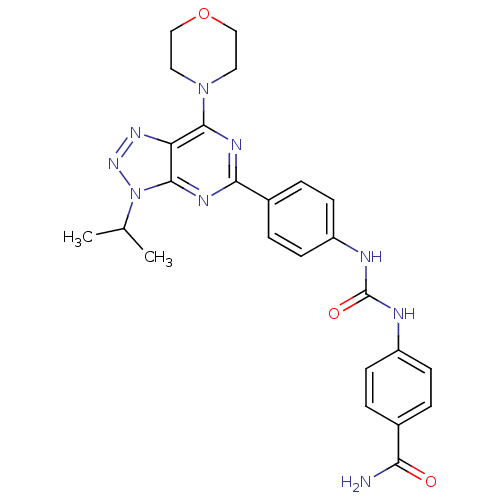
(4-{3-[4-(3-Isopropyl-7-morpholin-4-yl-3H-[1,2,3]-t...)Show SMILES CC(C)n1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(N)=O)cc1)N1CCOCC1 Show InChI InChI=1S/C25H27N9O3/c1-15(2)34-24-20(31-32-34)23(33-11-13-37-14-12-33)29-22(30-24)17-5-9-19(10-6-17)28-25(36)27-18-7-3-16(4-8-18)21(26)35/h3-10,15H,11-14H2,1-2H3,(H2,26,35)(H2,27,28,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327426

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-mo...)Show SMILES OCCc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2C3CCC2COC3)cc1 Show InChI InChI=1S/C28H33N7O4/c36-14-11-19-1-5-21(6-2-19)29-28(37)30-22-7-3-20(4-8-22)25-31-26(34-12-15-38-16-13-34)33-27(32-25)35-23-9-10-24(35)18-39-17-23/h1-8,23-24,36H,9-18H2,(H2,29,30,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 5869-73 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.104
BindingDB Entry DOI: 10.7270/Q2XP755V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308780
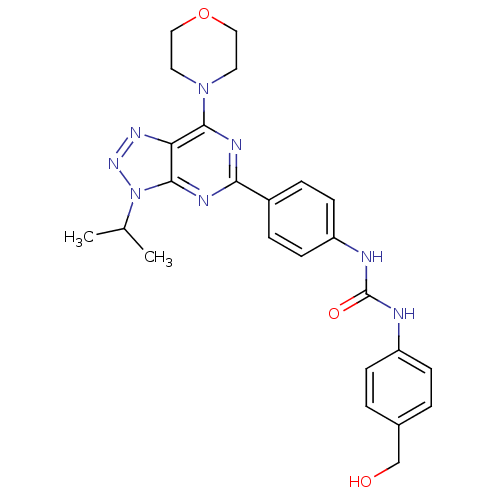
(1-(4-Hydroxymethylphenyl)-3-[4-(3-isopropyl-7-morp...)Show SMILES CC(C)n1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(CO)cc2)cc1)N1CCOCC1 Show InChI InChI=1S/C25H28N8O3/c1-16(2)33-24-21(30-31-33)23(32-11-13-36-14-12-32)28-22(29-24)18-5-9-20(10-6-18)27-25(35)26-19-7-3-17(15-34)4-8-19/h3-10,16,34H,11-15H2,1-2H3,(H2,26,27,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308784
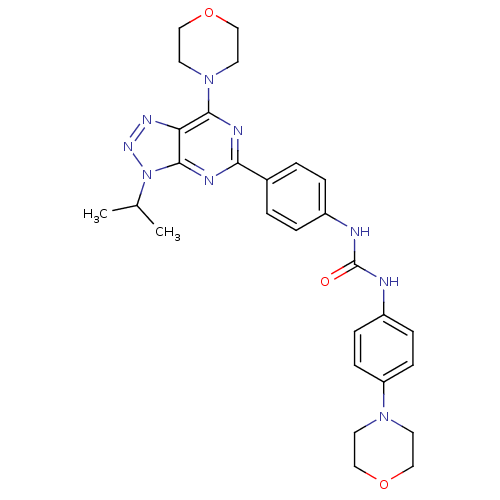
(1-[4-(3-Isopropyl-7-morpholin-4-yl-3H-[1,2,3]-tria...)Show SMILES CC(C)n1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)N2CCOCC2)cc1)N1CCOCC1 Show InChI InChI=1S/C28H33N9O3/c1-19(2)37-27-24(33-34-37)26(36-13-17-40-18-14-36)31-25(32-27)20-3-5-21(6-4-20)29-28(38)30-22-7-9-23(10-8-22)35-11-15-39-16-12-35/h3-10,19H,11-18H2,1-2H3,(H2,29,30,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308781
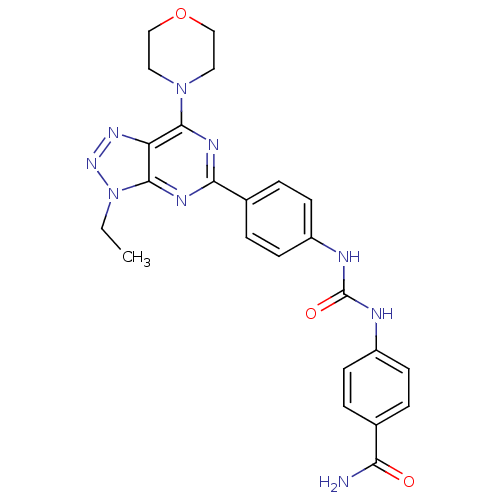
(4-(3-(4-(3-ethyl-7-morpholino-3H-[1,2,3]triazolo[4...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(N)=O)cc1)N1CCOCC1 Show InChI InChI=1S/C24H25N9O3/c1-2-33-23-19(30-31-33)22(32-11-13-36-14-12-32)28-21(29-23)16-5-9-18(10-6-16)27-24(35)26-17-7-3-15(4-8-17)20(25)34/h3-10H,2,11-14H2,1H3,(H2,25,34)(H2,26,27,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308135
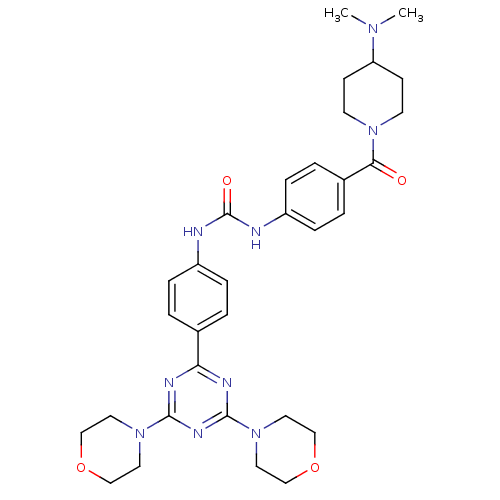
(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315759

(CHEMBL1092073 | N-[2-(Dimethylamino)ethyl]-4-[({4-...)Show SMILES CN(C)CCNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ccn(CC(F)(F)F)c3n2)cc1 Show InChI InChI=1S/C30H33F3N8O3/c1-39(2)14-12-34-28(42)21-5-9-23(10-6-21)36-29(43)35-22-7-3-20(4-8-22)25-37-26(40-15-17-44-18-16-40)24-11-13-41(27(24)38-25)19-30(31,32)33/h3-11,13H,12,14-19H2,1-2H3,(H,34,42)(H2,35,36,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 3169-82 (2010)
Article DOI: 10.1021/jm901783v
BindingDB Entry DOI: 10.7270/Q21J99XP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320103
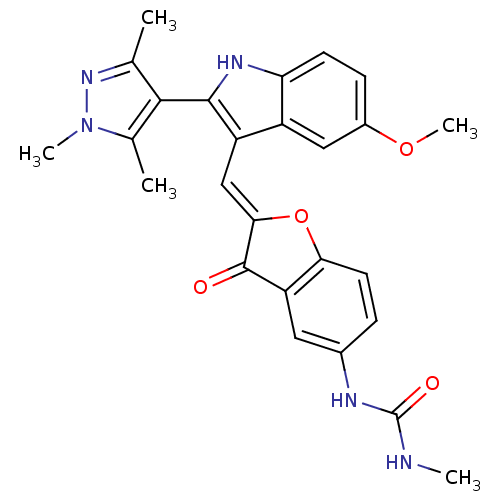
(1-(2-((5-methoxy-2-(1,3,5-trimethyl-1H-pyrazol-4-y...)Show SMILES CNC(=O)Nc1ccc2O\C(=C\c3c([nH]c4ccc(OC)cc34)-c3c(C)nn(C)c3C)C(=O)c2c1 |(-6.21,5.21,;-4.89,5.98,;-3.55,5.22,;-2.22,5.99,;-3.55,3.68,;-2.21,2.91,;-.87,3.69,;.45,2.92,;.45,1.39,;1.6,.37,;.97,-1.04,;1.74,-2.37,;1.14,-3.79,;2.05,-5.04,;1.14,-6.29,;-.32,-5.82,;-1.66,-6.59,;-2.99,-5.82,;-2.99,-4.28,;-4.33,-3.51,;-4.33,-1.97,;-1.66,-3.51,;-.32,-4.27,;3.59,-5.04,;4.49,-6.29,;4.01,-7.75,;5.95,-5.81,;5.95,-4.27,;7.2,-3.36,;4.49,-3.79,;4.01,-2.33,;-.56,-.88,;-1.59,-2.03,;-.87,.63,;-2.2,1.38,)| Show InChI InChI=1S/C26H25N5O4/c1-13-23(14(2)31(4)30-13)24-18(17-11-16(34-5)7-8-20(17)29-24)12-22-25(32)19-10-15(28-26(33)27-3)6-9-21(19)35-22/h6-12,29H,1-5H3,(H2,27,28,33)/b22-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha expressed in SF9 insect cells after 2 hrs by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308135

(1-(4-{[4-(Dimethylamino)piperidin-1-yl]carbonyl}ph...)Show SMILES CN(C)C1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C32H41N9O4/c1-38(2)27-11-13-39(14-12-27)29(42)24-5-9-26(10-6-24)34-32(43)33-25-7-3-23(4-8-25)28-35-30(40-15-19-44-20-16-40)37-31(36-28)41-17-21-45-22-18-41/h3-10,27H,11-22H2,1-2H3,(H2,33,34,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50349629

(CHEMBL1808987)Show SMILES CC(C)Oc1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)NCCN(C)C)cc1)N1CCOCC1 Show InChI InChI=1S/C28H36N8O4/c1-19(2)40-28-33-24(32-26(34-28)36-15-17-39-18-16-36)20-5-9-22(10-6-20)30-27(38)31-23-11-7-21(8-12-23)25(37)29-13-14-35(3)4/h5-12,19H,13-18H2,1-4H3,(H,29,37)(H2,30,31,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50349631
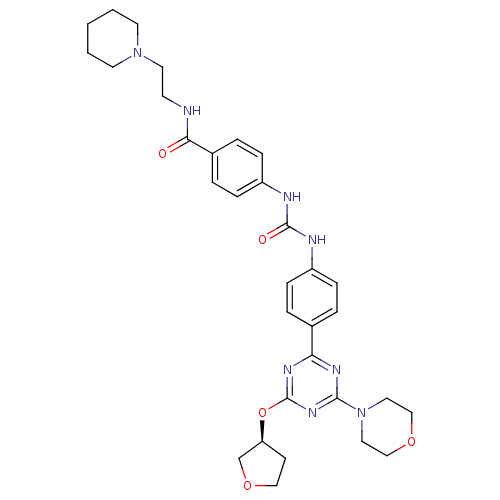
(CHEMBL1808989)Show SMILES O=C(Nc1ccc(cc1)C(=O)NCCN1CCCCC1)Nc1ccc(cc1)-c1nc(O[C@H]2CCOC2)nc(n1)N1CCOCC1 |r| Show InChI InChI=1S/C32H40N8O5/c41-29(33-13-16-39-14-2-1-3-15-39)24-6-10-26(11-7-24)35-31(42)34-25-8-4-23(5-9-25)28-36-30(40-17-20-43-21-18-40)38-32(37-28)45-27-12-19-44-22-27/h4-11,27H,1-3,12-22H2,(H,33,41)(H2,34,35,42)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50349621
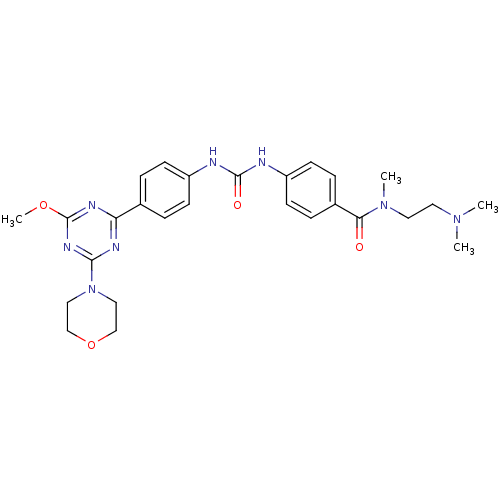
(CHEMBL1808979)Show SMILES COc1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)N(C)CCN(C)C)cc1)N1CCOCC1 Show InChI InChI=1S/C27H34N8O4/c1-33(2)13-14-34(3)24(36)20-7-11-22(12-8-20)29-26(37)28-21-9-5-19(6-10-21)23-30-25(32-27(31-23)38-4)35-15-17-39-18-16-35/h5-12H,13-18H2,1-4H3,(H2,28,29,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha expressed in SF9 insect cells after 2 hrs by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320095
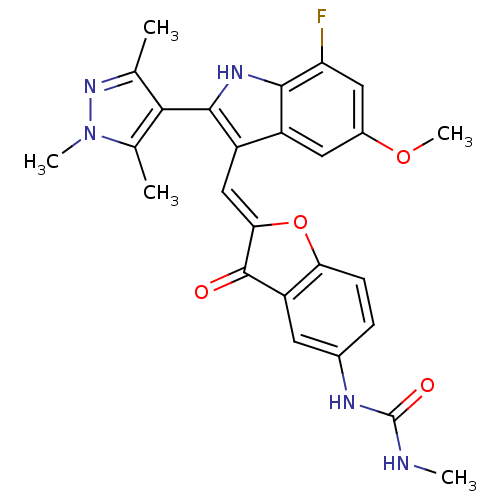
(1-(2-((7-fluoro-5-methoxy-2-(1,3,5-trimethyl-1H-py...)Show SMILES CNC(=O)Nc1ccc2O\C(=C\c3c([nH]c4c(F)cc(OC)cc34)-c3c(C)nn(C)c3C)C(=O)c2c1 |(15.41,-12.32,;16.73,-11.54,;18.07,-12.3,;19.4,-11.53,;18.07,-13.84,;19.41,-14.61,;20.75,-13.83,;22.07,-14.6,;22.07,-16.14,;23.22,-17.16,;22.59,-18.56,;23.36,-19.9,;22.76,-21.32,;23.67,-22.57,;22.77,-23.82,;21.3,-23.34,;19.96,-24.12,;19.97,-25.65,;18.63,-23.35,;18.63,-21.81,;17.29,-21.03,;17.29,-19.49,;19.96,-21.03,;21.3,-21.8,;25.21,-22.57,;26.11,-23.81,;25.63,-25.28,;27.57,-23.34,;27.57,-21.8,;28.82,-20.89,;26.11,-21.32,;25.63,-19.85,;21.06,-18.41,;20.03,-19.56,;20.75,-16.9,;19.42,-16.14,)| Show InChI InChI=1S/C26H24FN5O4/c1-12-22(13(2)32(4)31-12)24-17(16-9-15(35-5)10-19(27)23(16)30-24)11-21-25(33)18-8-14(29-26(34)28-3)6-7-20(18)36-21/h6-11,30H,1-5H3,(H2,28,29,34)/b21-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50378663

(CHEMBL1204015)Show SMILES CN(C)CCN(C)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C30H39N9O4/c1-36(2)12-13-37(3)27(40)23-6-10-25(11-7-23)32-30(41)31-24-8-4-22(5-9-24)26-33-28(38-14-18-42-19-15-38)35-29(34-26)39-16-20-43-21-17-39/h4-11H,12-21H2,1-3H3,(H2,31,32,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308156
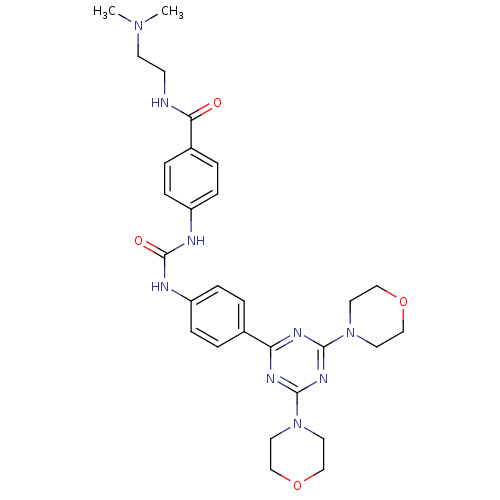
(CHEMBL592615 | N-[2-(Dimethylamino)ethyl]-4-({[4-(...)Show SMILES CN(C)CCNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C29H37N9O4/c1-36(2)12-11-30-26(39)22-5-9-24(10-6-22)32-29(40)31-23-7-3-21(4-8-23)25-33-27(37-13-17-41-18-14-37)35-28(34-25)38-15-19-42-20-16-38/h3-10H,11-20H2,1-2H3,(H,30,39)(H2,31,32,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327419

(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-mo...)Show SMILES O=C(Nc1ccncc1)Nc1ccc(cc1)-c1nc(nc(n1)N1CCOCC1)N1C2CCC1COC2 Show InChI InChI=1S/C25H28N8O3/c34-25(28-19-7-9-26-10-8-19)27-18-3-1-17(2-4-18)22-29-23(32-11-13-35-14-12-32)31-24(30-22)33-20-5-6-21(33)16-36-15-20/h1-4,7-10,20-21H,5-6,11-16H2,(H2,26,27,28,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 5869-73 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.104
BindingDB Entry DOI: 10.7270/Q2XP755V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327425
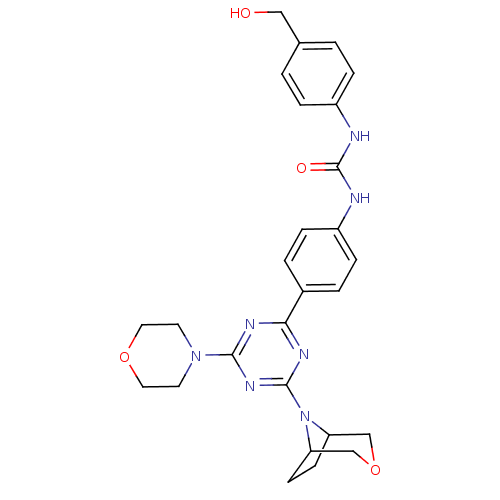
(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-mo...)Show SMILES OCc1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2C3CCC2COC3)cc1 Show InChI InChI=1S/C27H31N7O4/c35-15-18-1-5-20(6-2-18)28-27(36)29-21-7-3-19(4-8-21)24-30-25(33-11-13-37-14-12-33)32-26(31-24)34-22-9-10-23(34)17-38-16-22/h1-8,22-23,35H,9-17H2,(H2,28,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 5869-73 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.104
BindingDB Entry DOI: 10.7270/Q2XP755V |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320094
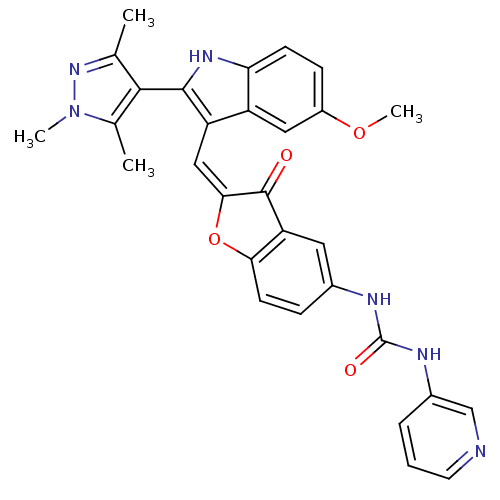
(1-(2-((5-methoxy-2-(1,3,5-trimethyl-1H-pyrazol-4-y...)Show SMILES COc1ccc2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5cccnc5)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(-3.98,-22,;-3.98,-23.54,;-2.64,-24.31,;-2.64,-25.86,;-1.31,-26.62,;.03,-25.85,;1.49,-26.32,;2.4,-25.07,;1.49,-23.82,;2.09,-22.4,;1.32,-21.07,;1.95,-19.66,;.8,-18.64,;.8,-17.11,;-.53,-16.34,;-1.86,-17.12,;-3.2,-16.35,;-3.2,-14.81,;-1.87,-14.03,;-4.54,-14.04,;-4.55,-12.51,;-5.88,-11.76,;-5.9,-10.23,;-4.57,-9.45,;-3.24,-10.21,;-3.23,-11.74,;-1.85,-18.65,;-.53,-19.4,;-.21,-20.91,;-1.24,-22.06,;.02,-24.3,;-1.31,-23.54,;3.94,-25.07,;4.84,-26.32,;4.36,-27.78,;6.3,-25.85,;6.3,-24.3,;7.55,-23.39,;4.84,-23.82,;4.36,-22.36,)| Show InChI InChI=1S/C30H26N6O4/c1-16-27(17(2)36(3)35-16)28-22(21-13-20(39-4)8-9-24(21)34-28)14-26-29(37)23-12-18(7-10-25(23)40-26)32-30(38)33-19-6-5-11-31-15-19/h5-15,34H,1-4H3,(H2,32,33,38)/b26-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327431
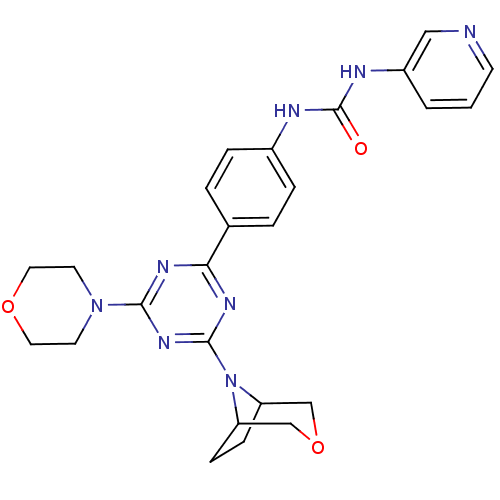
(1-(4-(4-(3-oxa-8-azabicyclo[3.2.1]octan-8-yl)-6-mo...)Show SMILES O=C(Nc1ccc(cc1)-c1nc(nc(n1)N1CCOCC1)N1C2CCC1COC2)Nc1cccnc1 Show InChI InChI=1S/C25H28N8O3/c34-25(28-19-2-1-9-26-14-19)27-18-5-3-17(4-6-18)22-29-23(32-10-12-35-13-11-32)31-24(30-22)33-20-7-8-21(33)16-36-15-20/h1-6,9,14,20-21H,7-8,10-13,15-16H2,(H2,27,28,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 5869-73 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.104
BindingDB Entry DOI: 10.7270/Q2XP755V |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315762

(CHEMBL1092402 | N-[2-(Dimethylamino)ethyl]-4-({[4-...)Show SMILES CCn1ccc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)NCCN(C)C)cc1)N1CCOCC1 Show InChI InChI=1S/C30H36N8O3/c1-4-37-15-13-25-27(37)34-26(35-28(25)38-17-19-41-20-18-38)21-5-9-23(10-6-21)32-30(40)33-24-11-7-22(8-12-24)29(39)31-14-16-36(2)3/h5-13,15H,4,14,16-20H2,1-3H3,(H,31,39)(H2,32,33,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 3169-82 (2010)
Article DOI: 10.1021/jm901783v
BindingDB Entry DOI: 10.7270/Q21J99XP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308788

(CHEMBL590587 | N-[2-(Dimethylamino)ethyl]-4-({[4-(...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)NCCN(C)C)cc1)N1CCOCC1 Show InChI InChI=1S/C28H34N10O3/c1-4-38-26-23(34-35-38)25(37-15-17-41-18-16-37)32-24(33-26)19-5-9-21(10-6-19)30-28(40)31-22-11-7-20(8-12-22)27(39)29-13-14-36(2)3/h5-12H,4,13-18H2,1-3H3,(H,29,39)(H2,30,31,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50378662
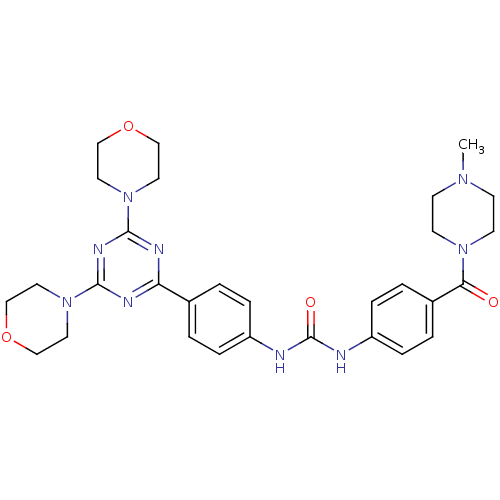
(CHEMBL1204014)Show SMILES CN1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C30H37N9O4/c1-36-10-12-37(13-11-36)27(40)23-4-8-25(9-5-23)32-30(41)31-24-6-2-22(3-7-24)26-33-28(38-14-18-42-19-15-38)35-29(34-26)39-16-20-43-21-17-39/h2-9H,10-21H2,1H3,(H2,31,32,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308156

(CHEMBL592615 | N-[2-(Dimethylamino)ethyl]-4-({[4-(...)Show SMILES CN(C)CCNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C29H37N9O4/c1-36(2)12-11-30-26(39)22-5-9-24(10-6-22)32-29(40)31-23-7-3-21(4-8-23)25-33-27(37-13-17-41-18-14-37)35-28(34-25)38-15-19-42-20-16-38/h3-10H,11-20H2,1-2H3,(H,30,39)(H2,31,32,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50320100
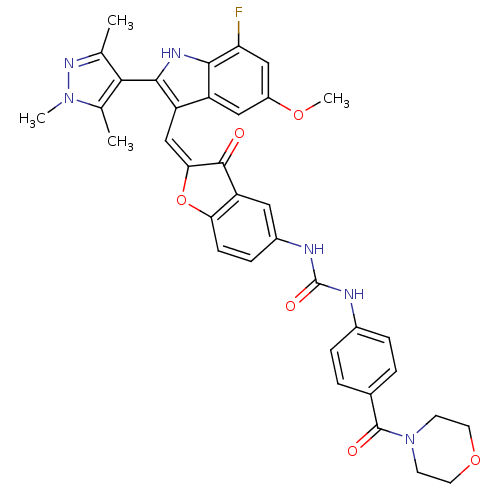
(1-(2-((7-fluoro-5-methoxy-2-(1,3,5-trimethyl-1H-py...)Show SMILES COc1cc(F)c2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N5CCOCC5)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(-4.1,-10.42,;-4.1,-11.96,;-2.77,-12.73,;-2.77,-14.28,;-1.43,-15.05,;-1.43,-16.58,;-.1,-14.27,;1.37,-14.74,;2.27,-13.49,;1.37,-12.24,;1.96,-10.82,;1.2,-9.49,;1.82,-8.08,;.67,-7.06,;.67,-5.53,;-.65,-4.75,;-1.98,-5.53,;-3.32,-4.77,;-3.33,-3.22,;-2,-2.45,;-4.67,-2.46,;-4.68,-.93,;-3.35,-.16,;-3.36,1.37,;-4.7,2.13,;-6.02,1.35,;-6.01,-.18,;-4.71,3.67,;-3.39,4.45,;-6.04,4.42,;-6.05,5.96,;-7.38,6.72,;-8.71,5.95,;-8.7,4.41,;-7.36,3.65,;-1.98,-7.07,;-.65,-7.82,;-.33,-9.33,;-1.36,-10.48,;-.1,-12.73,;-1.44,-11.96,;3.81,-13.49,;4.72,-14.74,;4.24,-16.21,;6.18,-14.27,;6.18,-12.73,;7.43,-11.82,;4.71,-12.24,;4.24,-10.78,)| Show InChI InChI=1S/C36H33FN6O6/c1-19-31(20(2)42(3)41-19)33-26(25-16-24(47-4)17-28(37)32(25)40-33)18-30-34(44)27-15-23(9-10-29(27)49-30)39-36(46)38-22-7-5-21(6-8-22)35(45)43-11-13-48-14-12-43/h5-10,15-18,40H,11-14H2,1-4H3,(H2,38,39,46)/b30-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308769
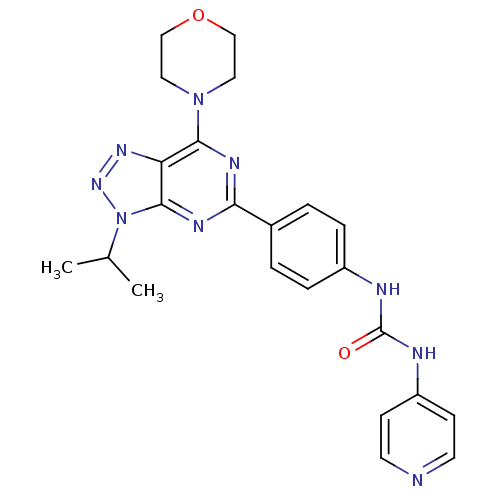
(1-[4-(3-Isopropyl-7-morpholin-4-yl-3H-[1,2,3]-tria...)Show SMILES CC(C)n1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccncc2)cc1)N1CCOCC1 Show InChI InChI=1S/C23H25N9O2/c1-15(2)32-22-19(29-30-32)21(31-11-13-34-14-12-31)27-20(28-22)16-3-5-17(6-4-16)25-23(33)26-18-7-9-24-10-8-18/h3-10,15H,11-14H2,1-2H3,(H2,24,25,26,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308785
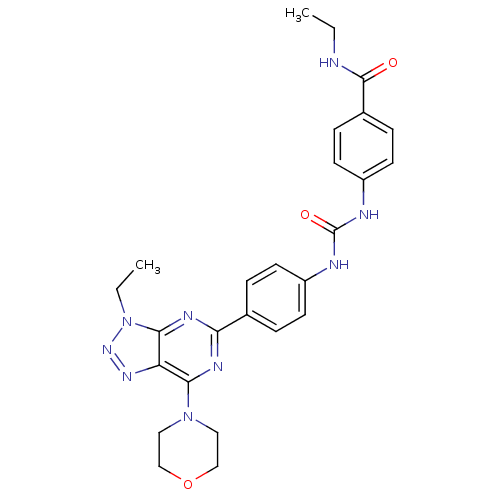
(CHEMBL591357 | N-Ethyl-4-({[4-(3-ethyl-7-morpholin...)Show SMILES CCNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3nnn(CC)c3n2)cc1 Show InChI InChI=1S/C26H29N9O3/c1-3-27-25(36)18-7-11-20(12-8-18)29-26(37)28-19-9-5-17(6-10-19)22-30-23(34-13-15-38-16-14-34)21-24(31-22)35(4-2)33-32-21/h5-12H,3-4,13-16H2,1-2H3,(H,27,36)(H2,28,29,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308772

(1-[4-(3-Isopropyl-7-morpholin-4-yl-3H-[1,2,3]-tria...)Show SMILES CC(C)n1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2cccnc2)cc1)N1CCOCC1 Show InChI InChI=1S/C23H25N9O2/c1-15(2)32-22-19(29-30-32)21(31-10-12-34-13-11-31)27-20(28-22)16-5-7-17(8-6-16)25-23(33)26-18-4-3-9-24-14-18/h3-9,14-15H,10-13H2,1-2H3,(H2,25,26,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308774
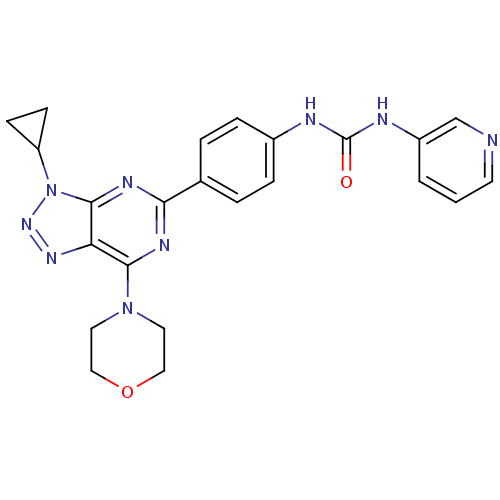
(1-(4-(3-Cyclopropyl-7-morpholino-3H-[1,2,3]-triazo...)Show SMILES O=C(Nc1ccc(cc1)-c1nc(N2CCOCC2)c2nnn(C3CC3)c2n1)Nc1cccnc1 Show InChI InChI=1S/C23H23N9O2/c33-23(26-17-2-1-9-24-14-17)25-16-5-3-15(4-6-16)20-27-21(31-10-12-34-13-11-31)19-22(28-20)32(30-29-19)18-7-8-18/h1-6,9,14,18H,7-8,10-13H2,(H2,25,26,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50349626

(CHEMBL1808984)Show SMILES CN1CCN(CC1)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(OC3COC3)nc(n2)N2CCOCC2)cc1 Show InChI InChI=1S/C29H34N8O5/c1-35-10-12-36(13-11-35)26(38)21-4-8-23(9-5-21)31-28(39)30-22-6-2-20(3-7-22)25-32-27(37-14-16-40-17-15-37)34-29(33-25)42-24-18-41-19-24/h2-9,24H,10-19H2,1H3,(H2,30,31,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha expressed in SF9 insect cells after 2 hrs by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50349622
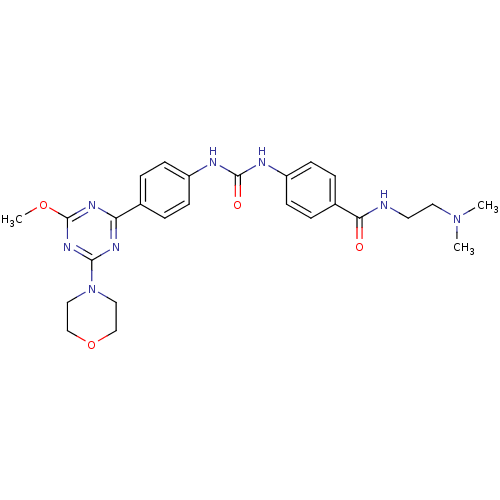
(CHEMBL1808980)Show SMILES COc1nc(nc(n1)-c1ccc(NC(=O)Nc2ccc(cc2)C(=O)NCCN(C)C)cc1)N1CCOCC1 Show InChI InChI=1S/C26H32N8O4/c1-33(2)13-12-27-23(35)19-6-10-21(11-7-19)29-25(36)28-20-8-4-18(5-9-20)22-30-24(32-26(31-22)37-3)34-14-16-38-17-15-34/h4-11H,12-17H2,1-3H3,(H,27,35)(H2,28,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha expressed in SF9 insect cells after 2 hrs by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50378663

(CHEMBL1204015)Show SMILES CN(C)CCN(C)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C30H39N9O4/c1-36(2)12-13-37(3)27(40)23-6-10-25(11-7-23)32-30(41)31-24-8-4-22(5-9-24)26-33-28(38-14-18-42-19-15-38)35-29(34-26)39-16-20-43-21-17-39/h4-11H,12-21H2,1-3H3,(H2,31,32,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50320101

(CHEMBL1082621 | N-(2-(dimethylamino)ethyl)-4-(3-(2...)Show SMILES COc1cc(F)c2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N(C)CCN(C)C)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(16.64,-8.95,;16.64,-10.49,;17.98,-11.27,;17.98,-12.81,;19.32,-13.58,;19.32,-15.12,;20.65,-12.81,;22.12,-13.28,;23.02,-12.03,;22.12,-10.78,;22.71,-9.36,;21.95,-8.02,;22.57,-6.62,;21.42,-5.6,;21.42,-4.06,;20.1,-3.29,;18.76,-4.07,;17.42,-3.3,;17.42,-1.76,;18.75,-.99,;16.08,-1,;16.07,.53,;17.4,1.31,;17.39,2.84,;16.05,3.6,;14.73,2.81,;14.74,1.29,;16.04,5.13,;17.36,5.91,;14.71,5.89,;14.69,7.42,;13.38,5.11,;12.05,5.87,;10.73,5.09,;9.39,5.85,;10.74,3.56,;18.77,-5.6,;20.1,-6.36,;20.42,-7.87,;19.38,-9.02,;20.65,-11.26,;19.31,-10.49,;24.56,-12.03,;25.46,-13.28,;24.99,-14.74,;26.93,-12.8,;26.93,-11.26,;28.18,-10.35,;25.46,-10.78,;24.99,-9.32,)| Show InChI InChI=1S/C37H38FN7O5/c1-20-32(21(2)45(6)42-20)34-27(26-17-25(49-7)18-29(38)33(26)41-34)19-31-35(46)28-16-24(12-13-30(28)50-31)40-37(48)39-23-10-8-22(9-11-23)36(47)44(5)15-14-43(3)4/h8-13,16-19,41H,14-15H2,1-7H3,(H2,39,40,48)/b31-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3delta |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308137

(1-(4-(1,4'-bipiperidine-1'-carbonyl)phenyl)-3-(4-(...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCC(CC1)N1CCCCC1)Nc1ccc(cc1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C35H45N9O4/c45-32(42-16-12-30(13-17-42)41-14-2-1-3-15-41)27-6-10-29(11-7-27)37-35(46)36-28-8-4-26(5-9-28)31-38-33(43-18-22-47-23-19-43)40-34(39-31)44-20-24-48-25-21-44/h4-11,30H,1-3,12-25H2,(H2,36,37,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50315758
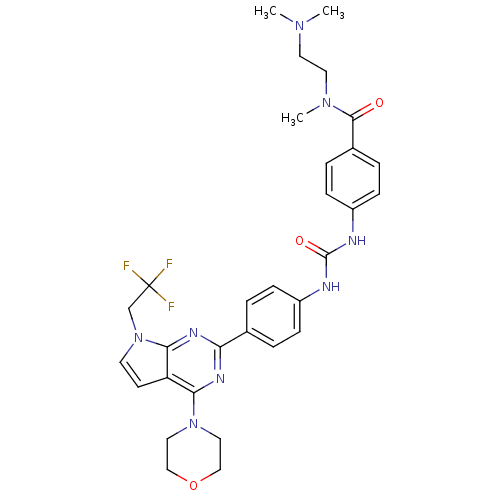
(CHEMBL1092072 | N-[2-(Dimethylamino)ethyl]-N-methy...)Show SMILES CN(C)CCN(C)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(N3CCOCC3)c3ccn(CC(F)(F)F)c3n2)cc1 Show InChI InChI=1S/C31H35F3N8O3/c1-39(2)14-15-40(3)29(43)22-6-10-24(11-7-22)36-30(44)35-23-8-4-21(5-9-23)26-37-27(41-16-18-45-19-17-41)25-12-13-42(28(25)38-26)20-31(32,33)34/h4-13H,14-20H2,1-3H3,(H2,35,36,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 3169-82 (2010)
Article DOI: 10.1021/jm901783v
BindingDB Entry DOI: 10.7270/Q21J99XP |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308768
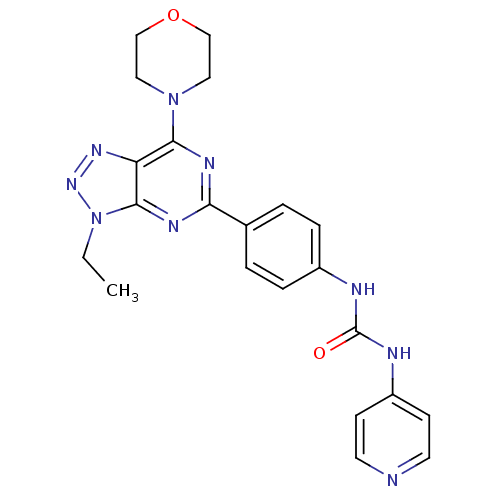
(1-[4-(3-Ethyl-7-morpholin-4-yl-3H-[1,2,3]triazolo-...)Show SMILES CCn1nnc2c(nc(nc12)-c1ccc(NC(=O)Nc2ccncc2)cc1)N1CCOCC1 Show InChI InChI=1S/C22H23N9O2/c1-2-31-21-18(28-29-31)20(30-11-13-33-14-12-30)26-19(27-21)15-3-5-16(6-4-15)24-22(32)25-17-7-9-23-10-8-17/h3-10H,2,11-14H2,1H3,(H2,23,24,25,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR by DELFIA |
J Med Chem 53: 798-810 (2010)
Article DOI: 10.1021/jm9014982
BindingDB Entry DOI: 10.7270/Q2NZ87RZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50320101

(CHEMBL1082621 | N-(2-(dimethylamino)ethyl)-4-(3-(2...)Show SMILES COc1cc(F)c2[nH]c(c(\C=C3\Oc4ccc(NC(=O)Nc5ccc(cc5)C(=O)N(C)CCN(C)C)cc4C3=O)c2c1)-c1c(C)nn(C)c1C |(16.64,-8.95,;16.64,-10.49,;17.98,-11.27,;17.98,-12.81,;19.32,-13.58,;19.32,-15.12,;20.65,-12.81,;22.12,-13.28,;23.02,-12.03,;22.12,-10.78,;22.71,-9.36,;21.95,-8.02,;22.57,-6.62,;21.42,-5.6,;21.42,-4.06,;20.1,-3.29,;18.76,-4.07,;17.42,-3.3,;17.42,-1.76,;18.75,-.99,;16.08,-1,;16.07,.53,;17.4,1.31,;17.39,2.84,;16.05,3.6,;14.73,2.81,;14.74,1.29,;16.04,5.13,;17.36,5.91,;14.71,5.89,;14.69,7.42,;13.38,5.11,;12.05,5.87,;10.73,5.09,;9.39,5.85,;10.74,3.56,;18.77,-5.6,;20.1,-6.36,;20.42,-7.87,;19.38,-9.02,;20.65,-11.26,;19.31,-10.49,;24.56,-12.03,;25.46,-13.28,;24.99,-14.74,;26.93,-12.8,;26.93,-11.26,;28.18,-10.35,;25.46,-10.78,;24.99,-9.32,)| Show InChI InChI=1S/C37H38FN7O5/c1-20-32(21(2)45(6)42-20)34-27(26-17-25(49-7)18-29(38)33(26)41-34)19-31-35(46)28-16-24(12-13-30(28)50-31)40-37(48)39-23-10-8-22(9-11-23)36(47)44(5)15-14-43(3)4/h8-13,16-19,41H,14-15H2,1-7H3,(H2,39,40,48)/b31-19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta |
Bioorg Med Chem Lett 20: 3526-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.139
BindingDB Entry DOI: 10.7270/Q2FQ9WSC |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50308136
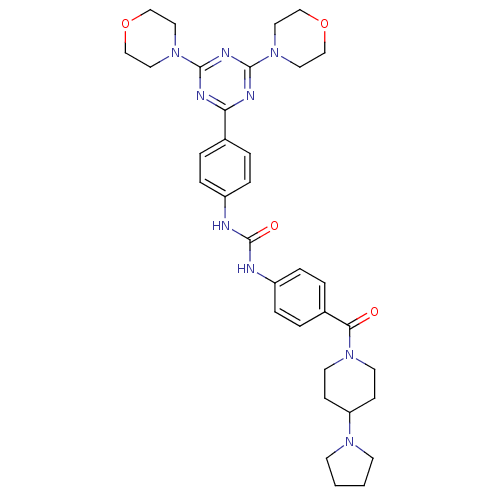
(1-[4-(4,6-Dimorpholin-4-yl-1,3,5-triazin-2-yl)-phe...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCC(CC1)N1CCCC1)Nc1ccc(cc1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C34H43N9O4/c44-31(41-15-11-29(12-16-41)40-13-1-2-14-40)26-5-9-28(10-6-26)36-34(45)35-27-7-3-25(4-8-27)30-37-32(42-17-21-46-22-18-42)39-33(38-30)43-19-23-47-24-20-43/h3-10,29H,1-2,11-24H2,(H2,35,36,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308137

(1-(4-(1,4'-bipiperidine-1'-carbonyl)phenyl)-3-(4-(...)Show SMILES O=C(Nc1ccc(cc1)C(=O)N1CCC(CC1)N1CCCCC1)Nc1ccc(cc1)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C35H45N9O4/c45-32(42-16-12-30(13-17-42)41-14-2-1-3-15-41)27-6-10-29(11-7-27)37-35(46)36-28-8-4-26(5-9-28)31-38-33(43-18-22-47-23-19-43)40-34(39-31)44-20-24-48-25-21-44/h4-11,30H,1-3,12-25H2,(H2,36,37,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50308155
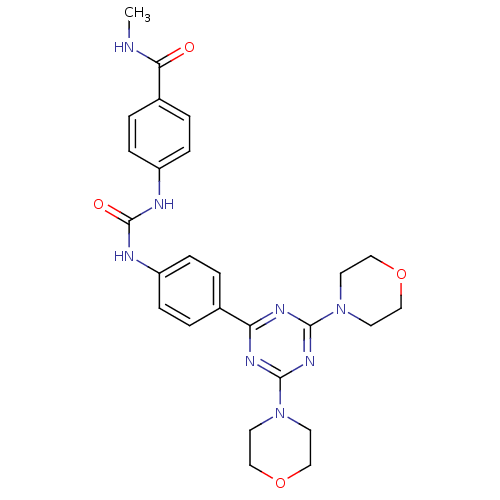
(4-(3-(4-(4,6-Dimorpholino-1,3,5-triazin-2-yl)pheny...)Show SMILES CNC(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(nc(n2)N2CCOCC2)N2CCOCC2)cc1 Show InChI InChI=1S/C26H30N8O4/c1-27-23(35)19-4-8-21(9-5-19)29-26(36)28-20-6-2-18(3-7-20)22-30-24(33-10-14-37-15-11-33)32-25(31-22)34-12-16-38-17-13-34/h2-9H,10-17H2,1H3,(H,27,35)(H2,28,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
J Med Chem 53: 2636-45 (2010)
Article DOI: 10.1021/jm901830p
BindingDB Entry DOI: 10.7270/Q2862HDH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50349619

(CHEMBL1808977)Show SMILES CN(C)CCN(C)C(=O)c1ccc(NC(=O)Nc2ccc(cc2)-c2nc(O[C@H]3CCOC3)nc(n2)N2CCOCC2)cc1 |r| Show InChI InChI=1S/C30H38N8O5/c1-36(2)13-14-37(3)27(39)22-6-10-24(11-7-22)32-29(40)31-23-8-4-21(5-9-23)26-33-28(38-15-18-41-19-16-38)35-30(34-26)43-25-12-17-42-20-25/h4-11,25H,12-20H2,1-3H3,(H2,31,32,40)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 21: 4773-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.063
BindingDB Entry DOI: 10.7270/Q20C4W4G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data